Changes in the Salmonine Community of Lake Michigan and Their Implications for Predator–Prey Balance
Abstract
We combined statistical stock assessment methods with bioenergetic calculations to assess historical changes in abundance and consumptive demand of the hatchery-supported salmonine community in Lake Michigan, with the goal of providing information needed to examine the lake's predator–prey balance. Especially for Chinook Salmon Oncorhynchus tshawytscha, the most dominant salmonine predator in the lake, our analysis revealed density-dependent changes in growth, survival, production levels, consumptive demand, and fishery characteristics, suggesting that increased salmonine abundance possibly had substantial impacts on prey abundance that led to predators being food limited. Indeed, the estimated changes in the salmonine community were consistent with historical changes in prey abundances that were previously documented for Lake Michigan. Specifically, higher salmonine abundance and consumption were estimated for the early 1980s, during which time Alewife Alosa pseudoharengus abundance experienced a marked decline, leading to a Chinook Salmon mass mortality event in 1987. Similarly, increased salmonine abundance and consumption were estimated for the years since the early 2000s, and the Alewife population in Lake Michigan has been driven to historically low levels during these years. Increased salmonine abundance estimates in recent years were attributable to improved survival rates and natural reproduction of Chinook Salmon. Although past revisions to stocking rates may have been reasonable measures taken to stabilize the predator–prey system, our analysis suggests that recent reductions in stocking have not been sufficient to reduce predatory pressure on the Alewife population; however, they may have ameliorated potential effects of increased natural reproduction of Chinook Salmon. Along with a complementary assessment of the production dynamics of key prey species, our retrospective assessment of the dynamics of the Lake Michigan predator community and their consumptive demands can provide the basis for making future fishery management decisions from an ecosystem perspective.
Given that predator–prey interactions are a major ecological process regulating the trophic structure of food webs in many aquatic ecosystems (Goodrich and Buskirk 1995; Christensen 1996; Bax 1998), altering the abundance of predatory fishes through fishing or stocking has the potential to shift predator–prey balance of a system and lead to major trophic restructuring (Carpenter et al. 1985; Pauly et al. 1998). However, because of the single-species nature of most fishery assessments, the ecosystem-level effects of altering predator abundance in aquatic systems can be hard to predict (Link and Garrison 2002). Therefore, adopting approaches that combine stock assessment methods with explicit or implicit consideration of predator–prey interactions can give better insights into potential ecosystem effects of fishery management decisions (Link and Garrison 2002). In view of the recurrent changes in predator and prey populations in the Laurentian Great Lakes (Madenjian et al. 2002; Bence et al. 2008; Claramunt et al. 2012), this study considers the importance of predator–prey balance for the management of salmonine fisheries in these lakes by focusing on the Lake Michigan fish community.
Owing to combined effects of overfishing, habitat degradation, eutrophication, and invasive species, predator–prey communities of the Great Lakes have undergone considerable changes over the last century. Many important native species have been either greatly reduced or extirpated, resulting in introduced and invasive species taking on more important roles in fish communities (Mills et al. 1993; Eshenroder and Burnham-Curtis 1999). Most notably, abundances of the once-dominant top predator, Lake Trout Salvelinus namaycush, were greatly reduced in the middle of the 20th century due to predation by Sea Lamprey Petromyzon marinus and overfishing, leading to the expansion of invasive Alewife Alosa pseudoharengus and Rainbow Smelt Osmerus mordax populations (Hansen et al. 1996; O'Gorman and Stewart 1999; Bence et al. 2008). Where they have become abundant, these invasive species have adversely affected native fish species through competition for food and predation on eggs and larvae (Crowder 1980; Eck and Wells 1987; Krueger et al. 1995; Mason and Brandt 1996). To reduce the abundance of exotic prey fishes and rehabilitate native species, millions of native (Lake Trout) and nonnative salmonines, including Chinook Salmon Oncorhynchus tshawytscha, steelhead O. mykiss (anadromous Rainbow Trout), Brown Trout Salmo trutta, and Coho Salmon O. kisutch, have been stocked into the Great Lakes since the 1960s (Claramunt et al. 2012). Due to its fast growth, high angling quality, low production cost, and high rate of Alewife predation, Chinook Salmon became the preferred species for stocking by both fishery managers and anglers (Hansen and Holey 2002). The stocking of salmonines led to reductions in Alewife and Rainbow Smelt densities and the recovery of several formerly depressed native species, such as Deepwater Sculpin Myoxocephalus thompsoni, Yellow Perch Perca flavescens, and Burbot Lota lota (Madenjian et al. 2002; Dobiesz et al. 2005) and created opportunities for the development of new recreational fisheries, bringing considerable economic benefits to the region (Kotchen et al. 2006; Fenichel et al. 2010; Dettmers et al. 2012).
Although the stocking of salmonines is credited with having led to the reduction of invasive species and re-establishment of native species in the Great Lakes, fishery managers have been concerned about the risk of overstocking as they sought to achieve a delicate balance between rehabilitating native fishes (mainly Lake Trout) by maintaining invasive prey species (mainly Alewife) at relatively low levels and sustaining an economically important recreational fisheries by maintaining adequate prey fish for the nonnative predators (i.e., Pacific salmonines) (Stewart et al. 1981; Madenjian et al. 2002; Dettmers et al. 2012). Throughout the years, there have been several instances across the Great Lakes suggesting that the predator–prey systems have been “out of balance.” In Lake Michigan, a massive die-off of Chinook Salmon, associated with an outbreak of bacterial kidney disease, occurred in the late 1980s following declines in Alewife abundances (Holey et al. 1998; Madenjian et al. 2002; Benjamin and Bence 2003). In Lake Huron, Alewife populations collapsed in the early 2000s, which was followed by a major decline in Chinook Salmon abundance (Riley et al. 2008; Roseman et al. 2008; Brenden et al. 2012). Even in Lake Superior, which is considered the least perturbed of the Great Lakes, Lake Trout abundance declines have occasionally been observed during periods of increased Rainbow Smelt densities (Mason et al. 1998; Kitchell et al. 2000; Cox and Kitchell 2005). Seeking to achieve a better predator–prey balance, Great Lakes fishery managers have adjusted stocking rates when evidence of limited predator food emerged, mostly in the form of reduced Chinook Salmon growth rates and low Alewife abundance (Bence et al. 2008; Jones and Bence 2009; Fenichel et al. 2010; Murry et al. 2010).
The Lake Michigan salmonine community has undergone some of the most striking changes in the Great Lakes, posing serious challenges to management (Madenjian et al. 2002; Claramunt et al. 2012). In the early years of the stocking program, salmonine abundance increased steadily along with stocking rates, leading many to conclude that the lake's prey populations could support more stocking (Stewart et al. 1981). Conversely, the Alewife decline and the subsequent Chinook Salmon die-off in the 1980s suggested that stocking levels had exceeded prey fish production capacity (Stewart and Ibarra 1991; Hansen and Holey 2002; Benjamin and Bence 2003). Although both the salmonine and Alewife populations rebounded following reductions in stocking rates after the Chinook Salmon die-off, salmonine and prey abundances have continued to fluctuate, which necessitated continuing adjustments to stocking (e.g., in 1998) (Madenjian et al. 2002; Claramunt et al. 2012). Similarly, major declines in Alewife abundance in the years since 2000 suggested a possible recurrence of poor feeding conditions for salmonines, prompting a 25% reduction in stocking rates in 2005 (Jones and Bence 2009; Claramunt et al. 2012). However, despite these adjustments in predator stocking levels, Alewife abundance in Lake Michigan remained low (Warner et al. 2011).
The predator–prey system of Lake Michigan was assessed quantitatively at several points during the last 30 years. Early studies, including those of Stewart et al. (1981), Stewart and Ibarra (1991), Koonce and Jones (1994), and Rutherford (1997), estimated historical predatory demand by combining results from simple age-structured population and bioenergetic models. As part of an effort to explore optimal salmonine stocking options for Lake Michigan (Jones et al. 2008), Szalai (2003) developed updated predator assessments and bioenergetics models to estimate lake-wide predatory demand using data collected through 1999. Although these studies led to a better understanding of the Lake Michigan fish communities, the predator and prey populations in the lake have undergone several major changes since the most recent study. First, as previously indicated, adult Alewife abundance remains at historically low levels (Warner et al. 2011). Second, due to the decreased importance of Diporeia in the diet of adult Alewives after the invasion of dreissenid mussels, adult Alewife energy density in Lake Michigan has declined by about 23%, increasing the amount of Alewife consumption needed to maintain preinvasion growth rates by about the same percentage (Madenjian et al. 2006; Nalepa et al. 2006). Third, just as in Lakes Huron (Johnson et al. 2010) and Ontario (Connerton et al. 2009), a large proportion of the Chinook Salmon population in Lake Michigan is now derived from natural reproduction (Williams 2012). In light of these changes, quantitative reassessment of the dynamics of Lake Michigan's salmonine populations and their consumptive demands is a prerequisite for the development of effective stocking or fishery management policies. By combining modern statistical stock assessment methods with bioenergetics calculations, we aimed to assess historical changes in the Lake Michigan salmonine community and their consumptive demand and examine their implications for predator–prey balance in the lake.
METHODS
We developed updated statistical catch-at-age (SCA) models for Chinook Salmon, steelhead, Brown Trout, and Coho Salmon in Lake Michigan using data collected through 2008 and by adding new model components (e.g., time-varying catchability) to previous assessments. A monthly time step was also incorporated in the SCA models to account for seasonal fluctuations in fishing effort and semelparous spawning by some of the species. Depending on the life history characteristics and evidence regarding population processes for individual species, the SCA models accounted for several sources of mortality, including (1) fishing mortality, (2) baseline natural mortality, (3) maturation (spawning) mortality, and (4) time-varying natural mortality. Estimated parameters were time-varying catchability (for all species), age-specific selectivities (for all species except Coho Salmon), and age-specific, time-varying natural mortality and maturation parameters (for Chinook Salmon). For Lake Trout, stock assessment models already existed for multiple management areas in portions of Lake Michigan ceded in the 1836 Treaty (Jonas 2011). We aggregated abundance estimates from these models, expanded estimates to cover unassessed areas, and calculated weighted averages of parameter estimates to provide lake-wide estimates. These existing SCA models for Lake Trout accounted for fishing, Sea Lamprey-induced, and baseline natural mortality. Finally, we generated time series of species-specific and lake-wide consumption estimates by combining age-specific abundance estimates from our salmonine SCA and the Lake Trout Treaty-ceded-water models with time series of growth data.
Model inputs and background.—Annual fishery-dependent data, collected as part of creel survey programs administered by the Michigan (MDNR), Indiana (INDNR), Illinois (ILDNR), and Wisconsin (WDNR) Departments of Natural Resources, were used as input to the stock assessment models for Chinook Salmon, steelhead, Brown Trout, and Coho Salmon. Depending on the information available for individual species, fishery-dependent data used in fitting the SCA models were: (1) total harvest from 1985 to 2008 (all species individually), (2) fishing effort directed at all salmonine species as a group from 1985 to 2008 (all species), (3) age composition of total harvest from 1985 to 2008 (all species except Coho Salmon), (4) age composition of harvest of mature fish from 1985 to 2008 (Chinook Salmon only), and (5) age composition of weir harvest (fish captured during the spawning run) from 1985 to 2008 (Chinook Salmon only). Because fishing mostly occurs during the summer months, annual summaries of the age composition of harvest were obtained using harvest data for July and August. Total salmonine targeted effort was used as an input for all models because this was consistently recorded across jurisdictions and time, and because many trips were directed more generally at the group rather than at a single species. We believe this was the best index of fishing pressure available for the salmonine fisheries.
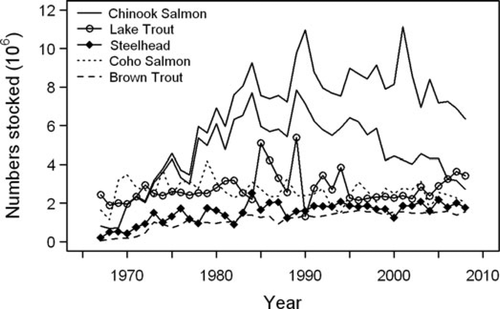
Salmonine recruitment in Lake Michigan in numbers stocked (lower solid line) and sum of stocked and wild (upper solid line) for Chinook Salmon and numbers stocked for all other species.
In addition to fishery-dependent data, other types of data were used as model input: (1) natural mortality (for steelhead, Brown Trout, and Coho Salmon) (2) stocking numbers and/or recruitment, (3) weight at age, and (4) diet composition. While natural mortality was estimated as a parameter for Chinook Salmon, we used existing natural morality estimates as input into the models for the other salmonines because previous studies found no evidence of increased natural mortality rates of these salmonines as prey abundance declines (Rutherford 1997; Szalai 2003) (Table A.1). Recruitment (Figure 1) was assumed to occur at the beginning of the year for all species. Because of shorter stream residency of Chinook Salmon juveniles, and thus better survival, and the production costs involved (Cochrane et al. 1992), Chinook Salmon are typically stocked as fingerlings and the other salmonines as yearlings. Therefore, recruitment was incorporated into the models as the number of age-0 fish for Chinook Salmon and age-1 fish for the other species. Total annual recruitment was calculated as the sum of wild recruitment and stocking numbers, and stocking numbers were adjusted for poststocking survival rates of 0.75 for Chinook Salmon, 0.5 for steelhead and Coho Salmon, and 0.75 for Brown Trout (Rutherford 1997). Annual salmonine stocking numbers were extracted from a database compiled by the U.S. Fish and Wildlife Service (USFWS 2009). Estimates of wild recruitment of Chinook Salmon from 1967 to 2008 were obtained from the Salmonid Working Group (SWG) of the Lake Michigan Technical Committee. These data were obtained using stream surveys conducted in 1979 and 1983 and oxytetracyclene (OTC) marking studies from 1992 to1995, 2001–2004, and 2007 to the present (Jonas et al. 2008; Williams 2012). Recruitment levels for the intervening years were obtained through linear interpolation. Natural recruitment was assumed to be negligible for all other salmonines; there is no evidence of substantial natural recruitment for any of the other salmonines including Lake Trout. The difference in recruitment success between Chinook Salmon and the other nonnative salmonines is believed to be because the other salmonines have longer stream residency that extends to the summer months, when streams may be too warm for their juveniles. The inability of Lake Trout to reproduce naturally in Lake Michigan is believed to be due to Sea Lamprey predation, fishing, and negative impacts from nonnative species (Jonas et al. 2008).
Salmonine stocking into Lake Michigan increased steadily during the 1960s and 1970s, reaching peak levels in the mid 1980s (Figure 1). Since 1975, Chinook Salmon stocking rates have exceeded those of all other species. Principally as the result of lake-wide management decisions in 1991, 1998, and 2005 to reduce stocking rates, Chinook Salmon stocking has generally declined since 1990. The other salmonine species have experienced variable stocking rates since the mid-1970s without any clear trends. Estimates of wild Chinook Salmon recruitment have risen steadily since the early 1970s, and data from recent years indicate that annual wild smolt production has exceeded 3 million fish (Figure 1).
Weight-at-age data (Tables A.2, A.3), collected from recreational fishers as part of the MDNR creel survey program, were used as model inputs for the estimation of consumption rates. For Chinook Salmon, we generated mean weight at age at time of harvest and at annulus formation (1985–2008) as weights on August 1 and January 1, respectively, using a linear regression of the natural log (ln) of weight on date of capture (Table A.2). Weight-at-age data for Chinook Salmon between 1965 and 1978, which were obtained based on weight data from fish during spawning runs, were derived from Rybicki (1973) and Wesley (1996). Weight-at-age estimates for the period between 1978 and 1985 were obtained through linear interpolation. For Lake Trout, weight-at-age estimates, which showed slight changes over time, were calculated as weighted averages of management area-specific, weight-at-age values from the assessment models for the 1836 Lake Michigan Treaty-ceded waters. For steelhead, Brown Trout, and Coho Salmon, we assumed constant weights over time as in previous studies (Table A.3) given that there has been no evidence of variable growth rates that can be associated with changes in prey abundance in Lake Michigan (Rutherford 1997; Madenjian et al. 2002; Szalai 2003).
Diet composition data were also used as a model input during the estimation of prey consumption. For years before 1999, we used diet composition estimates from earlier studies, including Stewart et al. (1981), Stewart and Ibarra (1991), and Rand et al. (1993). The diet composition data for Chinook Salmon, Coho Salmon, and Lake Trout were given as the proportion of small Alewives (<8 g), large Alewives (≥8 g), and other fish, using diet categories defined by Stewart et al. (1981). The diet composition data for Brown Trout and steelhead were taken from Szalai (2003). For years after 1999, we generated annual diet composition data for Chinook Salmon and Lake Trout using stomach content data compiled by the SWG. These data include weight and length category of major prey species (Alewife, Rainbow Smelt, and others) consumed by individual predators collected from multiple management areas and over several months covering the open-water fishing season. The SWG assembles lake-wide Lake Michigan salmonine diet data from fish collected from gill-net surveys conducted by MDNR as well as from angler-caught fish according to the Lake Michigan Technical Committee lake-wide diet assessment protocol (Elliott et al. 1996). Diet compositions for steelhead, Brown Trout, and Coho Salmon were assumed to remain the same as that observed for years before 1999 because more recent data were not available.
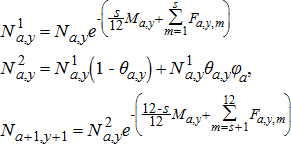 (1)
(1)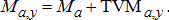 (2)
(2)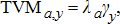 (3)
(3)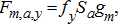 (4)
(4)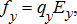 (5)
(5)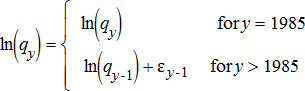 (6)
(6)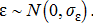
The random walk deviations, ɛ, were assumed to be normally distributed with a mean of zero and a SD of σɛ. These deviations combine both measurement error, representing the difference between measured and actual fishing effort, and process error, representing deviations from the direct proportionality assumption between fishing mortality and fishing effort.
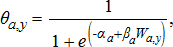 (7)
(7)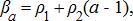 (8)
(8)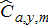 ) for all modeled species was predicted using the Baranov catch equation,
) for all modeled species was predicted using the Baranov catch equation,
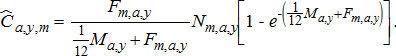 (9)
(9)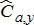 ) was calculated by summing monthly values. Age-specific harvest of mature fish (
) was calculated by summing monthly values. Age-specific harvest of mature fish (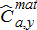 ) was calculated by multiplying age-specific harvest by the predicted maturation probability as follows:
) was calculated by multiplying age-specific harvest by the predicted maturation probability as follows:
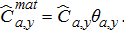 (10)
(10)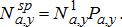 (11)
(11)Predicted age composition of spawning runs was calculated by dividing the age-specific spawning run estimates by the spawning run total. We did not include total spawning run estimates in the objective function when fitting the Chinook Salmon SCA model as the observed weir harvest data only included a small subset of spawning tributaries.
Model fitting.—The SCA models were fit by minimizing the negative log posterior probability of parameter estimates using Automatic Differentiation Model Builder (Fournier et al. 2012). This approach to estimation is also known as highest posterior density or penalized likelihood estimation (Schnute 1994). Estimated parameters consisted of q1985, ɛy (for all species), Sa (for all species except Coho Salmon), and λa, γy, βα, ρ1, and ρ2 (for Chinook Salmon). Uniform priors (on a ln scale) were specified for all parameters so that the resulting posterior distributions were mainly influenced by observed data. Given the number of data types used in model fitting for each species, the objective function used in the optimization of model fits consisted of five log-likelihood or log-prior (penalty) components for Chinook Salmon, three for Brown Trout, and steelhead, and two for Coho Salmon.
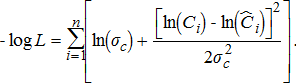 (12)
(12)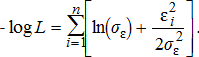 (13)
(13)The standard dispersion of observation error for total annual harvest (σc) and catchability deviations (σɛ) were assumed known for all species and were set equal to 0.08 and 0.04, respectively. Benjamin and Bence (2003) used these same values for previous assessments of Chinook Salmon. We chose to use these same values for all other species because no previous estimates were available.
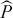 ) proportions at age using:
) proportions at age using:
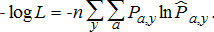 (14)
(14)The effective sample size was assumed known for each likelihood component and was set at n = 100 for age composition of total harvest, n = 50 for age composition of mature fish harvest, and n = 50 for age composition of weir harvest. The effective sample sizes weight the relative importance of likelihood components corresponding to age compositions. The use of effective sample size, rather than actual sample size, is based on the recognition that the number of independent sample units is smaller than the actual number of fish aged due to nonrandomness of samples. The effective sample sizes we used were based on analyses from other systems (e.g., Crone and Sampson 1998) and our impressions regarding the relative qualities of the age-composition data sets. Similar approaches are widely used (Merritt and Quinn 2000) given the challenges in assessing effective sample sizes analytically (Crone and Sampson 1998).
We conducted a retrospective examination of model fits, which showed that the standard deviations and effective sample sizes we assumed were reasonable. In particular, the mean square error of model fits (for harvest and catchability) closely matched the prespecified standard dispersions (σc = 0.08, σɛ = 0.04), and the deviations between observed and predicted proportions at age were consistent with the variances of the multinomial distributions given the effective sample sizes. This latter approach for evaluating effective sample sizes was generally endorsed by Francis (2011).
The fitted SCA models were considered to have converged on a highest posterior density of parameter estimates when the gradient of the objective function was less than 1.0 × 10−4 with respect to each parameter. To assess uncertainty associated with parameter estimates, posterior probability distributions for parameter estimates were obtained by Markov chain Monte Carlo (MCMC) simulations through a Metropolis Hastings algorithm (Fournier et al. 2012). The MCMC chain was run for 1 million steps and sampled every 100th step. The initial 1,000 saved steps were discarded as a burn-in period. The MCMC chains were evaluated for adequacy (convergence and sufficient information) using trace plots for each estimated parameter and derived variable as a visual check to ensure the chain was well mixed and did not show long-term patterns, the effective sample size of the saved MCMC chains, and similarity of the first 10% and last 50% of the saved chains using Geweke's (1992) tests. All MCMC chain diagnostics were conducted in R (R Development Core Team 2010) using the CODA package (Plummer et al. 2010).
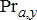 ) is divided by gross conversion efficiency (GCE) to estimate consumption. Gross production was estimated as a function of fishery yield (Ya, y), production lost to natural mortality (Da, y), production lost to spawning mortality (Ra, y) (which applies to all species other than Lake Trout), and change in biomass of the standing stock (Ba + 1, y + 1 − Ba, y), i.e.,
) is divided by gross conversion efficiency (GCE) to estimate consumption. Gross production was estimated as a function of fishery yield (Ya, y), production lost to natural mortality (Da, y), production lost to spawning mortality (Ra, y) (which applies to all species other than Lake Trout), and change in biomass of the standing stock (Ba + 1, y + 1 − Ba, y), i.e.,
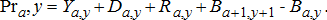 (15)
(15)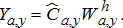 (16)
(16)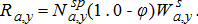 (17)
(17)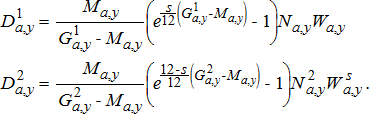 (18)
(18)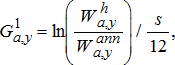 (19)
(19)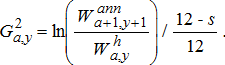 (20)
(20) (21)
(21)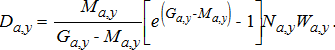 (22)
(22)Age-specific consumption rates were calculated by dividing age-specific production estimates by age-specific GCEs, which represent the fraction of consumed biomass that is converted to growth. To account for the observed drop in Alewife energy density after the dreissenid mussel invasion (Madenjian et al. 2006), we used lower GCE values for the periods after (post-1995) the invasion (Table A.5). Finally, to obtain the proportion of consumption by prey type, the total consumption calculated using the production–conversion efficiency method was partitioned into different prey types based on stomach-content data for the different salmonines (see Model inputs and background section above).
RESULTS
Model Fits
Each of the predator SCA models successfully converged on a solution, in which the maximum gradient of the objective function was less than 1.0 × 10−4. Predicted values generally matched observed data quite well for both the harvest and age compositions, and the mean absolute percent error between observed and predicted values was less than 5% for all species. Based on all criteria used to evaluate convergence, the MCMC chain for each of the parameter estimates was judged to have converged to the underlying posterior probability distribution and to contain enough information to characterize the distribution. Trace plots showed no “stickiness,” effective sample sizes were similar to actual number of saved MCMC samples, and the means of the first 10% and last 50% of the saved samples were similar based on Geweke's (1992) Z-score. Parameters were estimated with varying degrees of uncertainty, with estimates of catchability, selectivity, and maturation parameters having narrower 95% Bayesian credible intervals than estimates of year and age effects on natural mortality (Table 1). Similarly, annual abundance, biomass, and consumption were estimated with low degree of uncertainty, and CV (100·SD/mean) was estimated at less than 10% for each derived quantity.
| Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Estimate | ln(γ) | ln(λ) | ρ1 | ρ2 | α | ln(q) | ln(ɛ) | ln(Sa) |
| Chinook Salmon | HPD | −29.80, 0.61 | −22.21, −0.85 | 0.38 | −0.09 | 0.08, 2.29 | −16.59 | −0.01, 0.17 | −3.81, −0.93 |
| Lower CL | −34.95, 0.48 | −23.71, −2.05 | 0.28 | −0.16 | −0.05, 1.86 | −16.83 | −0.07, 0.07 | −3.91, −1.18 | |
| Upper CL | −11.36, 1.52 | −3.31, −0.75 | 0.62 | −0.01 | 0.30, 2.49 | −16.43 | 0.05, 0.22 | −3.49, −0.82 | |
| Steelhead | HPD | −19.67 | −0.15, 0.25 | −3.23, −0.74 | |||||
| Lower CL | −19.80 | −0.27, 0.12 | −3.46, −0.83 | ||||||
| Upper CL | −19.54 | −0.03, 0.36 | −3.05, −0.65 | ||||||
| Brown Trout | HPD | −19.69 | −0.15, 0.05 | −3.94, −0.08 | |||||
| Lower CL | −19.83 | −0.22, −0.02 | −4.19, 0.22 | ||||||
| Upper CL | −19.54 | −0.08, 0.12 | −3.69, 0.06 | ||||||
| Coho Salmon | HPD | −17.45 | −0.23, −0.42 | ||||||
| Lower CL | −17.87 | −0.61, 0.10 | |||||||
| Upper CL | −16.98 | 0.20, 0.80 | |||||||
Changes in Salmonine Fisheries
Model fits as well as observed data showed large temporal fluctuations in total salmonine harvest, and Chinook Salmon experienced greater levels of change than the other species. Chinook Salmon harvest declined sharply between the mid-1980s and the early 1990s from approximately 4.5 million kg (∼900,000 fish) to less than 1 million kg (∼200,000 fish) (Figure 2). Since the early 1990s, Chinook Salmon harvest has increased steadily to a mean of approximately 4 million kg (∼800,000 fish) between 2005 and 2008 (Figure 2). Brown Trout harvest has declined steadily throughout the modeled time period from approximately 300,000 kg in the 1980s to 100,000 kg in the late 2000s. Steelhead harvest increased from approximately 200,000 kg in the mid-1980s to approximately 500,000 kg in the early 2000s, but declined to the mid-1980 levels by the late 2000s (Figure 2). Coho Salmon harvest has fluctuated without much of a trend (Figure 2). Lake Trout harvest has sharply declined since 2000 (Figure 2), which reflected the decline in recreational fishing effort (Figure 3), but may also have been due to an increase in the minimum size limit for Lake Trout established for fishing in some management areas (Jonas 2011). The proportion by weight of Chinook Salmon in total salmonine harvest steadily fell from over 60% in the early1980s to about 30% by the mid-1990s, but has since increased annually and reached over 70% in the late 2000s.
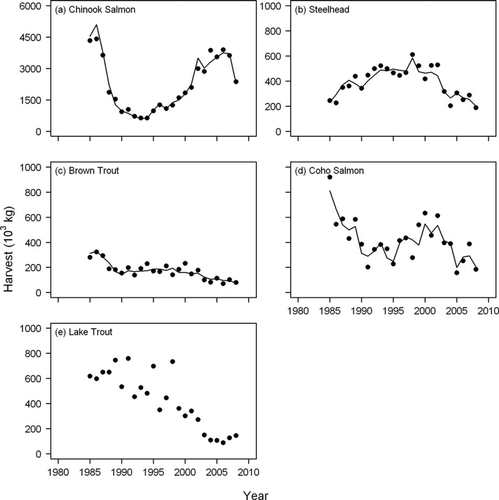
Observed (symbols) and predicted (lines) values of recreational fishery harvest of (a) Chinook Salmon, (b) steelhead, (c) Brown Trout, (d) Coho Salmon, and (e) Lake Trout in Lake Michigan.
Recreational fishing effort declined throughout the late 1980s, from a peak of over 8 million angler-hours to a low of approximately 2 million angler-hours (Figure 3), which paralleled the decline in Chinook Salmon and Lake Trout harvests. Total fishing effort has been relatively low (∼4 million angler-hours) for the past 15 years, while Chinook Salmon harvest increased throughout the 1990s and 2000s. These increases in Chinook Salmon harvest may be attributable to increased fish abundance, but there was also a large increase in catchability from the 1980s to the late 2000s (Figure 3). Chinook Salmon weight at age in the recreational fishery harvest was the highest in the early years of the time series and dropped sharply in the mid-1980s, primarily for older age-groups (Figure 4). Chinook Salmon weight at age rose again in the 1990s, albeit not to historic levels, but has decreased somewhat since 2004. Lake Trout weight at age fluctuated with no clear trends (Figure 4).
Model fits as well as observed data indicated that harvest-age composition has fluctuated over time for most of the species (Figures 4, 5). For Chinook Salmon, recreational harvest of age-1 fish was proportionally higher in the early 1990s than it was in the mid-1980s, but has declined in recent years (Figure 5). Proportions of recreational harvest of age-1 and age-2 mature fish increased from the mid-1980s to the late 1980s and have since been relatively stable, with fish of ages 2 through 4 comprising 97% of the recreational harvest of mature fish (Figure 5). Age composition in weir harvest fluctuated slightly over time, with age-2 and age-3 fish alternately comprising the highest proportion of harvest (Figure 5). The differences between the age compositions of mature fish from the recreational fisheries and weir returns largely reflect the varying selectivities of the two fisheries. For steelhead and Brown Trout, age compositions of the recreational harvest were fairly stable. For steelhead, age-3 and older fish comprised between 70% and 80% of the recreational harvest (Figure 6). For Brown Trout, the proportion of age-2 fish was consistently greater than age-3 and older fish (Figure 6).
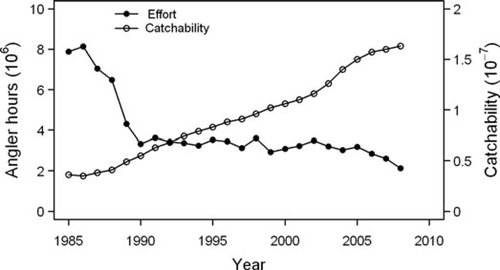
Recreational fishery effort in Lake Michigan in millions of angler-hours targeted at salmonines and average salmonine catchability.
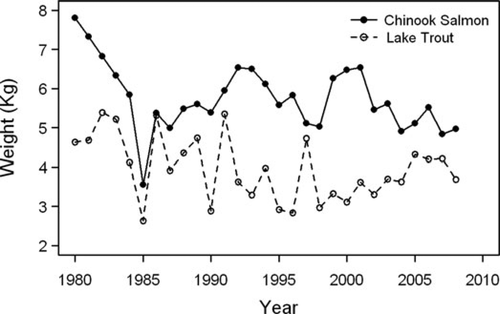
Average weight of age-3 Chinook Salmon and age 5 and older Lake Trout in the Lake Michigan recreational fishery harvest.
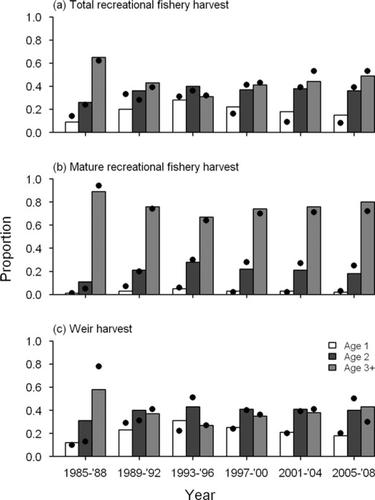
Predicted (bars) and observed (dots) age composition of (a) total recreational fishery harvest, (b) mature recreational fishery harvest, and (c) weir harvest of Chinook Salmon in Lake Michigan.
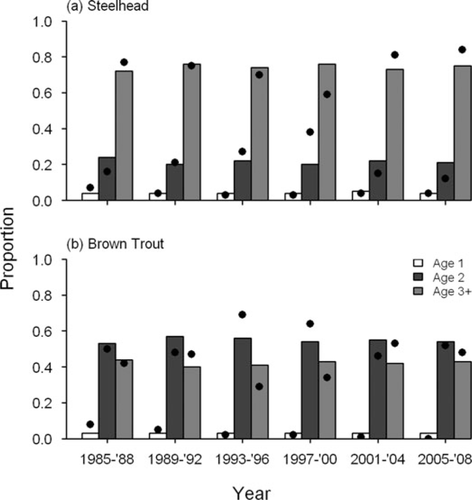
Predicted (bars) and observed (dots) age composition of recreational fishery harvest of (a) steelhead and (b) Brown Trout in Lake Michigan.
Changes in Salmonine Abundance
Model estimates indicated that total Chinook Salmon abundance increased steadily from near zero in 1968 to over 10 million fish by the early 1980s (Figure 7). From the early 1980s to the late 1990s, Chinook Salmon abundance has generally fluctuated without a trend between 10 million and 14 million fish. Overall, Chinook Salmon abundance has remained high and relatively stable despite a sharp reduction in older Chinook Salmon abundance in the late 1980s (Figure 7), indicating that total Chinook Salmon abundance during these years was dominated by age-0 and age-1 fish. Thus, fluctuations in their abundance largely reflected changes in recruitment (Figures 1, 7). During these years, total Chinook Salmon recruitment has remained relatively high at approximately 8 million fish, with increases in natural recruitment compensating for declines in the stocking of hatchery-reared fish (Figure 1). Since 2000, there has been a general downward trend in Chinook Salmon abundance as well as total recruitment. Although there has been a decline in total Chinook Salmon recruitment during these years, declines in natural mortality (particularly for age-1 and older fish) from the elevated rates seen during the late 1980s and 1990s and increased wild recruitment have helped ameliorate changes in total abundance as a result of lower stocking rates (Figures 1, 7). Age-2 and older Chinook Salmon experienced more drastic declines than age-1 fish, indicating that the die-off event mostly affected older fish (Figure 7). For steelhead, Coho Salmon, and Brown Trout, total abundance estimates increased from the beginning of the stocking program in the late 1960s until the early 1980s, and appear to have been fairly stable ever since. For these salmonines, changes in abundance generally reflected changes in stocking numbers, given that their natural mortalities were assumed to be constant over time. Estimated abundance levels for steelhead, Brown Trout, and Coho Salmon in 2008 were approximately 3.5, 2.5, and 2.0 million fish, respectively (Figure 7). For Lake Trout, abundance was predicted to have increased from approximately 4 million fish in the late 1960s to approximately 8 million fish throughout the late 1980s and mid-1990s. Lake Trout abundance subsequently declined during the late 1990s. This decline was attributable to increased mortalities during these years (Figure 7) and slightly lower stocking numbers in the preceding few years. Lake Trout abundance has since increased to approximately 8 million fish as stocking numbers increased.
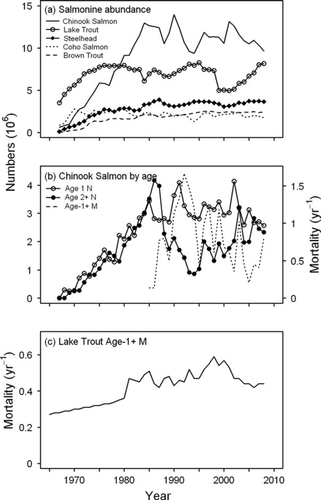
(a) Estimated lake-wide salmonine abundance, (b) abundances of age-1 and age-2 and older Chinook Salmon and average natural mortality of age-1 and older Chinook Salmon, and (c) average natural mortality of age-1 and older Lake Trout in Lake Michigan.
Changes in Salmonine Biomass, Production and Consumption
Estimated salmonine biomass increased steadily from less than 1 kilotonne (kt; 103 metric tons) in 1965 to more than 25 kt in 1986 (Figure 8), which is in concert with stocking numbers (Figure 1). Since the mid-1980s, salmonine biomass has remained relatively high, averaging approximately 23 kt, with Chinook Salmon comprising, on average, over 40% of the total salmonine biomass. Owing to the declines in abundance and weight at age of older Chinook Salmon (Figure 4), total salmonine biomass declined during the late 1980s. However, total salmonine biomass did not decline as much as Chinook Salmon biomass, indicating that increases in the abundance of other salmonines (especially Lake Trout and steelhead) made up for the decline in Chinook Salmon abundance. Chinook Salmon biomass increased moderately during 1994–1998, apparently due to the recovery of the population after the mass mortality event in the late 1980s. Although there has been a general downward trend in total Chinook Salmon abundance since 2000, Chinook Salmon biomass remained high (around 10 million kt), principally because there has been a greater proportion of older Chinook Salmon (Figure 7). Total salmonine biomass has remained relatively stable since the late 1990s despite fluctuations in biomass of Chinook Salmon and Lake Trout (Figure 8). For the other salmonines, trends in biomasses reflected changes in their abundance, given that the weight at age of these salmonines did not change over time.
In concert with total biomass, estimated salmonine production increased steadily from less than 1 kt in 1965 to close to 20 kt in the mid-1980s (Figure 8). Estimated lake-wide salmonine production has averaged around 17 kt/year since 1980. Compared with the major decline in biomass that occurred in the late 1980s as a result of the Chinook Salmon mass mortality event, declines in production were not as pronounced, mainly because the mass mortality event primarily affected the older, less productive segments of the population. Compared with the other salmonines, Chinook and Coho salmon showed relatively higher levels of production per biomass, meaning that these species have faster growth rates.
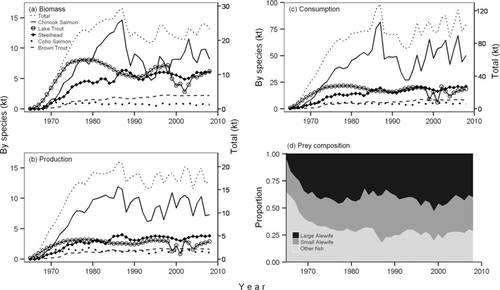
Estimated lake-wide salmonine (a) biomass, (b) production, (c) consumption, and (d) prey composition in Lake Michigan, 1965–2008, total and apportioned by species (note: total values are on the secondary y-axes).
Estimated total salmonine consumption increased steadily from less than 1 kt in 1965 to about 100 kt in the mid-1980s (Figure 8), which also largely reflected changes in stocking numbers (Figure 1). In contrast to total production, total salmonine consumption did show a decline in concert with the Chinook Salmon mass mortality event, suggesting that older age-classes accounted for a larger proportion of total consumption. Yet, the decline in total salmonine consumption was not as steep as the decline in Chinook Salmon consumption, indicating that the other salmonines played a considerable role as predators. Total salmonine consumption increased to historic high levels (∼100 kt/year) in the mid-1990s and remained relatively high since that time (Figure 8). This more recent increase in estimated total consumption is partly a consequence of the lower GCE we used to account for the decline in Alewife energy density that occurred after 1995. Just as with production, Chinook and Coho salmon showed relatively higher levels of consumption per biomass compared with the other salmonines. Finally, large Alewives represented the most common prey item (by weight) for all salmonines combined throughout the time series, with the exception of a brief period in the late 1980s when smaller Alewives became relatively more important (Figure 8). Small Alewives accounted for an increasingly larger proportion of salmonine diets in recent years.
Density-dependent Demographic Changes
Our analysis revealed evidence for two density-dependent demographic processes operating for Chinook Salmon: growth and mortality. Growth rate of Chinook Salmon appeared to be inversely related to numbers stocked (Figure 9). Similarly, growth rate of Chinook Salmon as inferred from mean weight of age-3 fish (Figure 4) appeared to be inversely related to total Chinook Salmon abundance (Figure 7). The highest weights at age for Chinook Salmon were observed during the early years of the stocking program (Figure 4; Table A.2), when their abundance was relatively low (Figure 7). The lowest weights at age for Chinook Salmon were observed prior to the mass mortality of the late 1980s, when their abundance was the highest. While there were no changes in the growth rates of the other salmonines modeled (given their constant weight at age over time), Lake Trout experienced periods of depressed growth rates (Figure 4). In contrast to growth rate, natural mortality rate of age-1 and older Chinook Salmon increased as stocking rates increased, suggesting an inverse relationship between survival rates of Chinook Salmon and number of fish stocked (Figure 9). Most significantly, the mass mortality of the late 1980s occurred during a time period when Chinook Salmon stocking rates and abundance were the highest (Figure 7). Conversely, the recent declines in natural mortality corresponded with reduced stocking rates in the 2000s.
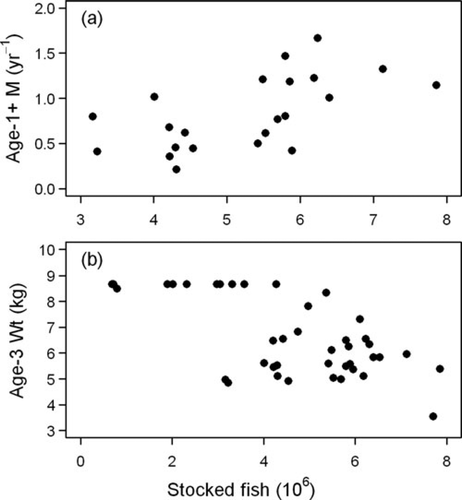
(a) Changes in average natural mortality of age-1 and older Chinook Salmon in Lake Michigan and (b) changes in average weight of age-3 Chinook Salmon in relation to stocking rate in the preceding year.
DISCUSSION
By incorporating historical and contemporary biological data into stock assessment and bioenergetics models, we assessed historical changes in the Lake Michigan salmonine community and their consumptive demand to examine their implications for the stability of the lake's predator–prey balance. Primarily due to a substantially longer time series of data than what was available for previous assessments, we were able to obtain better model fits to historical harvest data and to estimate abundances for all major salmonine predators. Our results revealed substantial changes in the abundance, biomass, and consumptive demand of predatory salmonines in Lake Michigan since the beginning of the stocking program in the mid-1960s. In light of its abundances and associated consumptive demands, the Chinook Salmon population has been a major driver of lake-wide salmonine community dynamics in Lake Michigan. Given that changes in lake-wide salmonine consumption were less pronounced than the fluctuations in Chinook Salmon consumption, the other salmonines (especially Lake Trout and steelhead) also played a considerable role as predators in the Lake Michigan fish community. On the other hand, while the changes in the abundance and biomass of the other salmonines generally reflected changes in their stocking rates because of their constant mortality and weigh at age over time, Chinook Salmon abundance and biomass changed more independently of stocking numbers due to density-dependent changes in growth and survival rates. Density-dependent changes in growth and survival rates of Chinook Salmon (Figure 9) suggested that changes in salmonine abundance and consumption had substantial impacts on the predator–prey balance. Specifically, the sharp decline in survival and growth rate that accompanied increasing salmonine abundances prior to the mass mortality of the late 1980s (Figure 7), as well as the decline in Chinook Salmon growth in the late 1990s and in the most recent years, possibly stemmed from reduced prey availability resulting from an excess of predators. Conversely, some of the improvements in Chinook Salmon survival and growth rates following reductions of stocking levels in the 1990s was likely due to improved prey availability arising from reduced predation pressure (Figure 7).
Indeed, the estimated changes in the salmonine community were consistent with historical changes in prey abundances that had previously been documented for Lake Michigan (Madenjian et al. 2002, 2005; Warner et al. 2008, 2011). Most notably, increased salmonine abundance and consumption were estimated for the early 1980s (Figure 8), during which time Alewife, the most dominant prey species, experienced a marked decline, with trawl-survey lake-wide abundance indices of age-3 and older fish falling from over 400 million fish in the early 1980s to below 200 million fish in the late 1980s (Stewart and Ibarra 1991; Madenjian et al. 2002; Warner et al. 2008, 2011). Before the Chinook Salmon mass mortality event in 1987, the decline in Alewife abundances apparently caused Chinook and Coho salmon to shift from feeding primarily on large Alewives to small Alewives, which are less preferred (Stewart and Ibarra 1991). Although the proximate cause of death for many of the fish during the mass mortality event was bacterial kidney disease, the low weight at age of Chinook Salmon in the years leading up to the event suggested that Chinook Salmon faced food limitation, which potentially increased the risk of epizootic events. Even if it was also believed that the initial outbreak of bacterial kidney disease stemmed from poor hatchery practices during the 1980s (Claramunt et al. 2012), it is likely that nutritional stress exacerbated the risk of mortality events.
Just as for the 1980s, increased salmonine abundance and consumption were estimated for the years since the early 2000s, and during this time the Alewife population in Lake Michigan has been estimated to be at very low levels, and trawl-survey lake-wide abundance estimates of age-3 and older fish have been well below 200 million fish (Warner et al. 2008, 2011). Total abundance, biomass, consumption, and production of salmonines during these years have remained high despite considerable reductions in Chinook Salmon stocking rates. This is most likely because increases in Chinook Salmon natural reproduction have made up for the stocking reductions. Based on analyses of a multiyear mass-marking program conducted throughout Lake Michigan, it has been estimated that since 2006 the proportion of naturally reproduced Chinook Salmon in the recreational fishery harvest has consistently exceeded 50% (Williams 2012). In addition to increases in wild reproduction, survey data and our assessment modeling results suggest that Chinook Salmon survival rates have changed since 1990 in a manner that has compensated for reduced stocking. Accompanying these changes in salmonine abundance, there have been fewer and smaller Alewives and fewer alternative prey species, especially during the late 2000s (Jacobs et al. 2012). Though recent levels of salmonine abundance may be large enough to have caused declines in Alewife numbers, these declines may have been exacerbated by the decline in Alewife energy density after the dreissenid mussel colonization, which led to a 22.1% increase in per capita consumption of Alewives by Chinook Salmon (Madenjian et al. 2006; Nalepa et al. 2006).
Though improvements in survival rates of Chinook Salmon in recent years could be a result of changes in environmental conditions or hatchery management practices, it is possible that they are in response to the decline in Chinook Salmon abundance, which points to the existence of a potentially important feedback mechanism that could limit the ability of managers to control the predator populations through stocking adjustments, as was suggested by Johnson et al. (2010). Similarly, though improvements in natural reproduction in recent years may be coincidental to cuts in stocking rates, increased natural reproduction could also undermine the ability of fishery managers to exert control over the predator–prey system (Claramunt et al. 2009). Conversely, it may be argued that feedback mechanisms could ultimately operate with increased Chinook Salmon abundance wherein nutritional stress leads to lower salmonine survival, thereby reducing predation pressure on Alewives and allowing prey fish recovery (Johnson et al. 2010). However, considering the 2004 Alewife collapse in the Lake Huron (Riley et al. 2008; Johnson et al. 2010; Brenden et al. 2012), we cannot be certain if self regulation will really prevent salmonine predators from overexploiting their forage base in Lake Michigan. Nevertheless, while improved survival and natural reproduction rates of Chinook Salmon may increase the risk of Alewife collapse, the resurgence of native species such as Lake Trout and Walleye Sander vitreus following periods of suppressed Alewife abundances in Lake Huron suggests some positive outcomes may result from increased Chinook Salmon predation. Furthermore, though current trends in survival and natural recruitment suggest increasing Chinook Salmon abundance, our assessment of the recreational fishery catchability (showing a large increase since the 1980s) suggests that if angler effort were to return to the levels observed in the mid-1980s, which may not be very likely in the near future given current trends in the culture of recreational fishing (O'Keefe and Miller 2011), then the Chinook Salmon population could actually decline, potentially leading to lowered predation pressure on prey fish (Claramunt et al. 2009).
Our analysis supports earlier assessments (Madenjian et al. 2002) that Chinook Salmon have been responsible for over 60% of the predation on Alewives by Lake Michigan salmonines since 1980, which is due to not only their higher abundance and higher consumption per biomass but also their heavy reliance on Alewife as a food source. The heavy reliance of Chinook Salmon on Alewife, which has also been documented by a multitude of other studies (Hagar 1984; Brandt 1986; Jude et al. 1987; Diana 1990; Elliott 1993; Warner et al. 2008), is in contrast to the more diverse diets of Brown Trout, steelhead, and Lake Trout, which tend to include a wider variety of prey fish (including Yellow Perch, Rainbow Smelt, and Bloater Coregonus hoyi) and macroinvertebrates (Rand et al. 1993). The tighter coupling between Chinook Salmon and Alewife abundance suggests that Chinook Salmon are more sensitive to declines in Alewife abundance in Lake Michigan. Given their constant annual weight at age, growth rates of the other salmonines have not been affected by Alewife abundance, supporting the contention that the other salmonines have sufficient alternative prey to make up for declines in Alewife abundance. While Chinook Salmon are the preferred species for stocking due in part to their faster growth rates, these varying feeding habits among the different salmonines point to the need to optimize the species composition of salmonines stocked in Lake Michigan.
In conclusion, fisheries managers for Lake Michigan, as for the other Great Lakes, face an interesting dilemma of whether to manage in the short term for a popular and economically important recreational fisheries or to aim for long-term sustainability by allowing recovery of native fish species that are presumed to be more suited to the ecosystem (Stewart and Ibarra 1991; Fenichel et al. 2010; Claramunt et al. 2012; Dettmers et al. 2012). While the stocking of nonnative salmonines is believed to have led to the reduction of invasive species and the creation of recreational fisheries, Lake Michigan fishery managers needed to implement major reductions stocking rates to maintain adequate prey supply for smaller, but healthier, salmonine populations. To this end, they implemented stocking reductions in 1991, 1998, 2006, and 2012 in light of evidence of predator food limitation, such as reduced Chinook Salmon growth rates or low Alewife abundance. Although the revisions to stocking rates implemented in the past may have been reasonable measures aimed at stabilizing the predator–prey system (Claramunt et al. 2012), our analysis of the salmonine community suggests that recent reductions in stocking have not been sufficient to reduce predatory pressure on the Alewife population and prevent the food limitation experienced by Chinook Salmon in Lake Michigan, although they may have ameliorated potential effects of increased natural reproduction of Chinook Salmon.
Our combined retrospective assessment of the Lake Michigan predator community and their consumptive demands can provide the basis for making future stocking decisions from an ecosystem perspective. However, the choice of appropriate stocking rates will also depend on the production capacity of the prey populations and what fraction of prey production is consumed by stocked and wild predators. This will require assessment of stock–recruitment relationships for the Lake Michigan prey species and an understanding of how consumption rates for major predator species in the lake would change depending on prey densities. Along with complementary assessments by Tsehaye et al. (in press), our retrospective assessment of the Lake Michigan predator community could ultimately contribute toward formal development of a decision model that objectively forecasts the consequences of alternative stocking options in terms of their expected success at achieving management objectives.
ACKNOWLEDGMENTS
Funding for this project was provided by the Great Lakes Fishery Trust (project number 2007.950). Additional funding was provided through the U.S. Fish and Wildlife Service Sportfish Restoration Project F-80R and a grant from Federal Aid in Sport Fish Restoration and the MDNR Game and Fish Protection Fund. The authors acknowledge the Lake Michigan Salmonid Working Group of the Lake Michigan Technical Committee for their willingness to share data and input on the project. Personnel at the MDNR Charlevoix Fisheries Research Station were instrumental in data collection and interpretation. This is manuscript 2014-04 of the Quantitative Fisheries Center at Michigan State University.
Appendix: Additional Input Data Used in the Construction of the Lake Michigan Salmonine Assessment Models
| Age | ||||||
|---|---|---|---|---|---|---|
| Species | 0 | 1 | 2 | 3 | 4 | 5 |
| Natural mortality | ||||||
| Chinook Salmona | 0.7 | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 |
| Steelhead | 0.1 | 0.1 | 0.1 | 0.5 | 1.0 | |
| Brown Trout | 0.3 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Coho Salmon | 0.5 | 0.1 | ||||
| Maturation probabilities | ||||||
| Chinook Salmonb | 0.00 | 0.12 | 0.33 | 0.99 | 0.99 | 0.99 |
| Steelhead | 0.04 | 0.14 | 0.42 | 0.62 | 0.62 | |
| Brown Trout | 0.05 | 0.50 | 0.65 | 0.70 | 0.99 | |
| Coho Salmon | 0.05 | 0.99 | ||||
- aBackground natural mortality.bFor years prior to 1981.
| Age | ||||||
|---|---|---|---|---|---|---|
| Year | 0 | 1 | 2 | 3 | 4 | 5 |
| 1967 | 0.005 | 0.586 | 3.176 | 8.193 | 9.830 | 11.800 |
| 1968 | 0.005 | 0.586 | 3.176 | 7.485 | 9.300 | 11.560 |
| 1969 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1970 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1971 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1972 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1973 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1974 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1975 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1976 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1977 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1978 | 0.005 | 0.586 | 3.176 | 7.485 | 9.630 | 12.400 |
| 1979 | 0.005 | 0.586 | 3.063 | 7.200 | 9.270 | 11.930 |
| 1980 | 0.005 | 0.586 | 2.936 | 6.766 | 8.650 | 11.070 |
| 1981 | 0.005 | 0.586 | 2.810 | 6.332 | 8.110 | 10.400 |
| 1982 | 0.005 | 0.586 | 2.683 | 5.898 | 7.570 | 9.730 |
| 1983 | 0.005 | 0.586 | 2.557 | 5.463 | 7.030 | 9.050 |
| 1984 | 0.005 | 0.586 | 2.430 | 5.029 | 6.490 | 8.380 |
| 1985 | 0.005 | 0.650 | 2.640 | 2.830 | 5.950 | 7.850 |
| 1986 | 0.005 | 0.770 | 1.730 | 4.260 | 4.470 | 7.670 |
| 1987 | 0.005 | 0.760 | 2.160 | 3.510 | 6.400 | 6.630 |
| 1988 | 0.005 | 0.750 | 2.230 | 4.260 | 5.920 | 9.100 |
| 1989 | 0.005 | 0.710 | 2.020 | 4.010 | 6.290 | 7.990 |
| 1990 | 0.005 | 0.780 | 2.110 | 4.080 | 6.590 | 9.170 |
| 1991 | 0.005 | 0.810 | 2.250 | 4.140 | 6.490 | 9.150 |
| 1992 | 0.005 | 0.890 | 2.610 | 4.880 | 7.350 | 10.100 |
| 1993 | 0.005 | 0.800 | 2.600 | 5.010 | 7.630 | 10.210 |
| 1994 | 0.005 | 0.770 | 2.240 | 4.700 | 7.420 | 10.100 |
| 1995 | 0.005 | 0.820 | 2.210 | 4.330 | 7.220 | 10.080 |
| 1996 | 0.005 | 0.840 | 2.380 | 4.330 | 6.810 | 9.790 |
| 1997 | 0.005 | 0.720 | 2.290 | 4.380 | 6.600 | 9.130 |
| 1998 | 0.005 | 0.730 | 1.850 | 3.820 | 5.990 | 8.090 |
| 1999 | 0.005 | 0.840 | 2.220 | 3.900 | 6.320 | 8.690 |
| 2000 | 0.005 | 0.870 | 2.850 | 5.160 | 7.500 | 10.530 |
| 2001 | 0.005 | 0.800 | 2.400 | 5.010 | 7.510 | 9.820 |
| 2002 | 0.005 | 0.780 | 2.240 | 4.460 | 7.450 | 10.020 |
| 2003 | 0.005 | 0.830 | 2.180 | 4.140 | 6.620 | 9.570 |
| 2004 | 0.005 | 0.760 | 2.330 | 4.200 | 6.480 | 9.060 |
| 2005 | 0.005 | 0.720 | 1.920 | 3.840 | 5.750 | 7.870 |
| 2006 | 0.005 | 0.780 | 2.110 | 3.840 | 6.140 | 8.200 |
| 2007 | 0.005 | 0.740 | 2.250 | 4.180 | 6.300 | 8.850 |
| 2008 | 0.005 | 0.740 | 1.870 | 3.730 | 5.710 | 7.690 |
| Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10+ |
| Lake Trout | 0.05 | 0.26 | 0.81 | 1.42 | 2.06 | 2.74 | 3.32 | 3.91 | 4.39 | 5.33 |
| Steelhead | 0.08 | 1.45 | 3.16 | 4.69 | 5.44 | |||||
| Brown Trout | 0.48 | 1.50 | 3.03 | 3.72 | 3.72 | |||||
| Coho Salmon | 0.23 | 1.61 | ||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.000 | 0.000 | 0.001 | 0.047 | 0.350 | 0.187 | 0.622 | 1.000 | 0.520 | 0.296 | 0.006 | 0.000 |
| Age | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Chinook Salmon | Before | 0.271 | 0.277 | 0.186 | 0.092 | 0.037 | 0.037 | |||||
| After | 0.243 | 0.224 | 0.152 | 0.075 | 0.030 | 0.030 | ||||||
| Lake Trout | Before | 0.205 | 0.192 | 0.162 | 0.144 | 0.144 | 0.128 | 0.116 | 0.105 | 0.095 | 0.085 | 0.205 |
| After | 0.199 | 0.180 | 0.148 | 0.132 | 0.131 | 0.116 | 0.105 | 0.095 | 0.087 | 0.077 | 0.199 | |
| Steelhead | Before | 0.238 | 0.221 | 0.213 | 0.154 | 0.144 | ||||||
| After | 0.222 | 0.196 | 0.185 | 0.134 | 0.125 | |||||||
| Brown Trout | Before | 0.231 | 0.221 | 0.213 | 0.154 | 0.154 | ||||||
| After | 0.216 | 0.196 | 0.185 | 0.134 | 0.134 | |||||||
| Coho Salmon | Before | 0.302 | 0.234 | |||||||||
| After | 0.285 | 0.199 | ||||||||||




