Evaluation of the Bone Injection Gun as a Method for Intraosseous Cannula Placement for Fluid Therapy in Adult Dogs
Supported by grants from University Small Research Grants, Kansas State University, and WaisMed Ltd, Caesarea, Israel 38900.
No reprints available.
Abstract
Objective— To evaluate the Bone Injection Gun (BIG) for placement of intraosseous cannulas through impact penetration and compare it with a standard Jamshidi bone marrow needle (JBMN) and to compare fluid delivery dynamics through each device.
Study Design— Randomized in vivo study.
Animals— Forty-eight mature dogs.
Methods— During surgical laboratories, dogs were randomly assigned to 2 groups (n = 24), and intraosseous access in the proximal tibial metaphysis was obtained using a BIG or JBMN. Variables measured during placement included insertion success, time required for placement, and alterations in respiratory rate (RR), heart rate (HR), and systolic blood pressure. After placement, maintenance fluids were administered to 6 dogs from each group, and fluids were administered under pressure to 6 dogs from each group to compare rates of delivery through each device. After euthanasia, the tibiae were harvested to evaluate and compare the morphologic consequences of needle and cannula placement.
Results— Successful placement occurred in 20 (83%) dogs for the BIG and 23 (96%) dogs for the JBMN, which was not significantly different (P= .3475). Time required for placement was significantly less (P= .0024) for the BIG (mean, 22.4 ± 8.2 seconds) compared with the JBMN (mean, 42.0 ± 28.1 seconds). Significant increases in RR occurred in both groups and in the HR for the BIG group, but significant differences were not noted between groups. Mean rate of pressurized fluid administration was similar for both groups. Two distinct patterns of cortical bone damage occurred, but the clinical significance of this observation is uncertain.
Conclusions— The BIG provides more rapid access to the intraosseous space for fluid administration than the JBMN.
Clinical Relevance— The BIG is an effective alternative for obtaining rapid access to the intraosseous space for emergency fluid and drug administration.
Successful treatment of many emergency conditions often requires rapid access to the systemic vascular space to deliver resuscitative drugs or fluids. However, vascular collapse or inadequate cardiac output may impair access to the peripheral vascular system, which is the most commonly used route of emergency drug and fluid administration. Other factors that may preclude access to the vascular space are small patient size, morbid obesity, trauma to sites commonly used for intravenous catheter placement, and peripheral edema. Alternative administration routes include intratracheal, intraperitoneal, subcutaneous, and intraosseous routes. Significant limitations to the intratracheal route are that only small volumes of supportive drugs can be administered and not the larger volume of fluids that are generally required.1 Also, emergency support measures such as positive pressure ventilation and cardiac massage may have to be temporarily halted during drug administration.2–4 If peripheral circulation is reduced or vascular collapse exists, the intraperitoneal and subcutaneous routes are inadequate because these conditions result in poor absorption into the systemic circulation.1
The rigid, tubular nature of bones with well-defined venous drainage that empties quickly into the systemic circulation provides a means to overcome the problem of inaccessible central or peripheral veins as well as other problems described for alternative routes.1,4,5 Intraosseous infusion (IOI) of fluids, blood products, and various drugs has been shown to be very effective as an alternative to intravenous infusion.1,5–14 An additional advantage to the intraosseous route is the relative ease and speed in which access to the vascular space can be attained. Medical and paramedical personnel, even if inexperienced in the technique of intraosseous cannula placement, can gain access to the intraosseous space in humans using a variety of cannula types within 2.5 minutes, and generally the time required is about 1 minute.11,15 The advantages of IOI have prompted the American Heart Association, the American Academy of Pediatrics, and the American College of Surgeons to recommend IOI in emergency situations when venous access is not immediately possible.14,16
The purpose of this study was to evaluate a relatively new device, the Bone Injection Gun (BIG) (WaisMed Ltd, GMS Marketing, West Hempstead, NY) that allows rapid placement of an IOI cannula through impact penetration. We hypothesized that this device would allow more rapid access to the intraosseous space than a standard Jamshidi bone marrow needle (JBMN) that requires a surgical approach through the skin and subcutaneous tissues and a boring technique for placement into the bone. Further, we speculated that the speed of placement would result in less apparent discomfort than conventional placement. We also attempted to compare fluid delivery dynamics and morphologic consequences of bone cortex penetration using each device.
Materials and methods
The Institutional Animal Care and Use Committee of Kansas State University approved the animal care and experimental protocol. Concurrent with a student surgical training laboratory (12 dogs per laboratory session), 48 mature dogs were randomly divided into 2 groups of 24. All dogs were premedicated with acepromazine (0.1 mg/kg subcutaneously [SC]), meperidine (4 mg/kg SC), and atropine (0.04 mg/kg SC). Anesthesia was induced using thiopental sodium (10 mg/kg intravenously [IV]) followed by endotracheal intubation and maintenance with halothane and oxygen. Veterinary student anesthetists performed anesthesia and monitored each dog at 5-minute intervals for respiratory rate, heart rate, ocular position, and jaw tone. Physiologic variables indicating less than a surgical anesthetic plane were addressed immediately with the help of a faculty anesthesiologist or staff anesthetist.
The same clinician investigator, who had no previous experience using the BIG, placed all cannulas and needles. The devices were inserted into the medial aspect of the right or left proximal tibial metaphysis below the medial condyle at a point level with the tibial tuberosity. Insertion was performed at a time when there were no concurrent surgical manipulations occurring. The first group had a 15-gauge intraosseous needle placed using a standard JBMN (82 mm; outside diameter [OD] = 0.0715 inches; internal diameter [ID] = 0.0525 inches). The placement technique was standardized and included a sterile presurgical preparation of the skin and a stab incision approach through the skin and subcutaneous tissue to the bone. The JBMN was then inserted through the incision to the bone and rotated alternately clockwise and counter-clockwise while applying pressure to the bone. Rotations were stopped when an abrupt loss of resistance was felt. The second group had a 15-gauge intraosseous cannula (44 mm; OD = 0.075 inches; ID = 0.055 inches) placed in the medial aspect of the right or left proximal tibia using an automatic device, the BIG, which allows rapid placement of an IOI cannula through impact penetration (Fig 1). This device uses a precharged coil to propel a trocar-cannula combination at high speed through the skin, subcutaneous tissues, and bone cortex. Adjusting the outer sleeve of the BIG to the desired level before placement can control the depth of trocar-cannula penetration (Fig 2). In order to estimate the correct insertion depth, tibial radiographs (cranial-caudal projection) of 30 randomly selected dogs that had been evaluated for various orthopedic problems were evaluated. The radiographs were separated according to the dog's weight (kg), and a chart was made that indicated median depth and range from the skin surface to the center of the medullary cavity for dogs in 4 weight groups (Table 1). For the BIG group, the BIG was adjusted to a penetration depth that corresponded to median depth for dogs in that weight range. After surgical skin preparation, the BIG was placed in contact with the skin, perpendicular to the bone surface, and the trigger was pulled to place the cannula.
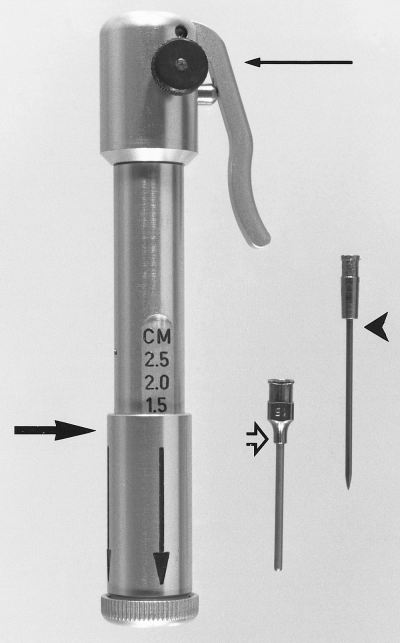
The BIG and intraosseous cannula (open arrow) with trocar (arrowhead). The assembled gun has an adjustable sleeve (large arrow) for varying the depth of trocar-cannula penetration and a trigger assembly with safety device (small arrow).
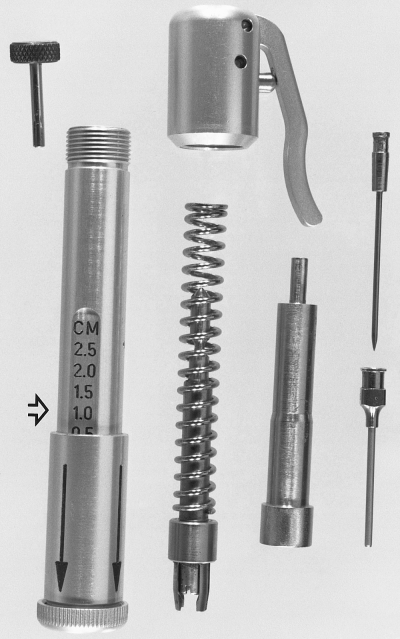
Exploded view of the BIG. Left to right are the safety catch pin, housing assembly with adjustment sleeve and end cap, trigger assembly and spring coil with trocar grip, plunger device for compressing the spring, and the trocar and cannula. Note the variable depth graduations on the housing assembly (open arrow).
| Weight (kg) | Median Depth (cm) | Range (cm) |
|---|---|---|
| 0−13.6 | 0.4 | 0.3−0.6 |
| 13.7−22.7 | 0.4 | 0.4−0.5 |
| 22.8−45.5 | 0.7 | 0.5−0.8 |
| 45.5+ | 1.0 | 1.0 |
Once positioned, the stylet from the JBMN and the trocar from the BIG cannula were withdrawn and aspiration of 2 mL of marrow confirmed correct placement. Data recorded during placement included success of device insertion, time required for placement (defined as the time from contact of scalpel blade or BIG to the skin to withdrawal of bone marrow), and changes in the physiologic variables of heart rate (HR), respiratory rate (RR), and systolic blood pressure measured using a noninvasive Doppler technique (SBP-D). The physiologic variables were obtained immediately before and after successful placement.
After placement was confirmed, lactated Ringer solution was administered to 6 dogs from each group by gravity flow through the intraosseous cannula or needle using a standard 10 drop/mL infusion set at a target rate of 10 mL/kg/h. Success of gravity flow was determined when drops/second provided the target rate. Lactated Ringer solution was administered at the same rate to the remaining dogs in each group through an IV catheter placed in one cephalic vein. At the conclusion of one of the surgical training laboratories, intraosseous lactated Ringer solution was administered to 6 dogs from each group by use of a pressure bag (constant pressure of 300 mm Hg for 8 minutes) to determine the maximum rate for isotonic crystalloid fluid administration through each device.
After completion of the surgical training exercises and intraosseous fluid administration trials, the dogs were euthanized by administration of pentobarbital sodium (88 mg/kg IV). The tibiae used for intraosseous device placement were harvested and stored in 10% neutral buffered formalin for later morphologic evaluation.
Statistical analysis of the data was performed using nonparametric methods. Comparisons of the physiologic variables between pre- and post-placement of the cannula within the 2 groups were made using the Wilcoxon's signed rank test. Comparisons between the 2 groups were made using Fisher's exact test for success of cannula placement and Wilcoxon-Mann-Whitney test for differences in physiologic variables. Results were reported as mean ± standard deviation. Significance was set at P≤ .05.
Results
Based on the presence of complete adult dentition, all dogs were judged to be mature; the exact ages were unknown. Thirty-three (69%) dogs were female, and 15 (31%) were male. Dogs weighed from 10.2 to 37.1 kg.
Successful placement of the intraosseous device occurred in 23 dogs (96%) in the JBMN group and 20 dogs (83%) in the BIG group. (Differences in successful placement were not significant [P= .3475].) Placement failures occurred because the device was positioned too far cranially, resulting in insertion in the area of the tibial crest rather than into the medullary cavity. Placement was significantly faster (P= .0024) with BIG (22.4 ± 8.2 seconds) than with JBMN (42.0 ± 28.1 seconds).
RR increased significantly in both groups during placement; the mean increase for JBMN was 6 ±11 breaths/min (P= .0045) and for BIG was 11 ± 18 breaths/min (P= .0006); however, differences between groups were not significant. Mean HR increased significantly for BIG (6 ± 10 beats/min; P= .0249) but not for JBMN (<0.5 beats/min; P= .3979); differences between groups were not significant. SBP-D did not change significantly for either group during insertion, and no difference was detected between groups. The mean SBP-D for JBMN actually decreased by 0.18 mm Hg (P= .3735), and for BIG it increased by 1.78 mm Hg (P= .4950).
Fluid delivery by gravity flow through the intraosseous devices was successful for the 6 dogs from each group. However, in 2 dogs from the BIG group and 3 dogs from the JBMN group, the fluid flow slowed noticeably but was restored by flushing the cannula or needle under pressure using a 3-mL syringe and heparinized saline.
Volumes and rates of intraosseous fluid delivery under pressure were similar for both groups. One dog in the BIG group had extensive fluid extravasation from the intraosseous space and therefore the data collected was excluded. Mean total volume administered over 8 minutes through the JBMN was 190.5 mL (rate, 74.6 mL/kg/h) and through the BIG was 220.5 mL (rate, 76.4 mL/kg/h); the delivery rates were not significantly different between groups.
On gross examination, the cortical entry points for the 2 devices could not be differentiated. Histopathologic evaluation of sections of each tibia containing penetration tracts was performed after decalcification and staining with hematoxylin and eosin. Two distinct patterns of microscopic damage were observed. Cannula placement with the BIG induced microfractures that were commonly seen extending away from both sides of the penetration tract toward the endosteal surface in a diagonal pattern. This created a conical crater circumferentially around the cortical bone tract with a narrow apex near the periosteal surface and a widened base at the endosteal surface. Fragments of cortical bone were frequently displaced into the medullary cavity from the edges of the conical cortical crater (Fig 3). The tracts from the JBMN insertion had less prominent microfractures, which were oriented perpendicular to the penetration tract, with evidence of bone debris and smaller fragments of cortical bone within the medullary cavity. Subjectively, these specimens also had more intramedullary hemorrhage (Fig 4).
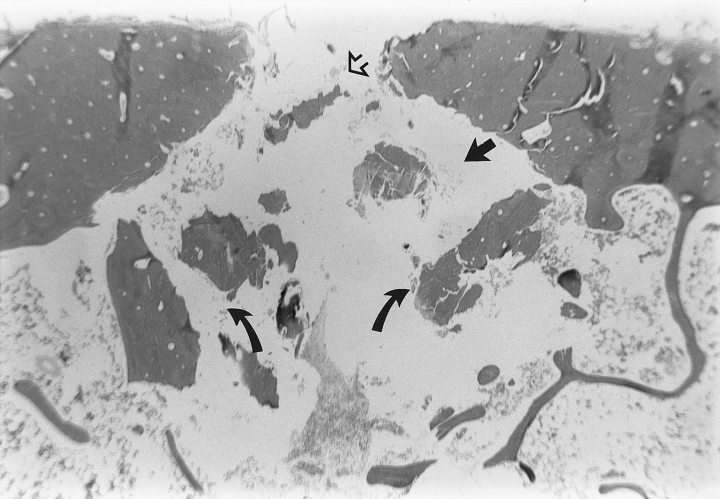
Photomicrograph of bone cortex after intraosseous cannula placement with the BIG. A conical tract through the cortical bone with a narrow periosteal surface (open arrow) and a widened endosteal surface (closed arrow) is present. Microfractures resulting from impact penetration extend away from the cannula tract in a diagonal pattern. Fragments of cortical bone are present within the medullary cavity (curved arrows).
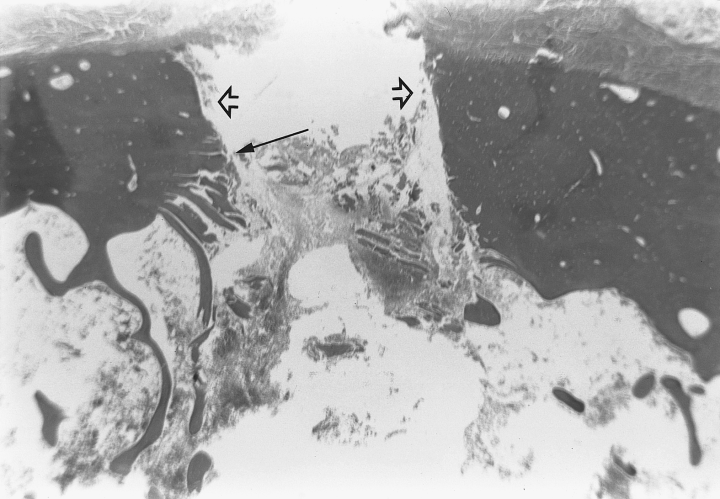
Photomicrograph of bone cortex after intraosseous needle placement with the Jamshidi bone marrow needle. The penetration tract exhibits parallel sides (open arrow) with microfractures oriented perpendicular to the tract (closed arrow). Fragments of cortical bone debris and hemorrhage are present within the medullary cavity.
Discussion
The lower successful placement rate with the BIG was likely because of an initial lack of familiarity with the device. All placement failures occurred during the first laboratory session. Although the BIG was easy to operate, there was an apparent learning curve associated with its use, indicating the need to become familiar with the device before use in an emergency situation to reduce the likelihood of incorrect placement. Another possible cause for the lower rate of success was failure to adequately identify topographic landmarks, which could result in failure to enter the intraosseous space. In a 5-year human study evaluating prehospital IOI, errors identifying landmarks was one of the most frequent causes of unsuccessful placement.14 Although it did not occur in our study, incorrect depth setting of the BIG could potentially lead to excessive or inadequate penetration of the bone and be another cause of insertion failure. Initially the JBMN was subjectively easier to use but after becoming accustomed to the BIG device, no other placement failures occurred. The one failure that occurred using the JBMN was caused by penetration of both the cis and trans cortex, which resulted from failure to correctly identify topographic landmarks.
Rapid access to the vascular space is imperative in emergency situations. We observed that the time required for placement was significantly less for the BIG. Likely reasons for this were that the BIG technique does not require either an incisional approach to the cortical surface or the repetitive twisting motion for cannula insertion. For the BIG, depression of the trigger releases the spring mechanism and the trocar-cannula is instantly propelled through the skin, subcutaneous tissue, and bone. The clinical importance of the reported significant difference in mean placement times between methods is difficult to determine from our study. However, in a recent study of IOI devices, which included the BIG and JBMN, more emergency paramedical personnel chose the BIG as their first or second preference in human medicine when the characteristics of simplicity, accuracy, and speed of placement were combined.15
The alterations in some of the monitored physiologic variables were not completely unexpected. In a previous report, an increase in HR, RR, and blood pressure occurred during insertion of an intraosseous device in ostensibly, adequately, anesthetized dogs.2 In that study, no attempt was made to determine whether the increases were significant. The increases were inferred to be an indication of the painful nature of intraosseous cannula placement and led to a recommendation to use local anesthetics during cannula placement in conscious animals. In our study, the variables that had a significant increase were RR in both groups and HR in the BIG group. The SBP-D did not change appreciably in either group. It is reasonable that the variable response to the stimulus may have been because of differences in anesthetic depth for each dog. Despite attempts to ensure a similar anesthetic depth, individual variation in response to anesthetic drugs, variability in observed patient response to noxious stimulus, and inexperience of the monitoring individual likely resulted in an apparent disparity of anesthetic levels. This may also explain why there was no significant difference in the altered variables between groups. It is also reasonable that the changes in the physiologic variables were seen because inhalation anesthetics may not block adrenergic responses to noxious stimuli during surgical procedures. A possible explanation for the lack of change in the SBP-D is that the measurement technique does not allow evaluation of pressure on a beat-to-beat basis and variations may have been noted if direct pressure measurement had been performed. Also, the time required to obtain the measurement may prevent seeing a rapid change and return of the SBP-D.
It was somewhat surprising that there was an increase in the physiologic variables in the BIG group. In humans, pain in conscious patients was related more to the infusion of fluids under pressure, and placement of the intraosseous cannula by impact penetration was considered nearly painless. The high speed of insertion is speculated to contribute to a decrease in the painful stimulus.8,16 However, it was also reported that use of local anesthetic infiltration in the skin and periosteum reduced pain on insertion. The changes in HR and RR noted in our study support the inference that intraosseous cannula placement could be painful in the conscious patient, and appropriate steps should be taken to prevent unnecessary discomfort.
An increase in intraosseous pressure associated with IOI into the proximal tibia of conscious goats has been reported to contribute to clinical signs of limb discomfort.17 We noted no elevations in monitored variables of 12 dogs during high-pressure infusion. It is possible that the level of anesthesia was sufficient during the time of infusion that the stimulus was insufficient to invoke a response.
We have shown that fluids can be administered by gravity flow into the intraosseous space. However, periodic pressurized flushing of the cannula or a needle with heparinized saline may be needed to maintain flow. Fluid delivery to dogs in emergency situations often requires rates of up to 90 mL/kg/h.1,18 The fluid rate achieved during 300-mm Hg pressure infusion did not reach this recommended rate. This is likely because of the resistance to flow at or near the venous exit points from the intraosseous cavity.17 Intraosseous flow rates are primarily affected by flow through the medullary cavity.12 Our findings concur with others and indicate that IOI of isotonic crystalloids through a single device may not be adequate as the only means for rapid volume replacement.19 However, the fluid volume that can be administered may facilitate access to other peripheral veins or might be increased by use of multiple IOI devices.
Morphologic observations show that placement of an intraosseous device using the BIG and JBMN created different types of cortical damage. It has been reported that as a projectile perforates bone, a crater or cone lesion is created with the larger diameter at the exit point.20,21 The characteristics of the bone damage created by impact penetration of the trocar-cannula using the BIG were similar. Microscopically, the microfractures seen extending away from the cannula tract in a diagonal pattern would translate into a cone shape when viewed in 3 dimensions. This would be similar to the crater caused by a projectile hitting a pane of glass.20 This type of bone damage occurred consistently within the BIG group. Structural damage caused by insertion of the JBMN would be similar to the damage seen when smooth orthopedic fixation pins are inserted by hand.22 Wobble that occurs during placement, which is also likely with JBMN placement, may contribute to cortical damage.23 It is difficult to determine from our study whether one type of damage is clinically more important than the other.
The technique and value of IOI of fluids and drugs have been known for many years; however, routine use of this potentially life-saving procedure is fairly uncommon. The limited use of IOI in veterinary medicine seems to parallel its use in the human field. A recent study in the human field concerning IOI in adults has shown that despite a relatively high (74%) awareness of the technique as an option in resuscitative efforts, very few personnel (7%) actually used the technique. Similarly, in 157 emergency medicine departments surveyed that actively trained the medical and paramedical staff, only 11% taught the technique of emergency IOI.24 In 1 author's experience at 5 veterinary teaching hospitals, the value of IOI is mentioned rarely and the technique is only briefly described if at all. Reasons for limited use include lack of familiarity with the technique and concern or fear of producing unwanted complications.16 Potential complications include introducing infection into the bone, inducing fat embolism, compartment syndrome, and perhaps causing pain and discomfort. The complication rate for IOI may be less than what occurs with standard IV infusion. A review of more than 4,000 cases of IOI detected a 0.6% bone infection rate,1,2,6,7,11,12,16 considerably less than the 3.7% infection rate seen with IV infusions.16,25 Fat embolization is seemingly more an academic concern in that despite knowing embolization can occur there have been no reported clinical problems.26 The incidence of fat embolization was no higher when IOI was used during cardiopulmonary resuscitation (CPR) than when CPR was performed without IOI.27 Another potential and relatively uncommon complication that has not been reported clinically in animals is compartment syndrome, which may be caused by extravasation of fluids during prolonged infusion or failure of the microvasculature within muscles adjacent to the bone.13,28–30
The BIG has been shown to be an effective means of gaining rapid access to the intraosseous space for fluid administration. Manufacturer recommendations for use of the BIG include severe trauma, shock, or other conditions that require emergency resuscitation. The BIG can also be used as alternative when unsuccessful IV catheter placement occurs and for diagnostic bone marrow aspiration. Proper training with this device would likely diminish incidents of failure of adequate insertion. The clinical significance of the differences in insertion time and induced bone trauma between the BIG and the JBMN could not be determined from our study.




