Evidence for Cooperativity in the Rejection of Cardiac Grafts Mediated by CD4+ TCR Tg T Cells Specific for a Defined Allopeptide
Abstract
Understanding the mechanisms of rejection of organs transplanted between unrelated individuals is confounded by the complexity of the alloantigens and the diversity of T cells responding to these alloantigens. To circumvent these problems, we developed a transgenic (Tg) C57BL/6 model system in which the T-cell receptor (TCR) expressed by CD4 T cells is specific for a defined allogeneic H-2Kd peptide and the cardiac donor expressed H-2Kd as a transgene on the C57BL/6 background (B6.Kd). These TCR Tg T cells were previously shown to mediate rapid rejection of a B10.D2 cardiac allograft when transferred to Rag1 recipients, demonstrating that the “indirect” pathway of allorecognition is sufficient for complete rejection in the absence of other T cells or antibody. Here, we report that B6.Kd hearts were rejected in an accelerated fashion by Rag1−/− TCR Tg T cells adoptively transferred to normal B6 recipients. Rejection in this model was associated with large myocardial infarcts and significant coronary artery inflammation. Moreover, transferred TCR Tg CD4+ cells mediated allograft injury without the requirement for cytotoxic function from recipient-derived CD8 T cells. A non-linear relationship was observed between the initial precursor frequency of the antigen-specific TCR Tg cells and the ultimate tempo of acute rejection, which is taken as evidence for cooperativity between components of the system.
Introduction
A central concept in our understanding of allograft rejection is that the initial precursor frequency of alloantigen-specific cells is a key determinate of the rate of allograft rejection. Rapid rejection is typically interpreted as evidence of a high precursor frequency prior to transplant, while rejection at longer times post-transplant is taken as evidence of a weaker immune response due to a lower number of initial alloantigen-specific precursor T cells. The more rapid transplant rejection associated with an increased number of discrete alloantigenic differences between donor and recipient is similarly thought to reflect the recruitment of a larger number of naïve precursor cells of different peptide/MHC (pMHC) epitope specificities into the functional antiallograft immune response.
To gain further insight into the dynamic behavior of individual allopeptides-specific T cells during the course of transplant rejection, we produced Tg mice that allow the restriction of the overall immune response in vivo to a single pMHC epitope. The T cells from the TCR Tg C57BL/6 (B6) mice recognize a single peptide derived from the H-2Kd MHC class I molecule presented by the I-Ab class II molecule (Kd54-68/I-Ab). The TCR Tg mice were crossed to the B6.Rag−/− line so that endogenous TCR chain rearrangements were blocked. In addition, we have produced a B6 Tg mouse line that expresses the native Kd molecule with the same tissue expression pattern as normal MHC I molecules. The Kd Tg mice were crossed on the CD901/1 background (B6.PLthy1.1) so that the monoclonal T cells can be physically identified in congenic B6.CD901/1 recipient mice by mAb staining of CD90.2.
In this report, we analyze the in vivo response to a B6.Kd vascularized cardiac allograft of both the TCR Tg mice themselves and the response of TCR Tg Rag1−/− T cells adoptively transferred into normal immunocompetent mice. We found an unexpected, non-linear, pattern of the time to rejection versus the initial precursor frequency of these allopeptide-specific cells.
Materials and Methods
Mice
C57BL/6J (B6; H-2b), B6.PL-Thy1.1 (B6.CD901/1; H-2b) and C57BL/6J-Rag1tm1 Mom (B6-Rag1−/−; H-2b) were purchased from the Jackson Laboratory (Bar Harbor, ME). Tg mice lines TCR34 and TCR75 expressing αβ T-cell receptors (TCR) specific for I-Ab/H-2Kd54-68 epitope were previously characterized (1). The H-2Kd Tg was crossed onto the B6.CD901/1 strain to obtain B6.Kd.CD901/1 mice to avoid detection of CD90.2 antigen in non-T lineage cells of the allograft. All mice were kept and bred in our facility in accordance with NIH regulations and the studies using these mice were approved by the Institutional Animal Care and Use Committee.
Cloning of genomic DNA encoding H-2Kd molecule
A 7.8 Kb Hind III fragment containing the entire genomic H-2Kd gene, including 2 Kb of 5' flanking DNA with the known promoter elements for regulated expression of class I MHC molecules (2), was obtained from DNA prepared from B10.D2 mouse liver. A sized selected fraction of Hind III digested DNA was ligated to the Hind III site of Zap express vector and packaged using the Gigapack Gold III system (Stratagene) to produce a phage library. A probe was produced by PCR amplification of reverse-transcribed cDNA from B10.D2 spleen cells resulting in a 1.2Kb H-2Kd cDNA probe. Using this probe, the phage library containing the full-length genomic H-2Kd sequence was screened, expanded and recloned several times. The genomic H-2Kd gene was excised and moved to a pBK-CMV phagemid, and the resulting material was partially sequenced and confirmed as the genomic H-2Kd gene. The resulting phagemid were used to isolate a linear 7.8 Kb Hind III genomic H-2Kd DNA (see supplementary material) and this DNA was microinjected into B6 mouse embryos in the UAB Transgenic Mouse Facility. B6.Kd Tg founder mice were screened by anti-H-2Kd staining of blood lymphocytes and analyzed by flow cytometry.
Flow cytometry and immunohistochemical analyses
Flow cytometric analysis of spleen cells from various strains that were stained with FITC-anti-Kd (clone SF1.1-1, Pharmingen), FITC anti-CD90.2 (clone 30H-12) and PE anti-CD4 (clone GK1.5) was performed using either a FACScan® or FACSCalibur® Instrument (Becton Dickinson, Mountain View, CA). Frozen sections of hearts from various strains were stained with FITC labeled anti-Kd mAb followed by HRP conjugated goat anti-FITC or with primary rat mAb specific for CD90.2 (clone 30H12), CD4 (clone H129) or CD8 (clone 53-6.7) obtained from Pharmingen, followed by biotin-anti-Rat-IgG that had been absorbed with mouse IgG (Vector Labs, Burlingame, CA), ABC-HRP complex (Vector), and the signal developed with DAB.
Adoptive transfer of TCR Tg T cells
Spleen cells were obtained from Rag−/− TCR Tg mice and the fraction of cells stained for CD4 and CD90.2 was determined by flow cytometry. Graded numbers of CD4+CD90.2+ TCR Tg T cells were injected intravenously and 1–2 days later the mice were transplanted.
Heterotopic cardiac transplantation
The surgical technique to place heterotopic cardiac allografts was adapted from the technique of Ono and Lindsey (3). Briefly, the donor aorta was anastomosed in end-to-side fashion to the recipient abdominal aorta and the pulmonary artery was similarly anastomosed to the vena cava. The heart was palpated daily; no detectable beating was presumed to be rejection, which was confirmed by laparotomy.
Results
Generation of B6.Kd Tg mice
To limit the antigenic complexity of a target allograft, we have constructed mice that express the H-2Kd molecule as a transgene in B6 mice to allow analysis of the population dynamics of these T cells during allograft rejection in an otherwise normal recipient immune system. A 7.8 Kb segment of DNA containing the entire genomic H-2Kd sequence along with several Kb of flanking sequence was cloned and microinjected into B6 embryos. The density of Kd expression by flow cytometry analysis and immunohistochemistry analysis is similar between the Tg B6.Kd mice and normal H-2d mice (Figure 1).
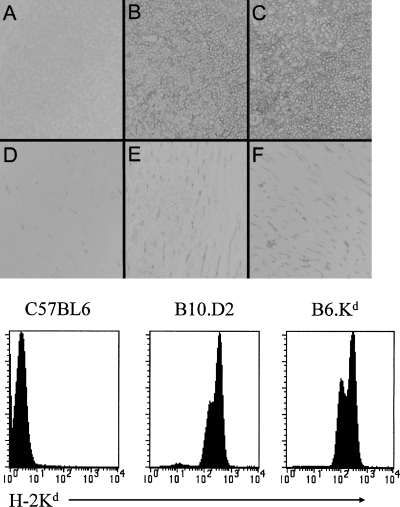
Expression of Tg H-2Kd compared to endogenous H-2Kd. Frozen sections of spleen (A–C) and heart (D–F) from normal, unimmunized B6, B10.D2 and B6.Kd mice were stained with FITC labeled anti-Kd mAb (clone SF1.1-1, Pharmingen) followed by HRP conjugated goat ant-FITC and the signal developed with DAB. The lower panel shows flow cytometric analysis of spleen cells from the same strains stained with FITC-anti-Kd. No differences were found in the density of Kd expression in multiple similar studies.
TCR Tg T cells were transferred into B6.CD901/1 mice and the frequency of TCR Tg T cells determined with a CD90.2 mAb. In preliminary studies, we found that CD90 was expressed in the vascular endothelium of cardiac tissue from B6.Kd mice after transplantation, which confounded the identification of CD90.2+ TCR Tg cells within the allograft myocardium (not shown). Thus, the H-2Kd transgene was crossed onto B6.CD901/1 mice so that CD90.2+ TCR Tg T cells could be uniquely identified in normal B6.CD901/1 mice. Endogenously rearranged TCRs were eliminated by crossing the TCR Tg mice onto the B6.Rag1−/− background.
Graft rejection by TCR Tg T cells
We have previously described a Kd-derived peptide Kd54-68 as potentially a dominant allopeptide recognized by H-2b mice and produced multiple T-cell clones expressing that specificity (4). Two of these CD4+ T-cell clones that differed in sensitivity to the Kd54-68 peptide were chosen for production of TCR Tg mice. To determine whether these TCR Tg mice could cause allograft rejection in vivo, full-thickness tail skin or heterotopic vascularized cardiac allografts were transplanted from either B6.Kd Tg, B10.D2 (H-2d) or B10.BR (H-2k), into the intact TCR Tg mice and the survival of these allografts were determined(Figure 2). Interestingly, the responses of non-Tg normal B6.CD901/1 mice to the tissue from the B6.Kd Tg mice that have only this single MHC class I alloantigenic difference were weak. Full-thickness tail skin grafts were rejected around day 14. Recipients of vascularized heterotopic heart allografts show a dichotomous pattern of transplant survival. About half of the transplanted B6 mice reject a B6.Kd heart in about 3 weeks, while the remaining animals had a “rejection episode” as detected by a decreased intensity of the ventricular beat around this time, but went on to long-term survival (>100 days) without any intervention. The TCR34 line rejected these Kd negative strain B10.BR allografts at a tempo equivalent to normal B6 mice. By contrast, TCR75 mice, which have a larger population of the CD4+ Tg TCR+ cells (and many fewer total CD8+ T cells) reject both skin and hearts from the Kd negative strain B10.BR more slowly than normal B6 mice. The data suggest that there is a lower precursor frequency of normal T cells specific for third party antigens in mice that efficiently select the TCR Tg T cells.
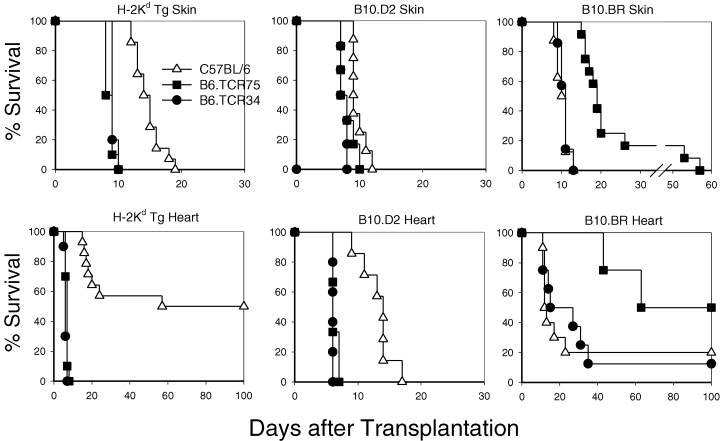
Survival of skin and cardiac grafts in TCR Tg versus normal B6 mice. Skin (upper panels) and cardiac grafts (lower panels) from B6.Kd, B10.D2 and third party B10.BR mice were grafted in B6 mice (Δ) mice, B6.TCR75 mice () or B6.TCR34 (=) Tg mice. The survival of grafts was monitored daily.
To further probe the capabilities of these TCR Tg cells to reject allografts in vivo, we crossed both TCR Tg lines onto the Rag1−/− background to block any endogenous TCR rearrangements. B6.Rag1−/−.TCR75 Tg T cells were adoptively transferred into B6.CD901/1 or into perforin knock-out mice (B6.pfp−/−). The survival of B6.Kd hearts in normal B6.CD901/1 mice was replotted from Figure 2 and shows the dichotomous survival pattern in normal mice to the isolated MHC class I difference presented by B6.Kd Tg tissue in normal non-TCR Tg mice. In contrast, transplantation of B6.Kd hearts into B6.pfp−/− mice that are deficient in perforin expression resulted in an even less vigorous response (1 mouse rejected at day 20 and 4 other mice developed long-term survival) (Figure 3). Thus, this single MHC class I alloantigenic difference stimulates a relatively weak immune response in normal B6 mice and rejection by even a fraction of the animals was dependent on the secretory pathway of CTL function in the recipient mice. In contrast, 1 × 106 Rag1−/−.TCR75 T cells transferred to either normal B6.CD901/1 recipients or B6.pfp−/− mice that were subsequently given a B6.Kd heart, induced rapid rejection with no significant difference in time to rejection (Figure 3). Although the extent of interstitial infiltrate is significant and patchy myocardial necrosis associated with dense infiltrates of the TCR Tg cells was present, the consistent morphological feature at complete rejection was the presence of large myocardial infarcts, usually in association with significant coronary artery inflammatory lesions (Figure 4).
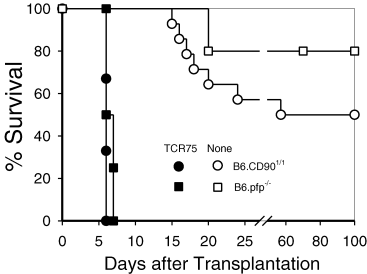
B6.Kd cardiac allografts were placed in either normal B6.CD901/1 mice (circles) or perforin knock-out mice (squares), either alone (open symbols) or 1 day after adoptive transfer of 1 × 106 Rag−/−.TCR75 T cells (closed symbols).

Representative photomicrographs of B6.Kd. CD901/1 cardiac allografts in normal B6.CD901/1 mice after adoptive transfer of 1 × 106 Rag−/−.TCR75 T cells at day 6, after palpable beating has stopped. Conventional H&E stain (A), immunoperoxidase stains for CD90.2 (B), CD4 (C) and CD8 (D). Horizontal bar represents 50 μm. Note the cellular vasculitis of a large coronary artery (large arrows) and extensive myocardial infarction (small arrows).
Threshold initial number of TCR Tg T cells required to mediate allograft rejection
Rejection of B6.Kd.CD901/1 hearts by Rag1−/− TCR Tg T cells, adoptively transferred into normal congenic mice, was dose-dependent (Figure 5). The survival of B6.Kd hearts in normal B6.CD901/1 mice, which was replotted from Figure 2, shows the dichotomous survival pattern in normal mice to the isolated MHC class I difference presented by B6.Kd Tg tissue. However, the relationship between median survival time (MST) and frequency of TCR Tg T cells was highly non-linear. At doses of 107–105 TCR Tg T cells, rejection is quite consistent with complete rejection at day 6 after transplant in most mice. With 104 TCR75 T cells, 6 out of 8 mice rejected between day 10 and 13, but the other 2 did not reject at all. With 104 TCR34 T cells, 5 out of 6 mice rejected between days 7 and 8 and 1 animal rejected at day 24. The apparent increased potency of the TCR34 TCR Tg cells is consistent with the 3-fold increase in sensitivity of these T cells to Kd54-68 in proliferation assays (data not shown). At a dose of 103 TCR Tg T cells, the TCR75 T cells did not accelerate rejection beyond what was found with no T-cell transfer, while the animals given TCR34 TCR Tg T cells showed a prolonged time to rejection compared to controls, although the difference was not statistically significant (p = 0.31 by the Wilcoxon rank sum test).
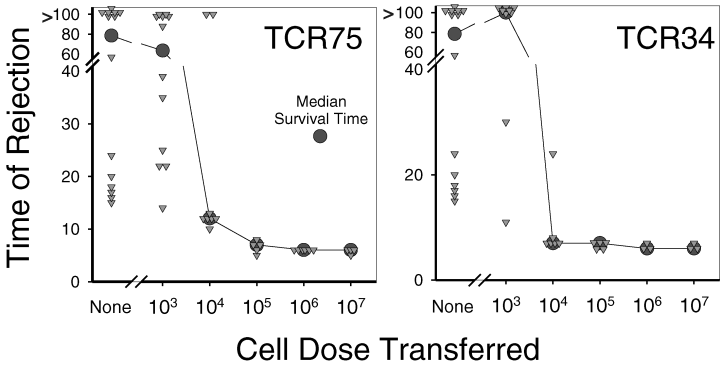
Survival of B6.Kd vascularized cardiac allografts in B6 mice after adoptive transfer of the indicated number (or no transfer) of either B6.Rag1−/ − .TCR75 or B6.Rag1−/ − .TCR34 CD4 TCR Tg T cells i.v. 1–2 days prior to transplantation. Each triangle represents an individual animal and the circle represents the median survival time for the group of animals with the same number of T cell Tg cells transferred.
Discussion
The simplest approach to studying TCR Tg T cells in allograft rejection is to examine the immune responses of the TCR Tg animals themselves. Using this approach, females from several different TCR Tg lines, expressing CD8 T cells specific for the male H-Y antigen, have been transplanted with male grafts. Some of these lines fail to reject male skin (5), while others show vigorous responses (6–5). In addition, two independent CD8+ TCR Tg mice that directly recognize an MHC class I antigen (the 2C line, a B6 strain TCR Tg specific for H-2Ld (9); and the BM3 line, a CBA strain TCR Tg specific for H-2Kb (10) have been shown to reject heart allografts. This approach suffers from the potential effector activity of T cells that express endogenously rearranged TCR genes, although treatment of the TCR Tg mice with anti-CD4 mAb has been used to argue that rejection was dependent on the TCR Tg cells (11,12). In this report, the TCR34 and TCR75 Tg mice rapidly rejected both skin and heart allografts from B10.D2 mice, as well as H-2Kd+ Tg mice. Although transplantation of grafts into TCR Tg mice has provided useful information, this approach suffers from the extremely high precursor frequency of T cells specific for the relevant pMHC epitope and limitations of T-cell response due to competition for limited pMHC on APC (13–16).
To avoid the limitations of the analysis in the intact TCR Tg mice, several investigators have used an alternative approach, pioneered by Kearney et al. (17), in which the TCR Tg T cells are adoptively transferred into otherwise normal syngeneic mice to follow the response within a more physiological microenvironment. The TCR Tg cells have been tracked in adoptive recipients with “clonotype”-specific antibodies or antibodies to allelic variants of molecules that differ between donors and recipients, such as CD90 (Thy-1) or CD45 (Ly5). Using this approach Gilot et al. (18) showed that transfer of 2 × 106 DES TCR Tg cells (CBA/Ca CD8+ T cells specific for H-2Kb) infiltrated the H-2Kb+ heart allografts and became activated, but they did not appreciably increase the tempo of rejection. Similarly, TCR Tg CD4+ T cells specific for an allopeptide (HYDbyp/I-Ab, termed Marilyn TCR Tg), tracked by crossing them to GFP-expressing mice, show T-cell activation, but their role in graft rejection was not reported (19). By contrast, BM3 TCR Tg T cells rejected heart allografts when adoptively transferred into T-cell deficient recipients (20). Interestingly, it required large numbers of TCR Tg T cells (6 × 106 BM3 TCR Tg T cells (21) and 2.5 × 106 TCR Tg CD4+ ABM Tg T cells specific for the bm12 variant of I-Ab (22) to mediate allograft rejection, whereas, as few as 104 TCR Tg T cells specific for Kd mediated rapid cardiac allograft rejection in immunocompetent recipients in our studies. Collectively, these studies illustrate that graft rejection by adoptive transfer of alloantigen-specific, TCR Tg T cells is quite variable for reasons that have not yet been delineated.
Another approach to analysis of allograft rejection is to combine TCR Tg T cells specific to a defined model peptide antigen that serves as a pseudo-alloantigen in a graft expressing the antigen as a transgene. For example, OT-I (CD8+ OVA-specific T cells) and OT-II (CD4+ OVA-specific T cells) have been transferred to mice given an allograft from Act-mOVA Tg mice. Although the OT-I and OT-II OVA-specific T cells accelerated rejection of transplanted Act-mOVA Tg skin allografts (23) normal B6 mice (without any TCR Tg adoptive transfer) rejected Act-mOVA Tg OVA grafts only slightly more slowly than the adoptive recipients of TCR Tg T cells. This same combination of TCR Tg cells and transgenic allografts has also been studied using trachea allografts that demonstrated specific T-cell activation and localization in the trachea allografts, but these did not mediate complete rejection (24). Similarly, rejection required several weeks to develop in adoptive recipients of a hemagglutin-specific CD4+ TCR Tg population (from TS1 mice), into mice with an allograft that expressed the relevant antigen as a transgene (25). Our model also employs a defined protein antigen (Kd) expressed as a transgene but differs from the above models in that the transgene bears the natural MHC class I promoter elements and is thus under physiological control. Moreover, the Kd-specific TCR34 and TCR75 Tg T cells induced more rapid rejection of both skin and cardiac grafts that are syngeneic except for the Kd transgene than observed with the antigen Tg donors described above.
One of the advantages of the adoptive transfer of our TCR Tg T cells into physiologically normal recipients is that the response by normal B6 mice to the Kd transgene is very weak, thereby permitting the detection of accelerated rejection of cardiac grafts by Kd-specific TCR Tg T cells. Both the intact TCR Tg animals and normal mice, transferred with extremely limited numbers of these cells, reject Kd+ cardiac and skin allografts at a clearly accelerated tempo compared to relevant control mice. Given that rejection by the TCR Tg T cells is more robust in our system, it is relevant to ask whether rapid rejection might be due to some random founder effect such as the site of integration of a given TCR Tg. However, two independently derived Tg mice expressing different TCR αβ genes (TCR34 and TCR75) behaved in a largely equivalent fashion making this possibility unlikely. The differences in rates of rejection in different TCR Tg systems are not yet understood mechanistically. These differences may include variations in the avidity of different TCR Tg to the relevant pMHC epitope or differences in the pattern of the relevant alloantigen expression.
A key finding in this study is the non-linear relationship between the initial precursor frequency of the antigen-specific TCR Tg cells and the ultimate tempo of acute rejection. These results indicate a complex “threshold” phenomenon for acute rejection based on initial precursor frequency between 103 and 104 transferred TCR Tg T cells. Above this threshold frequency, additional cells that are present at the initiation of the response contribute little to the overall “strength” of the response measured by time to rejection. Below this threshold, the allograft appears to be rejected at the same tempo as when no TCR Tg T cells are present. These observations not only verify the threshold effect in transferred CD8+ TCR Tg T cells reported by Jones et al. (21), but also extend their findings by showing that CD4 T cells exhibit a similar threshold effect and a substantially lower (600-fold) absolute number of required T cells. Such a non-linear response pattern is commonplace in biology and typically reflects the operation of cooperation between components of the system, each of which behaves in a linear fashion. For example, the binding kinetics of pentameric IgM antibody is non-linear, with a sharp transition between low-level binding and saturation, which reflects the cooperativity between individual binding sites. In the case of in vivo T-cell responses, it is most likely that cooperation between individual T cells is required to initiate a response within the population. Such a threshold density of responding T cells for graft rejection may reflect the requirement for cooperating T cells to interact with the same APC or might be required for close proximity to paracrine growth factors produced by only a fraction of the responding cells. Alternatively, a critical absolute number of effector cells might be required to arrive in the allograft in kinetic coordination to mediate irreversible allograft injury. The ability to track the fate of individual TCR Tg T cells during the response coupled with the ability to initiate the response with specific numbers of alloantigen-specific T cells in vivo provides a model system to work-out these dynamic mechanisms of T-cell cooperation.
Acknowledgments
This work was supported by NIH grant R01HL50724. The authors thank Lindsey Deckard and Rose Hogg for technical assistance.




