On the Use of Regulatory Regions from Pigmentary Genes to Drive the Expression of Transgenes in Mice
Abbreviations –
-
- Dct
-
- dopachrome tautomerase
-
- DRG
-
- dorsal root ganglia
-
- ES
-
- embryonic stem cells
-
- RPE
-
- retinal pigment epithelium
-
- Tyr
-
- tyrosinase
-
- Tyrp1
-
- tyrosinase-related protein-1
Dear Sir,
The combination of known regulatory regions from mammalian genes and the coding regions of exogenous genes has been instrumental for the generation and analysis of transgenic mice and influences the resulting expression pattern in specific cells or tissues. The transgenes can be of prokaryotic (lacZ, CRE) or eukaryotic origin. The proteins encoded by these genes might or might not have functions interfering directly with the biology of the cell. They can be used as a direct molecular reporter (such as lacZ, for its enzymatic activity), as a direct cellular reporter (such as diphtheria toxin-A for genetic ablation experiments) or as an indirect molecular marker (such as the bacteriophage P1-derived site-specific recombinase Cre, to be used for CRE/loxP recombination strategies).
However, it is also known that the artificial combination of some regulatory regions of a given gene, fused with the coding region of for example a reporter gene, can give rise to unexpected sites of expression in transgenic animals, which are not reproduced by the known expression pattern of the endogenous gene (Fig. 1). While recognizing the potential use of these ectopic patterns, this should be carefully taken into consideration before using the reporter transgenic lines in subsequent experiments.
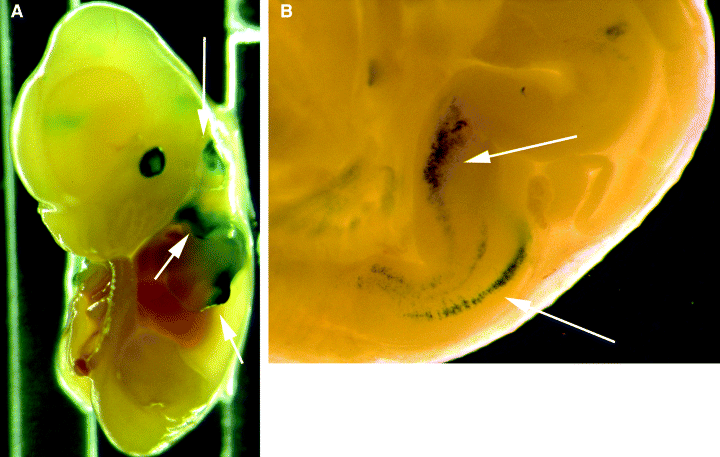
Ectopic expression of Tyrp1-lacZ transgenes in mice. (A) Transgenic Tyrp1-lacZ (9, 14) mouse embryo from +13.5 d.p.c. (days post coitum) stained for β-galactosidase activity (blue). Correct expression is depicted by the obvious β-galactosidase staining of RPE cells. Unexpected (ectopic) expression is indicated by arrows. (B) Close-up from a +15.5 d.p.c. embryo showing ectopic Tyrp1-lacZ expression in developing hindbrain.
This has been the case with pigmentary genes encoding tyrosinase, tyrosinase-related protein-1 and dopachrome tautomerase. Their corresponding regulatory regions have been successfully used to drive the expression of reporter transgenes in mice. In this regard, different 5′ upstream regulatory regions of the mouse dopachrome tautomerase (Dct) gene have been used to efficiently drive the expression of lacZ (1–4) or CRE (5) in transgenic mice. In all these cases, the observed patterns of β-galactosidase- or Cre-positive cells during embryo development extended the reported endogenous pattern for the mouse Dct gene (6). Expression of transgenes was found, as expected, in retinal pigment epithelium (RPE) cells, melanocytes and their migrating melanoblasts precursors and in the telencephalon (6), but unexpectedly, also in dorsal root ganglia (DRG) and caudal nerves. Moreover, the Dct promoter has been used for the expression of eukaryotic genes, such as fgf9 (2) and metabolic glutamate receptor 1 (7).
Different combinations of regulatory regions of the mouse tyrosinase-related protein-1 (Tyrp1) gene have also been used to drive the expression of lacZ (8, 9), CRE (10), diphtheria toxin-A (8), the T antigen from SV40 (11), the oncogene ret (9), the agouti signalling protein (12) or a dominant-negative form of FGF receptor 1 (13) in transgenic mice. In all these cases, the expression of the transgene was detected in RPE cells, matching the endogenous pattern for the Tyrp1 gene (6). However, other cell-types known to express the Tyrp1 gene, such as neural crest-derived melanocytes, present in skin, choroid and hair bulbs, did only rarely show an expression of transgenes (8). In addition, ectopic expression patterns, such as mesencephalon, hindbrain, DRG and optic stalk were reported in CRE (10) or lacZ (8, 9, 14) transgenic mice using the Tyrp1 promoter (Fig. 1). Such an ectopic expression might also explain the unexpected mortality of most transgenic mice expressing diphtheria toxin-A (8).
Finally, 5′ upstream regulatory regions of the mouse tyrosinase (Tyr) gene have been extensively used to drive the expression of lacZ (15, 16), CRE (17, 18), diphtheria toxin-A (16), the bacterial reverse tetracycline transactivator (rtTA) (19, 20), oncogenes (reviewed in 21) or TGFα (22), among other transgenes, in mice. Tyr-driven transgenes mostly reproduced the expression pattern of the endogenous gene in melanoblasts/melanocytes and in RPE cells (6, 23, 24). However, when proximal and distal regulatory regions of the Tyr gene were artificially combined in transgenic constructs, the absence of transgene expression in RPE cells (16, 19) or in skin melanocytes (20) was noted. In addition, ectopic expression in the brain (telencephalon), DRG and/or other tissues was reported for lacZ and CRE Tyr-driven transgenes (15–18), as places where the endogenous Tyr gene is not detected (24).
In the case of CRE transgenes, usually, the reported Cre activity cannot directly be related to the activity of promoters or the expression from the transgenic construct (10). In contrast, the expression is generally detected due to the Cre-mediated activation of a second transgenic system, using either lacZ, alkaline phosphatase or green fluorescent protein as reporters (5, 10, 17, 18). Thus, aberrant CRE transcription during early developmental stages has a wider impact in all cell lineages derived from affected precursors.
A number of reasons could explain the observed discrepancies between endogenous and transgenic reporter expression patterns:
First, the method of detection of the endogenous gene transcript or protein might not be sensitive enough to reveal low levels of expression. Equally, lack of activity of the exogenous protein (i.e. β-galactosidase staining) does not necessarily indicate lack of transcription of the transgene (i.e. lacZ mRNA).
Second, crucial regulatory elements that are required for the gene to be faithfully expressed in time and space would generally not be included in standard transgenic constructs. This lack of cis-regulatory sequences can also trigger the anomalous behaviour of transgenes inserted in the host genome, generally known as position effects. Several approaches can be used to overcome position effects of transgenes: (i) use of insulator elements (25); (ii) introduction of an artificial chromosome (26); the use of artificial chromosome-type transgenes normally guarantees the correct expression pattern and overcomes these position effects. Moreover, the bigger size of these constructs can accommodate the presence of most regulatory elements that are needed for the appropriate expression of the locus (26, 27); (iii) use of the entire set of endogenous regulatory elements of a given locus by a knock-in approach; using homologous recombination techniques in embryonic stem (ES) cells should ensure that the expression of the transgene recapitulates the expression pattern of the targeted gene. This has already been explored in the case of the mouse Dct (5), Kit (28) and EdnrB (29, 30) genes, generating both a knockout allele for the Dct, Kit and EdnrB genes and Dct::CRE, Kit::lacZ, EdnrB::tTA, EdnrB:rtTA or EdnrB::lacZ reporter mouse lines.
Third, the artificial combination of proximal and distal regulatory elements from a gene could generate regulatory cues resulting in the unexpected ectopic expression of transgenic constructs. The updated description of new activities associated with previously reported regulatory elements could explain the unexpected behaviour of some of these combinations (31).
Fourth, the use of prokaryotic reporter genes (such as lacZ, CRE, rtTA) can alter the adequate transcriptional control of eukaryotic regulatory sequences (32, 33).
In conclusion, ectopic expression from pigment gene promoters in transgenic mice may or may not have an influence on the outcome and subsequent interpretations of the results, which should be compared with the established pattern of expression for the endogenous gene to adequately assess the correct relevance of the observations. Keeping in mind such potential ‘aberrant’ or ‘unexpected’ results, and their interpretation, transgenic mice (including those generated using pigment gene promoters) are nevertheless valuable and attractive tools to study gene regulation and to approach biological questions in pigment cells.




