LMAN1 is a molecular chaperone for the secretion of coagulation factor VIII1
Portions of this work were supported by National Institutes of Health Grants RO1 HL52173 and PO1 HL057346.
Abstract
Summary. Combined deficiency of both coagulation factors (F)V and VIII is a rare autosomal recessive bleeding disorder caused by null expression of LMAN1 (previously termed ERGIC-53) in a majority of affected individuals. Previously, a requirement for a functional LMAN1 cycling pathway between the ER and Golgi was demonstrated for efficient secretion of FV and FVIII (Moussalli et al. J Biol Chem 1999; 274: 32569), however, the molecular nature of the interaction between LMAN1 and its cargo was not characterized. Using coimmunoprecipitation of LMAN1 and FVIII from transfected HeLa and COS-1cells, we demonstrate an interaction between LMAN1 and FVIII in vivo. The interaction was mediated via high mannose-containing asparagine-linked oligosaccharides that are densely situated within the B domain of FVIII, as well as protein–protein interactions. These results are interpreted based on the recent determination of the crystal structure of the carbohydrate recognition domain of LMAN1.
Combined factor (F)V and FVIII deficiency is a rare autosomal recessive genetic bleeding disorder that is distinct from the coinheritance of both FV deficiency and FVIII deficiency. Patients who present with this phenotype have between 5 and 30% of normal plasma levels of FV and FVIII antigen and activity, whereas the levels of all other plasma proteins studied are not altered. Coagulation factors V and VIII are plasma glycoproteins with molecular masses of 330 and 280 kDa, respectively (Fig. 1). They are both non-enzymatic cofactors necessary for efficient hemostasis as part of the coagulation cascade. FV and FVIII both have similar domain structures designated A1-A2-B-A3-C1-C2. FV has approximately 40% amino acid sequence homology to FVIII in the A and C domains. The amino acid sequences of the B domains are not conserved between FV and FVIII and have no identified function [1,2]. However, the structure of the FV and FVIII B domains share a similarity in that they contain 25 and 18 consensus sites for potential asparagine (N)-linked glycosylation, respectively. Both B domains are cleaved through limited proteolysis during thrombin-mediated activation of the cofactors and are dispensable for cofactor function [1,2].

Domain structures of factors V and VIII and ceruloplasmin. Homologous domains are designated by similar shading. Consensus sites of N-linked glycosylation are indicated by vertical dashes.
Homozygosity mapping and positional cloning were utilized to identify that a majority of families with combined FV/FVIII deficiency have mutations in LMAN1 (previously known as ERGIC-53) that result in null expression of LMAN1 [3], a protein that is localized to the endoplasmic reticulum (ER)-Golgi intermediate compartment (ERGIC) [4]. The ERGIC comprises vesicular-tubular clusters that mediate the transport of secretory proteins from the ER to the Golgi. Proteins destined for secretion are packaged in the ER into COPII-coated vesicles, and through anterograde transport are transported to the Golgi complex, after which they are ultimately secreted into the extracellular environment [5–7]. LMAN1 is a 53-kDa type I transmembrane protein that has homology to leguminous mannose-binding lectins [8] and is proposed to facilitate secretion of a subpopulation of glycoproteins [7,9–11]. LMAN1 forms homodimers and homohexamers, and selectively binds mannose sugars in a calcium-dependent manner [8]. Previous work by our group demonstrated that a functional LMAN1 cycling pathway was required for efficient secretion of FV and FVIII from cultured cells [12].
Chemical cross-linking of cells over-expressing LMAN1 demonstrated an interaction between LMAN1 and cathepsin Z-related protein that was mannose- and calcium-dependent [13]. Cathepsin Z-related protein has no currently known physiological function; thus, the direct interaction between LMAN1 and a physiological ligand remains elusive. In this report, we demonstrate that FVIII interacts with LMAN1 in a manner that requires carbohydrate–protein and protein–protein interactions.
Materials and methods
Materials
Fetal bovine serum, Dulbecco's modified Eagle's medium (DMEM), and methionine-free MEM were purchased from Invitrogen Corporation (Carlsbad, CA, USA). Phenylmethylsulfonyl fluoride (PMSF), iodoacetamide, tunicamycin and deoxymannojirimycin (DMJ) were purchased from Sigma (St. Louis, MO, USA). Aprotinin and soybean trypsin inhibitors were purchased from Roche Molecular Biochemicals (Indianapolis, IN, USA). Redivue Pro-mix in vitro cell labeling mix [[35S]methionine and [35S]cysteine; (>1000 Ci mmol−1)] was obtained from Amersham Pharmacia Biotech (Piscataway, NJ, USA). EN3hance was purchased from NEN Life Science Products, Inc. (Boston, MA, USA). Anti-heavy chain FVIII monoclonal antibody conjugated to CL-4B-Sepharose was a gift from Debra Pittman (Genetics Institute Inc., Cambridge, MA, USA). Anti-LMAN1 monoclonal antibody (G1/93) was prepared as described previously [4]. Anti-ceruloplasmin (Cp) antibodies were purchased from Dako Corporation (Carpinteria, CA, USA). Other materials used in this study were of the highest quality available.
Cell culture
The HtTA-1 cell line (HeLa) stably expressing a cDNA encoding a cytosolic tail mutant LMAN1 (KKAA) under the control of a tetracycline-repressible (tTA) promoter was described previously [14]. This expression construct incorporates a c-myc tag at the N-terminus of LMAN1. A carbohydrate recognition domain point mutant of KKAA LMAN1 (N156A-KKAA) was generated similarly to that previously described [14], whereby the KKAA LMAN1 construct was mutated at amino acid 156 (N156A) by site-directed mutagenesis. Both KKAA and N156A-KKAA are trapped within the ER. Additionally, N156A-KKAA is defective in carbohydrate binding. The N156A-KKAA construct also encodes a c-myc tag at its N-terminus and like KKAA LMAN, its expression is under the control of a tetracycline-repressible (tTA) promoter.
HtTA-1 cells were maintained in 400 µg mL−1 geneticin, 2 µg mL−1 tetracycline, and 500 ng mL−1 puromycin. COS-1 cells were maintained in media supplemented with 10% fetal calf serum, 2 mmol L−1 glutamine and 1% penicillin/streptomycin. Cells were transiently transfected with one of several mammalian expression vectors. The pMT2 plasmid [15] containing the cDNA of either full-length FVIII (WT FVIII) or B-domain-deleted FVIII (BDD-FVIII) was used to express the FVIII variants used in this study. The BDD-FVIII used in this study lacks amino acid residues 741 through 1647, inclusive, and results in the juxtaposition of arginine 740 and arginine 1648. This BDD-FVIII was designated 90/80 BDD-FVIII. A pCDNA3.1 plasmid encoding human ceruloplasmin was the generous gift of Dr Jonathan Gitlin (Washington University, St. Louis, MO, USA). The pED plasmid [16] containing the cDNA of either KKAA LMAN1 or N156A-KKAA LMAN1 was used to over-express the LMAN1 variants in COS-1 cells. Unlike the LMAN1 expressed in the HtTA-1 cell lines, the LMAN1 variants expressed in COS-1 cells were not c-myc-tagged. DNA was introduced into HtTA-1 cells by the calcium phosphate transfection method [17], while COS-1 cells were transiently transfected using the DEAE-dextran transfection technique [16]. HtTA-1 cells were maintained in the presence of tetracycline throughout transfection and metabolic labeling unless induction of recombinant LMAN1 was required. In these instances, tetracycline was removed from the cell media at the initiation of transfection. Where indicated, DMJ or tunicamycin were added at the initiation of metabolic labeling to final concentrations of 1 mmol L−1 and 10 µg mL−1, respectively. DMJ and tunicamycin were present throughout pulse and chase labeling conditions.
Metabolic labeling and immunoprecipitation
Analysis of protein synthesis and secretion was conducted by metabolic labeling of cells at 48 h post-transfection. Cells were incubated 15 min in methionine-free media and then pulse-labeled for 30 min with 250 µCi mL−1 Redivue Pro-mix in vitro cell labeling mix. Following a 3-h chase in medium containing excess unlabeled methionine and 100 µg mL−1 aprotinin, cell extracts and conditioned media were collected and immunoprecipitations were conducted and analyzed by SDS-PAGE under reducing conditions essentially as previously described [16] except that samples were not frozen prior to immunoprecipitation. Where indicated iodoacetamide or calcium chloride were added to the lysis buffer prior to cell lysis to concentrations of 10 µg mL−1 and 5 mmol L−1, respectively. Quantification of radiographic band intensities was performed using NIH Image software (public domain).
Results
To study whether FVIII interacts with LMAN1, FVIII was transiently expressed in HeLa cells that express wild-type and mutant LMAN1 under tetracycline-repressible control. The LMAN1 mutants studied were: KKAA LMAN1, that lacks the cytosolic carboxy-terminal diphenylalanine exit determinant and is trapped within the ER [14]; and N156A-KKAA LMAN1, that is mutated in the carbohydrate recognition domain and cannot bind high-mannose containing oligosaccharides [8]. N156A-KKAA LMAN1 is like KKAA LMAN1 in that it is also trapped within the ER and cannot cycle to the Golgi apparatus. KKAA and N156A-KKAA LMAN1 act as trans-dominant negative mutants because they interact with endogenous LMAN1 to prevent its trafficking from the ER [14], and in addition, the N156A-KKAA LMAN1 variant, has defective carbohydrate binding. We relied on expression of these variants of LMAN1 so that bound species would be concentrated within the ER.
Interaction of LMAN1 is specific to FVIII
HeLa cells expressing the ER retention mutant of LMAN1 (KKAA) were transiently transfected with either wild-type FVIII or B-domain deleted (BDD-FVIII). At 48 h post-transfection, cells were metabolically labeled with [35S]methionine and [35S]cysteine, and cell extracts containing equal trichloroacetic acid (TCA) precipitable protein counts were immunoprecipitated with either anti-FVIII or anti-LMAN1 antibodies. Products of the immunoprecipitations were analyzed by SDS-PAGE under reducing conditions and visualized by autoradiography.
Immunoprecipitation of extracts prepared from metabolically labeled KKAA LMAN1 HeLa cells expressing WT-FVIII or BDD-FVIII with anti-FVIII antibody yielded protein bands of either 280 or 160 kDa, respectively, corresponding to the expected masses of either WT-FVIII or BDD-FVIII (Fig. 2a; lanes 1 and 5). These bands were absent in mock-transfected cells (Fig. 2a; lanes 9 and 11). Immunoprecipitation employing anti-LMAN1 antibody yielded an intense band of approximately 56 kDa, corresponding to the expected size of the stably transfected inducible recombinant myc-KKAA LMAN1 (Fig. 2a; lanes 2, 6 and 10). In addition, a less-intense band of approximately 53 kDa, corresponding to the expected size of endogenous LMAN1, was identified by anti-LMAN1 antibody. Co-precipitating bands of 280 and 160 kDa were observed from the cell extracts expressing WT-FVIII and BDD-FVIII, respectively, suggesting both forms of FVIII interact with LMAN1 within the cell (Fig. 2a; lanes 2 and 6). The FVIII-specific bands were absent upon analysis of untransfected cells that do not express FVIII (Fig. 2a; lanes 9–12).

FVIII interacts directly with LMAN1 in transfected HeLa and COS-1 cells. (a) HeLa cells over-expressing either KKAA or N156A-KKAA LMAN1 were either mock transfected, or transfected with plasmids expressing wild-type FVIII or BDD FVIII, and metabolically labeled with [35S]methionine/cysteine. (b) COS-1 cells were either mock transfected, or transfected with plasmids expressing FVIII and KKAA LMAN1, and metabolically labeled with [35S]methionine/cysteine. (c) HeLa cells over-expressing either KKAA or N156A-KKAA LMAN1 were either mock transfected, or transfected with plasmids expressing wild-type FVIII or Cp. In all instances, cell extracts were immunoprecipitated (IP) with an anti-FVIII an anti-Cp or an anti-LMAN1 antibody. FVIIII, BDD FVIII, Cp, LMAN1, and molecular mass markers (kDa) are labeled.
WT-FVIII and BDD-FVIII were also transiently transfected into HtTA-1 cells that stably over-express the N156A-KKAA LMAN1 carbohydrate recognition domain mutant. This LMAN1 point mutant in the carbohydrate recognition domain is unable to bind to mannose columns in vitro[13]. N156A-KKAA LMAN1 also cannot cycle between the ER and Golgi because its C-terminal exit determinant is ‘inactivated’ by alanine substitution mutagenesis.
Immunoprecipitation with anti-FVIII antibody demonstrated that WT-FVIII and BDD-FVIII were expressed at similar levels in N156A-KKAA LMAN1 HtTA-1 compared with the KKAA LMAN1 HtTA-1 cells (Fig. 2a; lanes 1, 3, 5 and 7). Immunoprecipitation of cell extracts containing equivalent labeled protein from transfected N156A-KKAA LMAN1 expressing HtTA-1 cells with anti-LMAN1 antibody yielded coimmunoprecipitating FVIII bands of either WT-FVIII or BDD-FVIII variants (Fig. 2a; lanes 4 and 8), but to a lesser extent than that observed in FVIII transfected HtTA-1 cells expressing KKAA LMAN1 that had an intact carbohydrate recognition domain. NIH Image software was used to quantify the coimmunoprecipitation between FVIII and LMAN1 in cell extracts expressing FVIII and either KKAA or N156A-KKAA LMAN1. The amount of FVIII coimmunoprecipitating with LMAN1 was quantified by determining the amount of FVIII present in the LMAN1 immunoprecipitation lane as a percentage of FVIII present in the adjacent anti-FVIII immunoprecipitation lane (input control). Additionally, the percentage of FVIII coimmunoprecipitation was normalized against the amount of LMAN1 that was present in the LMAN1 immunoprecipitation lane. Compared with the coimmunoprecipitations of WT- and BDD-FVIII with KKAA LMAN1, WT- and BDD-FVIII had reductions in coimmunoprecipitation with N156A LMAN1 by 66% and 73%, respectively. Although FVIII and BDD-FVIII were detected upon immunoprecipitation of LMAN1, LMAN1 was not detected in the anti-FVIII immunoprecipitate (Fig. 2a).
In order to test the possibility that these observations were cell line specific, we transiently transfected COS-1 cells with FVIII and KKAA LMAN1. Immunoprecipitations employing either anti-FVIII or anti-LMAN1 antibodies after metabolic labeling 48 h post-transfection, demonstrated that both FVIIII and LMAN1 were efficiently expressed in COS-1 cells (Fig. 2b; lanes 3 and 4). As observed in transfected HtTA-1 cells, immunoprecipitation of cell extracts with anti-LMAN1 coimmunoprecipitated FVIII (Fig. 2b; lane 4). However, in contrast to observations in HtTA-1 cells, immunoprecipitation of transfected COS-1 cell extracts with anti-FVIII antibody coimmunoprecipitated LMAN1. One possible explanation for this observation is that the labeling conditions used in the transfected HeLa cells yielded less [35S]methionine incorporation into LMAN1 due to the lower rate of LMAN1 synthesis in the stably transfected HeLa cells.
Cp is a copper-binding plasma glycoprotein that contains 40% amino acid homology to FVIII within its A domains [18], but unlike FVIII, contains no B or C domains (Fig. 1). Cp was transiently expressed in HtTA-1 cells stably expressing KKAA LMAN1 (Fig. 2c). Immunoprecipitation of the cell extracts employing anti-Cp antibody, yielded a prominent protein of approximately 132 kDa, the anticipated molecular mass of Cp (Fig. 2c; lanes 3 and 5). Unlike those cells expressing WT-FVIII or BDD-FVIII, however, immunoprecipitation of cell extracts with anti-LMAN1 antibody did not detect Cp (Fig. 2c; lanes 4 and 6). Cp also did not coimmunoprecipitate with N156A-KKAA LMAN1 (Fig. 2c; lane 6). A protein band corresponding to the expected mass of Cp was also absent in mock-transfected KKAA HtTA-1 cells (Fig. 2c; lanes 1–2). Similar results were observed when Cp was transfected into COS-1 cells (data not shown). Additionally, non-FVIII-specific protein bands of approximately 180 kDa were observed in immunoprecipitations using either anti-FVIII or anti-LMAN1 antibodies. However, we did not identify these bands, as they were consistently observed in both mock-transfected and FVIII-transfected cell extracts.
Contribution of specific oligosaccharide structure to FVIII/LMAN1 interaction
To investigate the role of mannose trimming for FVIII interaction with LMAN1 and secretion, we studied the effect of deoxymannojirimycin (DMJ), a mannosidase inhibitor that prevents trimming of the outermost mannose residues of the GlcNac2Man9 core oligosaccharide structure [19] (Fig. 3a). KKAA LMAN1 stably transfected HtTA-1 cells were transiently transfected with WT-FVIII and metabolically labeled in the absence or presence of DMJ. Immunoprecipitations employing anti-FVIII antibody demonstrated efficient expression of WT-FVIII (Fig. 3b; lanes 1 and 3). Immunoprecipitation employing anti-LMAN1 antibody yielded protein bands corresponding to the expected size of LMAN1, as well as a polypeptide migrating around 280 kDa, the expected size of WT-FVIII (Fig. 3b; lanes 2 and 4).
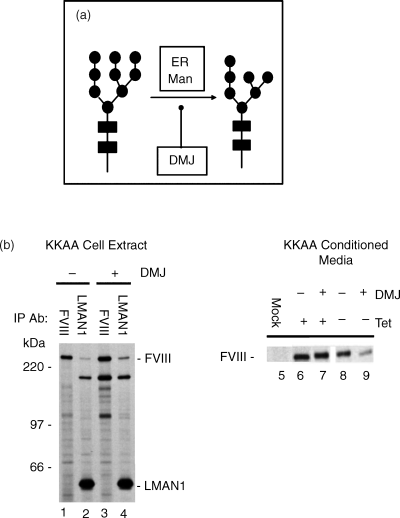
Recognition of specific mannose structures contributes to the LMAN1 and FVIII interaction. (a) Deoxymannojirimycin (DMJ) prevents trimming of terminal mannose residues from GlcNAc2Man9 oligosaccharide structures. Mannose (●), N-acetylglucosamine (▪) B: WT FVIII was immunoprecipitated from the conditioned medium (right panel) of metabolically labeled transfected HeLa cells expressing inducible (−;) KKAA LMAN1 under tetracycline (Tet) repression (+) in the absence (−) or presence (+) of deoxymannojirimycin (DMJ). FVIII and LMAN1 proteins were also immunoprecipitated from cell extracts (left panel) of KKAA LMAN1 over-expressing FVIII transfected HeLa cells.
Induction of KKAA LMAN1 reduced FVIII secretion by approximately 25% compared with an uninduced control expressing only wild-type endogenous LMAN1 (Fig. 3b; compare lanes 6 and 8). DMJ treatment of HtTA-1 cells induced to over-express KKAA LMAN1 further reduced the amount of WT FVIII secreted from the cells to approximately 40% of that observed in uninduced cells (Fig. 3b; compare lanes 7 and 9). In addition, DMJ treatment of WT FVIII transfected HtTA-1 cells stably expressing KKAA LMAN1 enhanced the interaction between FVIII and LMAN1 approximately 15%, compared with an untreated control (Fig. 3b; lanes 2 vs. 4). Importantly, DMJ treatment had no effect on the rate of FVIII secretion in uninduced HtTA-1 cells (Fig. 3b; compare lanes 6 and 7). These results support the hypothesis that a portion of the LMAN1–FVIII interaction is dependent upon exposure of high mannose-N-linked oligosaccharides.
Protein–protein interactions contribute to binding between FVIII and LMAN1
As specific high mannose-containing oligosaccharide structures appeared to enhance an interaction between FVIII and LMAN1, immunoprecipitations of transfected COS-1 cells extracts were conducted, as described in detail above, in the presence of tunicamycin in order to determine if oligosaccharide structures were required to observe an interaction between the two proteins. Tunicamycin was present during all steps of metabolic labeling to a final concentration of 10 µg mL−1 to prevent glycosylation during de novo protein synthesis. The results of SDS-PAGE after immunoprecipitation in the absence and presence of tunicamycin are shown in Fig. 4(a). Both BDD FVIII and wild-type FVIII coimmunoprecipitated with LMAN1 in the absence or presence of tunicamycin. Co-immunoprecipitation was observed when immunoprecipitations were conducted with either anti-FVIII or anti-LMAN1 antibodies. The migration of both BDD FVIII and wild-type FVIII was slightly greater when tunicamycin was present due to their smaller masses that result from a lack of carbohydrate addition.
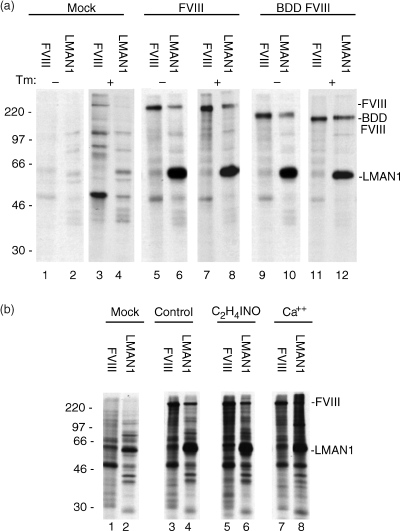
(a) Inhibition of glycosylation does not prevent FVIII interaction with LMAN1. COS-1 cells were either mock transfected, or transfected with plasmids expressing WT or BDD FVIII, and KKAA LMAN1, and metabolically labeled with [35S]methionine/cysteine. Cell extracts were immunoprecipitated with either anti-FVIII or anti-LMAN1 antibody. Tunicamycin was either absent or present at a final concentration of 10 µg mL−1 during metabolic labeling as described in Materials and methods. (b) The interaction of FVIII and LMAN1 is not affected by iodoacetamide or by the addition of calcium. COS-1 cells were either mock transfected, or transfected with plasmids expressing FVIII and KKAA LMAN1, and metabolically labeled with [35S]methionine/cysteine. Iodoacetamide (10 µmol L−1 final concentration) or calcium chloride (5 mmol L−1 final concentration) was added to cell lysis buffer just prior to cellular harvesting. Cell extracts were immunoprecipitated with an anti-FVIII antibody or an anti-LMAN1 antibody. FVIII, LMAN1 and molecular mass markers (kilodaltons) are labeled.
In order to test whether post lysis sulfhydryl cross-linking occurs between FVIII and LMAN1, immunoprecipitations were conducted using cell extracts of transfected HtTA-1 and COS-1 cells that had been lyzed in the presence of iodoacetamide. The presence of iodoacetamide had no effect on the coimmunoprecipitation profile observed in both cell lines studied (Fig. 4b; compare lanes 3 and 4 with lanes 5 and 6). It has previously been reported that LMAN1 binds to mannose in vitro in a calcium-dependent manner [8]. In order to test a possible requirement for additional calcium in facilitating an interaction between FVIII and LMAN1 in HtTA-1 cells, immunoprecipitations of cell extracts were repeated with the additional presence of 5 mmol L−1 calcium. This had no effect on the immunoprecipitation profile observed with any of the antibodies used (Fig. 4b; compare lanes 3 and 4 with lanes 7 and 8). Chelation of calcium in cell lysates by the addition of EDTA had no effect on the observed interaction between FVIII and LMAN1 (data not shown). This observation suggests that any requirement of calcium for promoting binding between the two proteins may be required to initiate the interaction (while the cells are still intact) and may not influence the duration or affinity of binding once the cells have been lyzed.
Discussion
Two-thirds of patients with a rare bleeding disorder caused by combined deficiency of FVIII and FV display null expression of LMAN1 [3]. LMAN1 is a calcium-dependent mannose-binding lectin proposed to interact with its ligands via binding to carbohydrate moieties present on target glycoproteins. The plasma levels of both FV and FVIII are equally reduced in combined FV and FVIII deficiency. Since these two proteins have similar domain structures, they presumably have unique properties that target them for LMAN1-mediated transport. It was suggested that the unique targeting element resides in the highly glycosylated B-domain. While this hypothesis is supported by previous work from our group [12], LMAN1 had not been shown to interact with either FV or FVIII. This study demonstrates an interaction between LMAN1 and a physiological ligand, coagulation FVIII. Our current results support that LMAN1 interacts with FVIII in a manner enhanced by the presence of high mannose-containing oligosaccharides, but our results also suggest that the interaction of LMAN1 and FVIII is mediated by protein–protein interactions.
We studied FVIII expression in cells that express a mutant LMAN1 (KKAA) that lacks the C-terminal diphenylalanine ER exit determinant and cannot exit the ER. Our previous study showed that a defect in FVIII secretion occurred when KKAA LMAN1 was over-expressed [12]. Based on those initial findings, our hypothesis was that if KKAA LMAN1 interacts with FVIII, then FVIII would be trapped in the ER and its secretion would be significantly impaired. Our results show that LMAN1 (KKAA) associates with FVIII upon coexpression in HtTA-1 cells. Although the interaction between FVIII and LMAN1 was most prominent when both LMAN1 and FVIII were over-expressed and anti-LMAN1 antibody was used for immunoprecipitation in HtTA-1 cells, FVIII expressed in transfected COS-1 monkey cells also coimmunoprecipitated with LMAN1 when anti-FVIII antibody was used. We did not detect association between transiently expressed recombinant FVIII and endogenous LMAN1, possibly due to the low expression level of endogenous LMAN1 in these cells.
Given that the B domain is highly glycosylated in FVIII and that LMAN1 is a mannose binding lectin, we predicted that the B domain would significantly contribute to the WT–FVIII and LMAN1 interaction. Additionally, our previous study suggested that efficient secretion of FVIII appeared to rely on the intracellular presence of high mannose containing oligosaccharides [12]. In order to determine the contribution of high mannose containing oligosaccharides in facilitating binding of FVIII with LMAN1, we conducted immunoprecipitations on cell extracts of transfected cells that had been treated with DMJ during metabolic labeling. DMJ not only reduced the rate of FVIII secretion (Fig. 3b), but the drug treatment also increased the proportion of FVIII that coimmunoprecipitated with LMAN1. These observations suggest that high mannose containing oligosaccharides provide a significant contribution to binding between FVIII and LMAN1.
Our data support that protein–protein interactions also contribute to the interaction between FVIII and LMAN1. WT-FVIII and BDD-FVIII both coimmunoprecipitated with the N156A-KKAA mutant LMAN1 that is defective in mannose binding. In addition, treatment of WT-FVIII and BDD-FVIII transfected cells with tunicamycin, an inhibitor of N-linked glycosylation, during metabolic labeling did not significantly reduce the interaction with LMAN1 (Fig. 4). Finally, N-glycanase treatment of cell-extracts prior to immunoprecipitation did not reduce the interactions detected (data not shown). The interaction between the two proteins appears specific, since over-expression of Cp, a protein with some homology to FVIII did not yield a visible interaction with LMAN1 through similar coimmunoprecipitation experiments.
The only phenotype observed with null expression of LMAN1 is that of combined FV/FVIII deficiency. Given the amount and variety of glycoproteins within the ER, the specificity of the FVIII–LMAN1 interaction is surprising. The results suggest FVIII-specific protein sequences contribute to LMAN1 interaction, in addition to high-mannose containing oligosaccharide structures. A recent crystal structure of the carbohydrate recognition domain of p58, the rat homologue of LMAN1 [20], identified a surface patch of conserved residues on the opposite side of the mannose-binding site that may provide protein–protein interactions that contribute to specificity for ligand binding.
While two-thirds of patients with combined FV/FVIII deficiency display null expression of LMAN1, the other one-third of individuals presenting with the same phenotype has normal LMAN1 expression [21,22]. This suggests that one or more genes may be associated with LMAN1 in providing a chaperone scaffold for transport of FV and FVIII from the ER to the Golgi. It is possible that a protein encoded by an additional gene may act in concert with LMAN1 to mediate LMAN1-dependent transport of FV and FVIII. In addition, interaction of the FVIII B domain with other components of an LMAN1 chaperone complex may contribute further specificity to the facilitated transport mechanism.
Acknowledgements
MAC was the recipient of a Research Fellowship from the Heart and Stroke Foundation of Canada. SWP is supported by a Career Development Award in Haemostasis from the National Haemophilia Foundation. BZ is a Judith Graham Pool Postgraduate Fellow of the National Haemophilia Foundation. We gratefully acknowledge Dr Florence Vollenweider for preparing the N156A-KKAA HtTA-1 cell line. We would like to thank Dr Chuan Yin Liu for helpful discussions. DG and RJK are investigators of the Howard Hughes Medical Institute.




