Abnormal Neuroimaging in Patients with Benign Epilepsy with Centrotemporal Spikes
Abstract
Summary: Purpose: Neuroimaging procedures are usually unnecessary in benign epilepsy of childhood with centrotemporal spikes (BECTS) but are often performed before a specific diagnosis has been reached. By definition, BECTS occurs in normal children; however, recent reports have shown that it also can affect children with static brain lesions. We evaluated the prevalence of abnormal neuroimaging in BECTS and assessed whether the lesions had influenced the clinical and EEG expression of this epilepsy.
Results: Among 98 consecutive cases first referred between 1984 and 1999, neuroimaging had been performed in 71 (72%) [magnetic resonance imaging (MRI), 20; computed tomography (CT), 59; MRI+CT, eight]. In ten (14.8%), neuroradiologic procedures were abnormal: enlargement of lateral venticles in five cases including a shunted hydrocephalus in two (no etiology in one, neonatal intraventricular hemorrhage in one), a moderate ventricular dilation in one (neonatal distress), a slight ventricular dilation and hypersignal intensities in the white matter in one (premature birth at 27 weeks), and a moderate enlargement of the right temporal horn in one. A right hippocampal atrophy, a biopercular polymicrogyria, a cavum septum pellucidum, a small cystic lesion located in the epiphysis, and an agenesis of the corpus callosum with macrocrania also were observed once each. The outcome was benign in all, in accordance with the overall prognosis of BECTS.
Conclusions: This study confirms that neuroimaging may be abnormal in patients with BECTS and shows that the presence of brain lesions has no influence on the prognosis. Conversely, BECTS can be diagnosed in patients with brain lesions with or without significant neurologic history or abnormalities.
Benign epilepsy of childhood with centrotemporal spikes (BECTS) or benign rolandic epilepsy is an idiopathic localization-related epilepsy with no underlying cause other than a possible hereditary predisposition (1,2). In a previous report, our group described three cases of BECTS occurring in brain-damaged patients (agenesis of the corpus callosum, lipoma of the corpus callosum, and congenital toxoplasmosis, respectively) (3). We also reported a boy with a typical form of BECTS in whom magnetic resonance imaging (MRI) documented a marked right hippocampal atrophy (4). We decided to review retrospectively a large, consecutive population of children with BECTS to evaluate the prevalence of neuroimaging anomalies and to assess whether the lesions had influenced the clinical and EEG expression of this epilepsy.
PATIENTS AND METHODS
All patients with rolandic spikes and seizures first referred to the Centre Saint Paul between 1984 and June 1999 were identified from a prospective database. All medical records were retrospectively reviewed in 1999. Patients with Landau–Kleffner syndrome and continuous spikes–and–wave activity during slow sleep were excluded from this study (5,6). Diagnosis of BECTS was based on age at onset, seizure types, and EEG. It was confirmed at the end of follow-up, when we reassessed the medical files of all to screen for those with abnormal neuroimaging. At least one routine waking EEG was performed in all children and one nap sleep EEG in 77%. In some cases, a routine waking EEG was performed in asymptomatic siblings of the same age group to validate further the diagnosis of BECTS. As a result of mis- or nondiagnosis of BECTS at referral, conventional neuroimaging procedures including computed tomography (CT) and MRI have been often performed. No standard method of neuroimaging was used because procedures were performed in various departments of neuroradiology over a long period. We can thus only take into account the global assessment on the presence or absence of gross neuroimaging abnormalities.
RESULTS
Over a period of 15.5 years, 98 new patients (31 girls, 67 boys) were diagnosed with BECTS. The demographic data were previously published (7). We found neuroradiologic documentation in 71 cases (72%): CT in 59, MRI in 20, and CT+MRI in eight. In 10 (14.8%), neuroimaging was abnormal: enlargement of lateral ventricles in five cases including a shunted hydrocephalus in two [CT, no etiology in one (pat. 1, Fig. 1); neonatal intraventricular hemorrhage in one (pat. 2)]; a moderate ventricular dilation in one (CT, neonatal distress, pat. 3, Fig. 2); a slight ventricular dilatation and hypersignal intensities in the white matter in one (MRI, premature birth at 27 weeks, pat. 4); and a moderate enlargement of the right temporal horn in one (MRI, pat. 5, Fig. 3). A right hippocampal atrophy (MRI, pat. 6), a biopercular polymicrogyria (MRI, pat. 7, Fig. 4), a cavum septum pellucidum (CT+MRI, pat. 8), a small cystic lesion located in the epiphysis (MRI, pat. 9), and an agenesis of the corpus callosum with macrocrania (CT, pat. 10) also were observed once each. Clinical characteristics of these 10 patients are summarized in Table 1. EEGs and neuroimaging of patients 10 and 6 were previously published in detail (ref. 3, case 10; and ref. 4, case 6). The EEG of patient 4 was published elsewhere (8). The outcome was benign in all (Table 1), in accordance with the overall prognosis of BECTS. The presence of brain lesions did not change the prognosis, although it influenced, in some patients, the intellectual development (pat. 3, 4, 7).
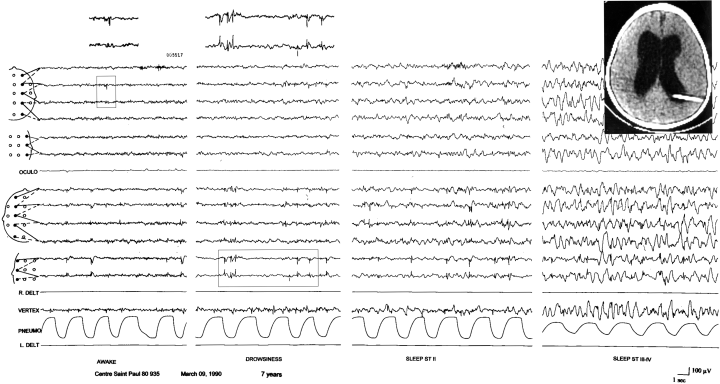
Patient 1. EEG at age 7 years; spike–waves over the left centrotemporal region at wake. Note the presence of less prominent, independent spikes over the right central area. Same recording during drowsiness and slow sleep showing the activation of focal sharp without changes in their morphology. Computed tomography scan (1986) showing shunted hydrocephalus and enlarged ventricles.
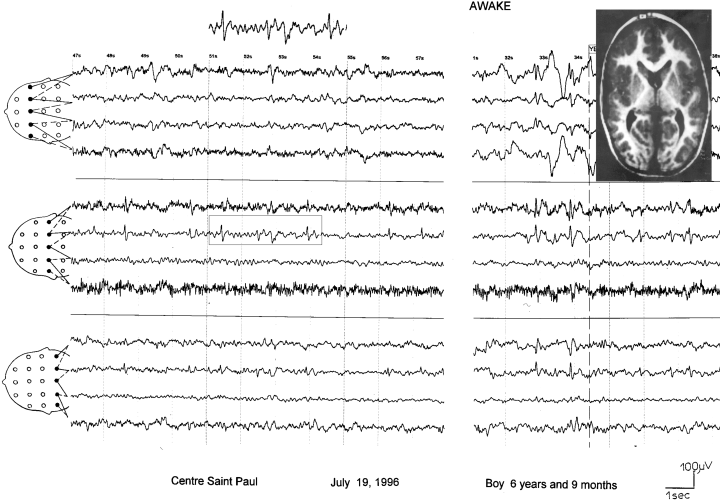
Patient 3. Presence of typical high-voltage diphasic spikes and slow waves over the left centrotemporal region, occurring in clusters. During drowsiness and light sleep, abnormalities are activated. Computed tomography scan (1985) showing ventricular dilatation but no hydrocephalus. The findings were compatible with the history of perinatal hypoxia.
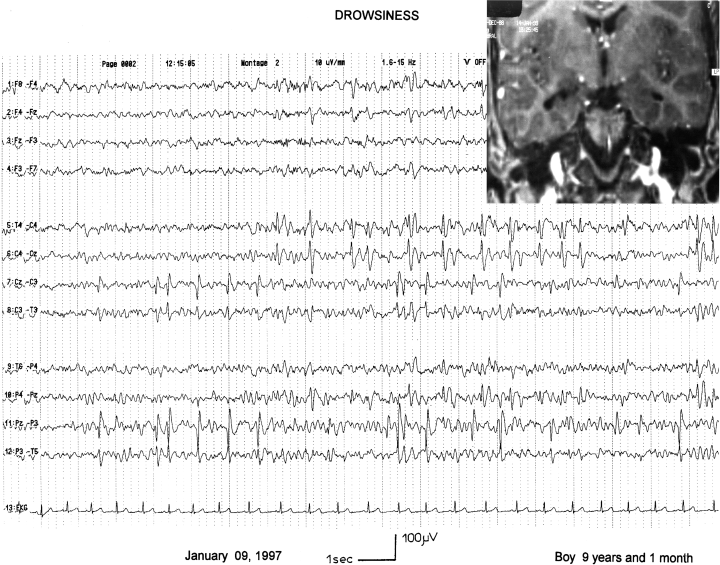
Patient 5. High-voltage diphasic spikes are apparent over the right centrotemporal region and independently over the left parietotemporal region. Magnetic resonance imaging showing moderate enlargement of the right temporal horn without hippocampal atrophy.
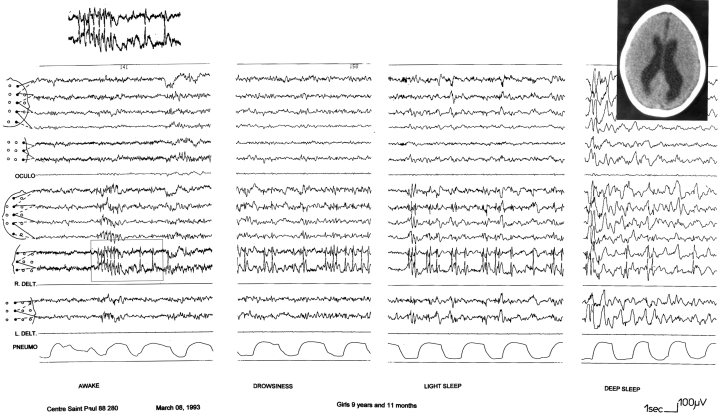
Patient 7. EEG recording showing diphasic spikes over the right centrotemporal region, associated with some slow waves. Magnetic resonance imaging showing extensive bilateral perisylvian polymicrogyria, in association with mild diffuse cerebral atrophy.
| Pat. | Sex | Age at onset (yr) | Age at the end of FU | Family history of sz | Personal history | Neurologic examination | Types of seizure |
|---|---|---|---|---|---|---|---|
| 1 | M | 2 | 17 | No | Hydrocephalus diagnosed at 6 mo | Normal | Nocturnal focal motor sz involving face and right leg |
| 2 | M | 8 | 14 | No | Neonatal hydrocephalus due to an intraventricular hemorrhage | Normal | Nocturnal focal motor sz involving face |
| 3 | F | 7 | 16.5 | No | Neonatal distress; status epilepticus in neonatal period | Moderate MR | Sleep sensory-motor sz involving right hemibody preserved consciousness |
| 4 | M | 6 | 15 | No | Premature birth at 27 wk | Mild MR | Right focal motor sz involving left hemibody |
| 5 | M | 8 | 11 | No | No | Normal | Nocturnal orofacial focal sz |
| 6 | M | 10.7 | 12 | Sister with RS (asymptomatic) | No | Normal | Nocturnal orofacial focal sz, one sz with generalization |
| 7 | M | 6.8 | 12 | Maternal aunt simple motor focal sz at age 6–8 recovery at puberty | Development delayed | Severe MR; dyspraxia; stiff limbs | One diurnal sz |
| 8 | M | 9 | 16 | No | No | Normal | Sleep focal motor sz involving face |
| 9 | M | 9.5 | 13.5 | Maternal aunt febrile sz | Asthma; simple febrile sz | Normal | Nocturnal focal motor sz involving left hemibody |
| 10 | M | 4.5 | 21 | No | Macrocrania; spondylothoracic dysplasia | Normal | Simple focal motor involving face |
| Pat. | Frequency of seizures | Antiepileptic treatment | EEG | Neuroimaging |
|---|---|---|---|---|
| 1 | Rare sz first yr | No | S over the left C-T region more rarely over the right C region. Increased by sleep | CT: shunted hydrocephalus (Fig. 1) |
| 2 | Single sz | VPA | S and slow S over the left C-T region increased by sleep. Abnormalities also found before the first sz | CT: shunted hydrocephalus |
| 3 | 4 sz first yr 5 sz second yr 1 sz third yr, then sz free without AED | VPA + CBZ + PB at referral then all were stopped | SW over the left C-T region increased by sleep with rare S over the right C region | CT: moderate ventricular dilatation compatible with the history of perinatal hypoxia (Fig. 2) |
| 4 | Single | VPA stopped at age 10 yr | Multifocal slow S over the C-T regions increasing during sleep | MRI: slight ventricle dilatation and hypersignals in the white matter (afteraffects of premature birth) |
| 5 | 3 sz first yr 2 sz second yr | VPA, CBZ | Independent slow S over the C-T regions | MRI: moderate enlargement of the right temporal horn (Fig. 3) |
| 6 | 3 sz first yr, then sz free | VPA | High-voltage S and SW predominating over the right F-T area | MRI: marked right hippocampal atrophy |
| 7 | Single | No | Multifocal S and slow S over the C-T regions | MRI; biopercular polymicrogyria (Fig. 4) |
| 8 | Single | CBZ stopped at age 13 yr | Independent S over the right and left C-T regions | CT, MRI: cavum septum pellucidum |
| 9 | 5 sz at age 9–10, then sz free without AED | VPA, CBZ first year, then stopped | Multifocal S and slow S over the C-T or F-T areas | MRI: small epiphysis cyst |
| 10 | Rare sz despite VPA; sz free since age 9 | VPA, CBZ stopped at age 11 yr | Independent S and slow S over the C-T regions | CT: agenesis of the corpus callosum with enlargement of the third ventricle but without hydrocephalus |
- PB, phenobarbital; CBZ, carbamazepine; VPA, valproate; FU, follow-up; S, spikes; SW, spike–waves; sz, seizure(s); AED, antiepileptic drug; C-T, centro-temporal; C, central; F-T, fronto-temporal; RS, rolandic spikes; CT, computed tomography; MRI, magnetic resonance imaging; MR, mental retardation.
DISCUSSION
Among 71 patients with BECTS who underwent neuroimaging, 10 (14%) had abnormal brain scans. This proportion is greater than that in the baseline nonepilepsy normal population. BECTS is a common childhood-specific epileptic syndrome that represents ∼15% of epilepsies in children younger than 15 years (9). Its EEG marker centrotemporal spikes, is much more common as it also is found in 2–3% of normal children (9). The presence of anatomic changes may contribute to the expression of seizure susceptibility in predisposed patients. It might thus be hypothetized that, even if they are present in only a minority of patients with BECTS and do not influence prognosis, brain lesions may increase the expression of epilepsy among the large number of children with rolandic spikes and logically be slightly overrepresented in this population. By virtue of high prevalence, BECTS also may affect children with static or residual brain lesions; however, most authors consider normal cognitive, neurologic, and anatomic findings to be prerequisites for the diagnosis of idiopathic forms of epilepsy, especially in localization-related epilepsies of childhood.
Basing themselves on a series of 260 cases, Dalla Bernardina et al. (10) described the characteristics common to the various forms of benign partial epilepsies of childhood. Subjects with neurologic and/or intellectual deficits were excluded. However, the authors mentioned, in a subject with a fixed or intellectual deficit, the diagnosis of benign partial epilepsy must not be a priori excluded but it must be considered with reserve and only when the other parameters are all present.
Indeed, several studies reported sporadic patients in whom different types of noncausative lesions were observed. Among 100 patients reported by Lerman and Kivity (11) three had cerebral palsy, and one had microcephaly. The authors pointed out that partial epilepsy can be benign, even in the presence of brain damage, to which it should not be etiologically attributed. Among the 40 cases reported by Blom et al. (12), three had slight hemiparesis. Since the appearance of modern neuroimaging procedures, precise findings have been reported (3,13–19). The literature are summarized in Table 2. Shevell et al. (17) described five patients with focal intracranial lesions. Histologic studies documented a mass lesion in four (low-grade astrocytoma in three, cavernous angioma in one. In the fifth patient, MRI displayed a mass suggestive of an oligodendroglioma, but the patient's family refused biopsy. Despite an electroclinical phenotype suggestive of BECTS, the authors underlined the presence of atypical clinical features in these five children: older age at onset, flurry of seizures, suggestion of possible subtle complex features in the seizure description, continued seizures with medication, purely unilateral spikes, and atypical localization of EEG abnormalities. More recently, Stephani and Doose (18) reported a girl who had head trauma from a dog bite at age 9 days. Despite multiple bilateral brain lesions, she had seizures and EEG changes evocative of benign partial epilepsy. We reported the case of a boy with a typical electoclinical form of BECTS in whom MRI disclosed an unilateral hippocampal atrophy (4). In this case (pat. 6), as in the case of Stephani and Doose, an EEG performed in an asymptomatic sister demonstrated focal sharp waves. This pattern is reportedly found in 34% of siblings of probands with BECTS submitted to waking and sleep EEGs (20). The presence of EEG changes in siblings further validates the diagnosis of BECTS. In another study, Degen and Degen (21) observed epileptic discharges in 26 of 69 siblings of 43 patients with rolandic epilepsy and/or centrotemporal spikes. However, these authors recorded foci in only four of the 26 and generalized spikes–wave complexes in 22.
| Authors | Year published | Procedure | No. | Neuroimaging findings |
|---|---|---|---|---|
| Morikawa et al. | 1979 | 13 CT scans in 41 pat. | 1 | Cavum septum pellicidum |
| 1 | Enlargement of the sylvian fissure on the L side | |||
| Mambelli et al. | 1985 | 17 CT scans in 40 pat. | 2 | Slight ventricular enlargement |
| 1 | R sylvian arachnoid cyst | |||
| Santanelli et al. | 1989 | CT scan | 1 | Agenesis of the corpus callosum (pat. 10 in the present study) |
| 1 | Lipoma of the corpus callosum | |||
| 1 | Congenital toxoplasmosis | |||
| Ambrosetto | 1992 | MRI | 1 | Unilateral R opercular macrogyria |
| Shevell et al. | 1996 | CT scan, MRI, or both | 1 | Cavernous angioma (L temporal parietal, close to the sylvian fissure) |
| 1 | Oligodendroglioma? (L globus pallidus/thalamus) | |||
| 2 | Low-grade astrocytoma (central convexity and R angular gyrus) | |||
| 1 | Mixed oligodendroglioma, low-grade astrocytoma (R occipital pole) | |||
| Sheth et al. | 1997 | MRI | 1 | Focal cortical dysplasia (L parietal) |
| Stephani and Doose | 1999 | MRI | 1 | Head trauma from a dog bite at age 9 days; multiple bilateral brain lesions |
| Lundberg et al. | 1999 | 18 MRIs | 10 | Hippocampal abnormalities (six children) Asymmetry in five Local high intensities on T2-weighted images in three |
| Heterotopic nodule above the R frontal horn (one case) | ||||
| Arnold–Chiari malformation (one case) |
- Pat, patients; CT, computed tomography; MRI, magnetic resonance imaging; R, right; L, left.
Lundberg et al. (1999) performed MRI in 18 BECTS children and found abnormalities in 10 (Table 2)(19). The two main abnormalities were asymmetric hippocampal size and local high signal intensities on T2-weighted images in the gray–white matter junction of the frontal and temporal lobes. None of the children had a history of complex febrile seizure. These authors suggested that the hypersignals may reflect a developmental abnormality, but offered no interpretation of the asymmetrics of hippocampi. However, the clinical symptoms of seizures in BECTS is not evocative of hippocampal involvement (4).
In our opinion, MRI should not be performed in all children with BECTS, but rather only in selected cases, when atypical clinical and/or EEG features according to Shevell et al. (17) are present. BECTS is a localization-related epilepsy determined by genetic factors. As a rule, it has a benign course with final remission before puberty. The discovery of structural brain lesions is coincidental, and epilepsy should not be ascribed to them. In typical cases of BECTS, extensive diagnostic procedures should be avoided.
Both history and EEG features are very important for the diagnosis of BECTS. The EEG characteristics common to idiopathic localization-related epilepsy were summarized by Dalla Bernardina et al. (10). An organic etiology may be suspected when focal fast rhythms or periods of flattening are observed, especially during sleep EEG recordings, when there is a shift in the morphology of the spike–waves, when the paroxysmal abnormalities disappear during rapid eye movement (REM) sleep (22). Such features were not present in our cases. All had typical EEG features of BECTS in terms of morphology, rhythmicity, and rate of activation during sleep. Furthermore, final recovery occurred in all cases who reached puberty, and all experienced a benign course during the period of seizure activity. This allows us to validate further the diagnosis of BECTS. No other focal epileptic syndrome is associated with constant remission of seizures before or at puberty and a dramatic increase of EEG changes during sleep, with the exception of the syndromes of continuous spikes-and-waves during slow sleep and the Landau–Kleffner syndrome (5,6), and none of our patients met the criteria of those.
The lesions found in these patients were not located at or near the rolandic are except in one patient with a bilateral perisylvian polymicrogyria (Fig. 4, pat. 7). Ambrosetto (15) reported a case of a patient with focal cortical polymicrogyric dysplasia and BECTS, in whom the follow-up confirmed a remission of epilepsy. Other types of age-related, self-limited epilepsies have been described in patients with brain lesions: a series of nine patients with sleep-related electrical status epilepticus and multilobar polymicrogyria was recently reported (23). These patients had a severe brain lesion and a very severe epilepsy, but in spite of the permanent brain lesion, all followed the syndrome-specific course toward terminal remission from seizures before puberty. Thus extensive brain dysplastic lesions can be associated with an age-related, self-limited expression of epilepsy. No consistent pattern emerges from the other lesions found in our patients, most of which appeared as subcortical, without any clear relation to the rolandic areas.
One might conclude that the coexistence of BECTS and brain lesion is purely coincidental, although it might be argued conversely that the existence of a brain lesion contributes to lower the epileptogenic threshold, thus constituting a supplementary factor transforming a genetic predisposition into a clinical condition. In most of our patients, no logical relation exists between the lesion and the clinically expressive rolandic cortical area (e.g., in the patient with hippocampal atrophy or with epiphyseal cyst); in some patients, a distant or possible relation might be discussed (e.g., in the presence of hydrocephalus or of bilateral or asymmetric ventricular enlargement). One patient had a potentially significant lesion (i.e., perisylvian polymicrogyria), but no literature discusses the pathogenesis of BECTS in relation with possible cortical anatomic abnormalities.
Our findings suggest that BECTS can be diagnosed in the presence of fixed neurologic deficits, and we demonstrated that abnormal neuroimaging findings do not change the course of this benign epileptic syndrome. We also used the same, retrospective method to evaluate the influence of abnormal findings on the clinical presentation and prognosis in other forms of idiopathic epilepsy: in juvenile myoclonic epilepsy, we recently found various abnormal findings in nine cases among 78 consecutive patients. The lesions were nonspecific and did not influence therapeutic decisions or prognosis (24).
CONCLUSIONS
This study confirms that BECTS can be diagnosed in patients with cerebral lesions with or without associated neurologic symptoms, as long as the other electroclinical traits of the syndrome are present. For all practical purposes, this association appears coincidental, as the presence of anomalies did not change the benign course of this epilepsy. Our aim was not to assess the exact prevalence of neuroimaging abnormalities in BECTS, a task that would have had to be prospective and would have had to use homogeneous methods of investigation. We want to demonstrate only that the presence of abnormal neuroimaging does not change the evolution of BECTS. This is, in our opinion, useful for clinicians, as many tend to discard the diagnosis of BECTS when some abnormality is found on neuroimaging. In clinical practice, such patients may thus also benefit from a very careful therapeutic approach that may include abstention from anticonvulsant therapy. Our findings also stress that a diagnosis of BECTS based on sound clinical and EEG data does not require confirmation by neuroimaging. In matters of epilepsy outcome and prognosis, the epileptic syndrome clearly outweighs the occasionally present associated pathologic condition.




