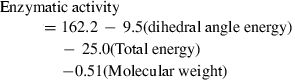Study of the mechanism of Flavobacterium sp. for hydrolyzing organophosphate pesticides
Abstract
The biotransformation by Flavobacterium sp. of the following organophosphate pesticides was experimentally and theoretically studied: phorate, tetrachlorvinphos, methyl-parathion, terbufos, trichloronate, ethoprophos, phosphamidon, fenitrothion, dimethoate and DEF. The Flavobacterium sp. ATCC 27551 strain bearing the organophosphate-degradation gene was used. Bacteria were incubated in the presence of each pesticide for a duration of 7 days. Parent pesticides were identified and quantified by means of a gas-chromatography mass spectrum system. Activity was considered as the amount (μmol) of each pesticide degraded by Flavobacterium sp. Also, structural parameters obtained by means of the CAChe program package for biomolecules, the reactivity index of phosphorus, of oxygen at the P=O function and of sulfur at the P=S function, and lipophilicity (log Poct) (ALOGPS v. 2.0) were obtained for each pesticide. Pesticides were hydrolyzed at the bond between phosphorous and the heteroatom, producing phosphoric acid and three metabolites. Enzymatic activity was significantly explained by the following multiple linear relationship: