Large-scale terrestrial gastropod community composition patterns in the Great Lakes region of North America
Abstract
Abstract: Previous ordination studies of land snail community composition have been limited to four or fewer habitat types from sites separated by no more than 300 km. To investigate the nature of large-scale patterns, North American land snail assemblages at 421 sites, representing 26 habitat types and covering a 1400 × 800 km area, were ordinated using global, nonmetric multi-dimensional scaling (NMDS). These data were then subjected to model-based cluster analysis and kmeans clustering to identify the main compositional groups and most important environmental covariables. Six primary compositional groups were identified. Three of these largely represent upland forest and rock outcrop sites, while the remaining largely represent either lowland forest, lowland grassland or upland grassland habitats. The geographical location and moisture level of sites also influences community composition. A strong compositional difference exists between sites having duff vs. turf soil surface layers. Only 8% of sites were improperly classified when soil surface architecture was used as the sole predictor variable. Fully 43% of taxa exhibited significant preferences towards one of these surface types, while only 15% of relatively common (10 + occurrence) taxa showed no preferences. Twelve groups of closely related taxa within the same genus had members that favoured different surface types, indicating that differential selection pressures have existed over evolutionary time scales. While turf faunas appeared unaffected by anthropogenic disturbance, duff faunas were strongly impacted, suggesting that their conservation will require protection of soil surface architecture.
INTRODUCTION
Land snails are regarded typically as generalist herbivores, fungivores and detritivores (Burch & Pearce, 1990) that exhibit weak levels of intraspecific competition (Cain, 1983; Cowie & Jones, 1987; Smallridge & Kirby, 1988; Barker & Mayhill, 1999). As land snail communities can consume less than 0.5% of annual litter input per year (Mason, 1970), some speculate that few resources, beyond CaCO3 (Boycott, 1934) and appropriate resting site availability (Pearce, 1997), will limit distribution. This concept is supported by high levels of microsympatry in land snail communities, where 13–35 (representing up to 50% of the regional fauna) co-occurring taxa can be found at < 1 m2 grains (Schmid, 1966; Cameron & Morgan-Huws, 1975; Nekola & Smith, 1999; Cameron, 2002).
However, at landscape scales, land snail population size and faunistic composition has been suggested to vary with habitat and vegetation types (e.g. Burch, 1956; Wäreborn, 1970; Van Es & Boag, 1981; Young & Evans, 1991; Stamol, 1991; Stamol, 1993; Ports, 1996; Theler, 1997; Nekola, 2002). Habitat preferences for individual species have also been discussed (without supporting empirical data) at subcontinental scales in both western Europe (Kerney & Cameron, 1979) and eastern North America (Hubricht, 1985).
Ordination techniques have documented significant species turnover along local environmental gradients in western Europe (e.g. Tattersfield, 1990; Magnin et al., 1995; Hermida et al., 2000; Ondina & Mato, 2001) and Pacific Island (e.g. Cowie et al., 1995; Barker & Mayhill, 1999) faunas. Unfortunately, these (and all other published snail ordination) studies have been conducted only at limited ecological (< four sampled habitat types) and geographical (maximum separation of no more than 300 km) scales.
The following study addresses these concerns by using global nonmetric multi-dimensional scaling (NMDS) ordination and model-based cluster analysis to analyse land snail composition patterns within 26 habitat types across a 1400-km extent of central North America. These data will be used to address: (1) if land snail community composition predictably varies across this subcontinental region; and (2) what environmental and geographical factors underlie any such patterns. This study represents not only the first use of NMDS in the analysis of land snail communities, but also represents the first time North American faunas have been subjected to ordination.
METHODS
Study region
Land snail faunas were sampled across a 1400 × 800 km area centred on the western portion of the Great Lakes basin in eastern and central North America (Fig. 1). This area covers a wide range of bedrock, climate and vegetation types. Both Palaeozoic sedimentary and Precambrian igneous bedrock outcrops in the region. One of the more prominent sedimentary exposures is the Niagaran Escarpment, a band of Silurian limestones and dolomites extending from western New York state to north-eastern Iowa. Outcrops along the western Lake Superior shore typically represent late-Precambrian mafic igneous rocks associated with the Keewenawan mid-continental rift system (Anderson, 1983). Average minimum winter temperatures range from −25 °C in northern Minnesota to −10 °C in western New York State. Average maximum summer temperatures range from 25 °C along the western Lake Superior shore to 30 °C in south-eastern Iowa. The average length of the 0 °C growing season varies from 100 to 110 days in northern Minnesota and Michigan to 180–190 days in southern Iowa, southern Ontario, and western New York state. In areas adjacent to or downwind from (east of) the Great Lakes (especially the Lower Peninsula of Michigan, southern Ontario, and western New York State), the climate tends to be buffered over that normally experienced in the continental interior, being warmer in the winter, cooler in the summer and having a longer growing season with more constant precipitation (Eichenlaub, 1979). Matrix vegetation varies from tallgrass prairie in the west to deciduous forest in the east to mixed boreal–hardwood forest in the north (Barbour & Billings, 1988).
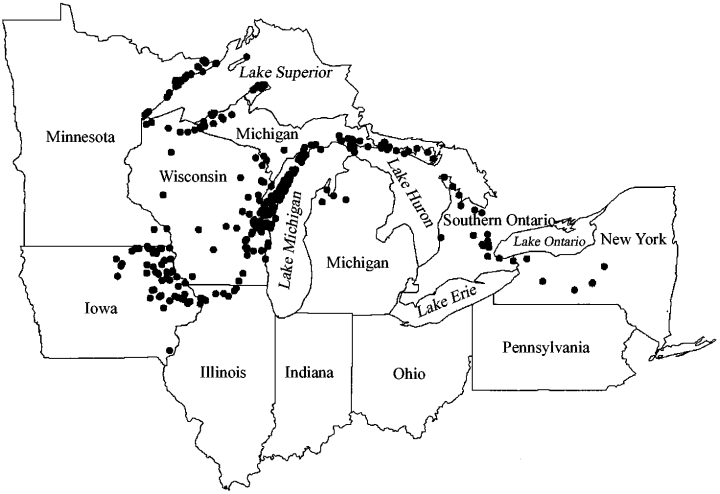
Map of the study region showing the location of the 443 sample sites.
Study sites
Four hundred and forty-three sites (Fig. 1) were surveyed across the range of habitats known to support diverse land snail assemblages (Nekola, 1999). The 26 habitats surveyed were broadly grouped into five major categories: rock outcrops, upland forests, lowland forests, upland grasslands and lowland grasslands (Table 1). While sites generally represent undisturbed examples of their respective habitats, an effort was also made to sample some (25 rock outcrop, 12 upland forest, nine lowland forest, four upland grassland and eight lowland grassland) that had been disturbed anthropogenically by grazing, logging, recreational/urban development or bedrock/soil removal. Examples of such sites include field-edge stone piles, abandoned agricultural fields, abandoned building foundations, old quarries, pastures, road verges and exploited forests.
| Group | Habitat type | Sites sampled | Geographic range |
|---|---|---|---|
| Rock outcrop | Carbonate cliff | 129 | Illinois, Iowa, Minnesota, Michigan, Ontario, New York, Wisconsin |
| Lakeshore carbonate ledge | 23 | Michigan, Ontario, Wisconsin | |
| Algific talus slope | 27 | Illinois, Iowa | |
| Igneous cliff | 72 | Michigan, Minnesota | |
| Sandstone/quartzite cliff | 5 | Wisconsin | |
| Shale cliff | 3 | New York | |
| Upland forest | Oak–hickory forest | 2 | Wisconsin |
| Maple–basswood forest | 3 | Wisconsin | |
| Hemlock–birch forest | 1 | Wisconsin | |
| Lakeshore forest | 16 | Michigan, Wisconsin | |
| Rocky woodland | 26 | Iowa, Michigan, Ontario, Wisconsin | |
| Lowland forest | Floodplain forest | 2 | Wisconsin |
| Black ash swamp | 6 | Wisconsin | |
| Tamarack swamp | 33 | Minnesota, Michigan, Ontario, Wisconsin | |
| White cedar swamp | 16 | Michigan, Ontario, Wisconsin | |
| Shrub-carr | 2 | Wisconsin | |
| Upland grassland | Tallgrass prairie | 1 | Iowa |
| Sand dune | 1 | Wisconsin | |
| Bedrock glade | 13 | Iowa | |
| Alvar | 6 | Michigan, Wisconsin | |
| Igneous shoreline | 4 | Michigan | |
| Successional old field | 4 | Wisconsin | |
| Lowland grassland | Sedge meadow | 5 | Michigan, Wisconsin |
| Fen | 29 | Iowa, Michigan, New York, Wisconsin | |
| Calcareous meadow | 7 | Michigan, Wisconsin | |
| Cobble beach | 7 | Michigan, Wisconsin |
Field methods
Documentation of terrestrial gastropods from each site was accomplished by hand collection of larger shells and litter sampling for smaller taxa from representative 100–1000 m2 areas. Soil litter sampling was primarily used as it provides the most complete assessment of site faunas (Oggier et al., 1998). As suggested by Emberton et al. (1996), collections were made at places of high micromollusc density, with a constant volume of soil litter (approximately 4 L) being gathered from each site. For woodland sites, sampling was concentrated: (1) along the base of rocks or trees; (2) on soil covered bedrock ledges; and/or (3) at other places found to have an abundance of shells. For grassland sites, samples consisted of: (1) small blocks (c. 125 cm3) of turf; (2) loose soil and leaf litter accumulations under or adjacent to shrubs, cobbles, boulders and/or hummocks; and (3) other locations observed to have an abundance of shells.
The latitude–longitude location of each sample was determined using either USGS 7.5 minute topographic maps or a hand-held GPS. To minimize bias from use of polar-coordinates, these locations were converted subsequently to Cartesian UTM Zone 16 coordinates using arcinfo.
The presence or absence of anthropogenic disturbance and soil surface architecture (duff vs. turf) was also recorded from each site. For purposes of this study ‘duff’ soils represent sites where the organic horizon was deep (> 4 cm) and subtended by a friable upper A horizon consisting largely of humus and mineral soil. ‘Turf’ soils represent sites where the organic horizon is thin (< 4 cm) and immediately subtended by an upper A horizon firmly bound together by living plant roots. While many habitats only supported a single soil architecture type (e.g. all carbonate cliffs were duff, and all bedrock glades were turf), some (such as white cedar swamps) could possess either turf or duff surface layers, depending upon individual site conditions. Thus, habitat type could not be used as a surrogate for soil surface architecture.
Laboratory procedures
Samples were dried slowly and completely in either a low-temperature soil oven (c. 80–95 °C) or in a greenhouse. Dried samples were then soaked in water for 3–24 h, and subjected to careful but vigorous water disaggregation through a standard sieve series (ASTME 3/8′ (9.5 mm), #10 (2.0 mm), #20 (0.85), and #40 (0.425 mm) mesh screens). These fractions were then dried and passed again through the same sieve series, and hand-picked against a neutral-brown background. All shells and shell fragments were removed.
All identifiable shells from each site were assigned to species (or subspecies) using the author's reference collection and the Hubricht Collection at the Field Museum of Natural History (FMNH). Identification of some additional specimens representing Holarctic taxa more common in western Europe were verified by Robert Cameron of the University of Sheffield, UK. All specimens have been catalogued and are housed in the author's reference collection at the University of Wisconsin — Green Bay. Nomenclature generally follows that of Hubricht (1985), with updates and corrections by Frest (1990, 1991) and Nekola (2002).
Statistical procedures
Ordination
Species lists were determined for each sample. Sites with four or fewer taxa were excluded from further analysis, as such samples can bias results and obscure compositional trends. The remaining sites were subjected to global nonmetric multi-dimensional scaling (NMDS) using decoda (Minchin, 1990). NMDS was used as it makes no assumptions regarding the underlying nature of species distributions along compositional gradients. As such, NMDS is the most robust form of ordination for detection of ecological patterns (Minchin, 1987).
To ordinate sites, a matrix of dissimilarity coefficients was calculated between all pairwise combinations of sites using the Czekanowski (Bray–Curtis) index (Faith et al., 1987). All species (including the most rarely encountered) were considered. NMDS in one to four dimensions was then performed, with 200 maximum iterations, a stress ratio stopping value of 0.9999, and a small stress stopping value of 0.01. Output was scaled in half-change units, so that an interpoint distance of 1.0 will correspond, on average, to a 50% turnover in species composition.
Because a given NMDS run may locate a local (rather than the global) stress minimum, multiple NMDS runs must be conducted on a given set of data from different initial random starting points to assess the stability of an individual solution (Minchin, 1987). For this ordination, decoda used a total of 20 random starting configurations. Solutions in each of the four dimensions were compared using a Procrustes transformation to identify those that were statistically identical. The number of unique solutions, and number of runs which fell into each, was then calculated across each of the four dimensions (Minchin, 1990). The modal solution of 20 runs was identified, and was considered a global optimum when it was achieved in at least 50% of starts.
Identification of compositional groups
The chosen optimal NMDS solution was then subjected to model-based cluster analysis (Banfield & Raftery, 1992) to identify the number of compositional groups most supported by the data. Clustering was performed on the selected ordination output, rather than raw data, as ordination results are more robust and less susceptible to sampling or other inadvertent errors (Equihua, 1990). A sum-of-squares model was chosen, as it assumes that clusters will be spherical in ordination space, making them maximally compact and similar in composition. The approximate weight of evidence for k clusters (AWEk) was calculated via the S + MCLUST algorithm (Statistical Sciences, 1995) for k= 1 to n − 1 clusters (where n= the total number of ordinated sites). The larger the AWEk, the more evidence exists for that number of clusters. After the optimum number of clusters was determined, kmeans iterative relocation (Hartigan, 1975) was used to assign each site to a cluster. Kmeans clustering was chosen as it operates under the same sum-of-squares criteria used for AWEk calculation.
Ordination interpretation
The number of occurrences (and frequency) of each species within each kmeans cluster was calculated. Species frequencies between clusters were compared using a Spearman's rank correlation. The 10 most frequent taxa, taxa reaching modal frequency and species richness for each cluster were calculated.
The frequency of the five major habitat groups between the compositional clusters was analysed using a contingency table. As predicted values were sparse (< 5) in more than one-fifth of cells, log-linear modelling was used to estimate significance (Zar, 1984).
The maximum correlation vectors for the four recorded environmental variables (UTM E coordinate, UTM N coordinate, soil surface type, presence of anthropogenic disturbance) was calculated by decoda. The significance of each was estimated through Monte-Carlo simulations using 1000 replications.
Discriminant analysis was used to help further describe the impact of soil surface type and anthropogenic disturbance on site position in ordination space. Three tests were conducted: (1) effect of soil surface architecture (duff vs. turf) and the effect of anthropogenic disturbance separately for (2) duff and (3) turf soils.
Lastly, the number of duff and turf sites containing and lacking each species was calculated. The significance of observed differences in these ratios between duff and turf sites was estimated using log-linear modelling. As this test was repeated for each species, a Bonferroni correction was used to adjust the significance threshold. This conservative adjustment was used so that only the most robust deviations would be used for data interpretation.
RESULTS
Site ordination
One hundred and eight terrestrial gastropod taxa were identified from the 443 inventoried sites (Appendix I). Twenty-two sites were species poor (four or fewer taxa) and removed from further analysis. These included eight igneous cliffs, three lakeshore forests, three tamarack wetlands and single shale cliff, oak–hickory forest, maple–basswood forest, floodplain forest, sand dune, old field, sedge meadow and cobble beach sites.
NMDS of the remaining 421 sites demonstrated that the only stable solution occurred along two axes of variation, where one was achieved in 50% of starts. The minimum stress configuration of this solution was 0.197412. In other dimensions, the most stable solution(s) were achieved in five (one dimension), three (three dimensions) and one (four dimensions) runs (Table 2).
| Dimensions | Stress level | Runs achieving minimum stress | Unique solutions | Number of runs in modal solution |
|---|---|---|---|---|
| 1 | 0.339103 | 20 | 11 | 5 |
| 2 | 0.197412 | 20 | 6 | 10 |
| 3 | 0.147405 | 18 | 16 | 3 |
| 4 | 0.116912 | 18 | 18 | 1 |
Identification and description of compositional clusters
Visual observation of the chosen NMDS ordination solution demonstrated apparent natural clustering in faunal composition, with at least one well-defined group existing in the lower-centre of the diagram (Fig. 2). AWEk analysis demonstrated that the maximum score (2131.5) was achieved at the 53rd cluster. As this result provides too many groups to be useful for generalization of faunistic trends, AWEk scores from k= 1–10 were calculated, along with the percentage increase in AWEk from k to k + 1 clusters (Table 3). These data demonstrate that over 50% of maximum AWEk was achieved by the 6th cluster. The percentage increase in AWEk fell by almost 50% for cluster 7 (7.3%), and decreased steadily to the 3.4% range by cluster 10. Based on this, the optimal number of clusters was set at six (Fig. 3), even though it does not represent maximum AWEk.
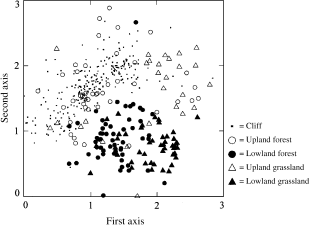
NMDS ordination of 421 sites with land snail richness of 5 or more, showing distribution of the five main habitat groups. Units are scales in half-change units, such that a distance of 1 represents a 50% turnover in fauna composition.
| No. of clusters | AWE k | % Change from AWEk to AWEk−1 |
|---|---|---|
| 1 | 0 | — |
| 2 | 298.3 | — |
| 3 | 767.8 | 157.4 |
| 4 | 919.4 | 19.7 |
| 5 | 1097.8 | 19.4 |
| 6 | 1244.0 | 13.3 |
| 7 | 1335.2 | 7.3 |
| 8 | 1411.4 | 5.7 |
| 9 | 1456.7 | 3.2 |
| 10 | 1508.9 | 3.6 |
| 53 | 2131.5 | — |

NMDS ordination of 421 sites with land snail richness of five or more. Letters represent each of the six compositional clusters assigned via Kmeans Clustering.
Contingency table analysis (Table 4) demonstrates that habitat representation significantly (P < 0.00005) varies between the six nonoverlapping kmeans clusters. Clusters A–C were equally (P = 0.3778) represented by rock outcrop and upland forest sites, while cluster D was dominated by lowland forests, cluster E by lowland grasslands and cluster F by upland grasslands. The 10 most frequent taxa also varied greatly, with approximately 50% turnover occurring between even the most similar groups (Table 5). Spearman's rank correlations of species occurrence frequencies indicated that clusters D and E were most similar (0.831), while clusters A and F were the most different (0.316). Six species possessed modal occurrence frequencies in cluster A, 25 in cluster B, 32 in cluster C, five in cluster D, 20 in cluster E and 20 in cluster F (Table 6). Forty-one total taxa were encountered in cluster A, 78 in cluster B, 87 in cluster C, 58 in cluster D, 60 in cluster E and 60 in cluster F (Appendix I).
| Compositional cluster | Habitat group | Total sites | Species richness | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| A | 57 | 6 | 2 | 4 | 0 | 69 | 41 |
| B | 100 | 20 | 2 | 1 | 0 | 123 | 78 |
| C | 86 | 9 | 4 | 3 | 0 | 102 | 87 |
| D | 2 | 2 | 37 | 2 | 11 | 54 | 58 |
| E | 0 | 1 | 10 | 2 | 34 | 47 | 60 |
| F | 5 | 5 | 0 | 15 | 1 | 26 | 60 |
| Comparison | Log-likelihood ratio statistic | d.f. | P | ||||
| Entire table | 451.898 | 20 | < 0.00005 | ||||
| Clusters A,B,C | 8.593 | 8 | 0.3778 | ||||
| Clusters D,E,F | 112.053 | 8 | < 0.00005 | ||||
| Rank order | Cluster A | Cluster B | Cluster C | Cluster D | Cluster E | Cluster F |
|---|---|---|---|---|---|---|
| 1 | Discus catskillensis (82.61%) | Discus catskillensis (93.50%) | Punctum vitreum (89.22%) | Carychium exiguum (88.89%) | Gastrocopta tappaniana (93.62%) | Hawaiia minuscula (57.69%) |
| 2 | Nesovitrea binneyana (82.61%) | Punctum minutissimum (91.06%) | Gastrocopta contracta (88.24%) | Striatura milium (83.33%) | Carychium exiguum (85.11%) | Vallonia costata (46.15%) |
| 3 | Zonitoides arboreus (82.61%) | Zonitoides arboreus (90.24%) | Carychium exile (88.24%) | Nesovitrea electrina (81.48%) | Nesovitrea electrina (85.11%) | Cochlicopa lubrica (46.15%) |
| 4 | Vertigo cristata (72.46%) | Strobilops labyrinthica (87.80%) | Vertigo gouldi (87.25%) | Strobilops labyrinthica (72.22%) | Vertigo elatior (74.47%) | Helicodiscus parallelus (46.15%) |
| 5 | Striatura milium (71.01%) | Anguispira alternata (84.55%) | Anguispira alternata (85.29%) | Striatura exigua (72.22%) | Euconulus alderi (70.21%) | Gastrocopta contracta (42.31%) |
| 6 | Punctum minutissimum (62.32%) | Vertigo gouldi (83.74%) | Strobilops labyrinthica (79.41%) | Zonitoides arboreus (68.52%) | Hawaiia minuscula (59.57%) | Gastrocopta similis (42.31%) |
| 7 | Zoogenetes harpa (53.62%) | Columella simplex (83.74%) | Gastrocopta holzingeri (78.43%) | Vertigo elatior (59.26%) | Gastrocopta contracta (57.45%) | Punctum vitreum (42.31%) |
| 8 | Euconulus fulvus (49.28%) | Striatura milium (73.98%) | Hawaiia minuscula (78.43%) | Punctum minutissimum (59.26%) | Oxylama retusa (55.32%) | Gastrocopta holzingeri (38.46%) |
| 9 | Vertigo paradoxa (49.28%) | Helicodiscus shimeki (66.67%) | Gastrocopta pentodon (74.51%) | Gastrocopta tappaniana (53.70%) | Stenotrema leai (55.32%) | Vallonia pulchella (38.46%) |
| 10 | Striatura exigua (46.38%) | Euconulus fulvus (65.85%) | Zonitoides arboreus (74.51%) | Euconulus alderi (48.15%) | Deroceras spp. (55.32%) | Vertigo pygmaea (38.46%) |
| Cluster A | Cluster B | Cluster C | Cluster D | Cluster E | Cluster F |
|---|---|---|---|---|---|
| Nesovitrea binneyana | Carychium nannodes | Allogona profunda | Carychium exiguum | Catinella avara | Catinella ‘vermeta’ |
| Vertigo cristata | Cochlicopa morseana | Anguispira alternata | Planogyra asteriscus | Catinella exile | Cepaea nemoralis |
| Vertigo modesta modesta | Columella simplex | Carychium exile | Striatura exigua | Cochlicopa lubricella | Cochlicopa lubrica |
| Vertigo modesta parietalis | Discus catskillensis | Catinella ‘gelida’ | Striatura milium | Discus cronkhitei | Gastrocopta armifera |
| Vertigo paradoxa | Discus patulus | Deroceras spp. | Vertigo nylanderi | Euconulus alderi | Gastrocopta procera |
| Zoogenetes harpa | Euconulus fulvus | Discus macclintockii | Gastrocopta tappaniana | Gastrocopta rogersensis | |
| Euconulus polygyratus | Gastrocopta contracta | Hawaiia n.sp. | Gastrocopta similis | ||
| Glyphyalinia rhoadsi | Gastrocopta corticaria | Helicodiscus n.sp. | Glyphyalinia wheatleyi | ||
| Helicodiscus shimeki | Gastrocopta holzingeri | Nesovitrea electrina | Helicodiscus inermis | ||
| Mesomphix cupreus | Gastrocopta pentodon | Oxyloma peoriensis | Helicodiscus parallelus | ||
| Mesomphix inornatus | Glyphyalinia indentata | Oxyloma retusa | Helicodiscus singleyanus | ||
| Oxychylus draparnaudi | Guppya sterkii | Pomatiopsis lapidaria | Pupilla muscorum | ||
| Paravitrea multidentata | Haplotrema concavum | Punctum n.sp. | Pupoides albilabris | ||
| Punctum minutissimum | Hawaiia minuscula | Stenotrema leai | Succinea indiana | ||
| Striatura ferrea | Hendersonia occulta | Strobilops affinis | Vallonia costata | ||
| Strobilops labyrinthica | Mesodon clausus | Triodopsis multilineata | Vallonia excentrica | ||
| Triodopsis albolabris | Mesodon pennsylvanicus | Vertigo elatior | Vallonia parvula | ||
| Triodopsis denotata | Mesodon thyroidus | Vertigo milium | Vallonia pulchella | ||
| Triodopsis tridentata | Oxychylus cellarius | Vertigo morsei | Vertigo pygmaea | ||
| Vallonia gracilicosta | Punctum vitreum | Vertigo ovata | Zonitoides nitidus | ||
| Vertigo n.sp. | Stenotrema barbatum | ||||
| Vertigo bollesiana | Stenotrema fraternum | ||||
| Vertigo hubrichti | Strobilops aenea | ||||
| Vitrina limpida | Succinea ovalis | ||||
| Zonitoides arboreus | Trichia striolata | ||||
| Triodopsis alleni | |||||
| Triodopsis fosteri | |||||
| Vallonia perspectiva | |||||
| Vertigo gouldi | |||||
| Vertigo meramecensis | |||||
| Vertigo tridentata | |||||
| Zonitoides limatulus |
Analysis of environmental co-variables
Monte Carlo testing of the maximum correlation vectors for the four recorded environmental variables (Fig. 4) demonstrated that all were highly significant (P < 0.0005), having maximum r-values ranging from 0.2111 to 0.7909 (Table 7). In duff sites, only UTM N coordinate and anthropogenic disturbance were found to correlate significantly (P < 0.0005) with the ordination diagram (Table 7). Northern sites tended to occur in the lower left of this group (maximum r= 0.8409), while disturbed sites tended to occur further to the right (maximum r = 0.4812; Fig. 5). In turf sites, only UTM N and E coordinates were found to correlate significantly (P < 0.0005) with the ordination diagram (Table 7). In this group, more northern (maximum r = 0.6690) and eastern (maximum r= 0.5067) sites tended to occur to the lower left (Fig. 5).
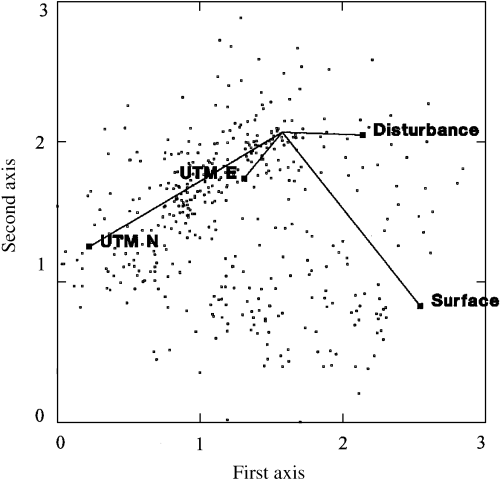
Environmental biplot for NMDS ordination. The direction of each vector represents the angle of maximum correlation, while the length represents the strength of the correlation.
| Variable | Maximum r | Angle to first axis | P |
|---|---|---|---|
| Entire ordination | |||
| UTM E-W coordinate | 0.2111 | 129.4 | < 0.0005 |
| UTM N-S coordinate | 0.7909 | 148.9 | < 0.0005 |
| Soil surface type | 0.7843 | 52.1 | < 0.0005 |
| Disturbance presence | 0.2829 | 1.7 | < 0.0005 |
| Duff soil sites only | |||
| UTM E-W coordinate | 0.1020 | 100.4 | 0.190 |
| UTM N-S coordinate | 0.8409 | 134.7 | < 0.0005 |
| Disturbance presence | |||
| Turf soil sites only | 0.4812 | 31.9 | < 0.0005 |
| UTM E-W coordinate | 0.5067 | 150.4 | < 0.0005 |
| UTM N-S coordinate | 0.6690 | 162.0 | < 0.0005 |
| Disturbance presence | 0.0671 | 1.3 | 0.820 |
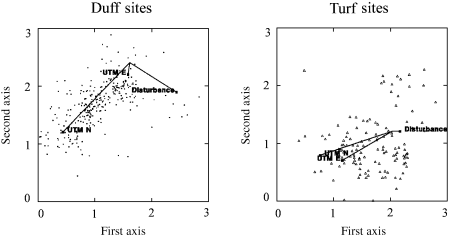
Environmental biplots for duff and turf sites. The direction of each vector represents the angle of maximum correlation, while the length represents the strength of the correlation.
Discriminant analysis demonstrated that the location of duff and turf sites in ordination space differs significantly (P < 0.0005) (Table 8), with duff sites being essentially limited to the upper left half of the diagram, and turf sites being found largely in the lower right (Fig. 6). The classification summary for this analysis indicates that only 33 of the 421 sites (7.8%) were classified improperly when soil surface type was used as the sole predictor variable.
| Factor | Comparison | ||
|---|---|---|---|
| Duff vs. turf (all sites) | Disturbed vs. pristine (duff sites only) | Disturbed vs. pristine (turf sites only) | |
| Canonical correlation | 0.784 | 0.482 | 0.067 |
| Eigenvalue | 1.596 | 0.302 | 0.005 |
| Likelihood ratio | 0.385 | 0.768 | 0.996 |
| Approximate F | 322.74 | 43.915 | 0.278 |
| Number d.f. | 2 | 2 | 2 |
| Density d.f. | 417 | 291 | 123 |
| P | < 0.0005 | < 0.0005 | 0.758 |
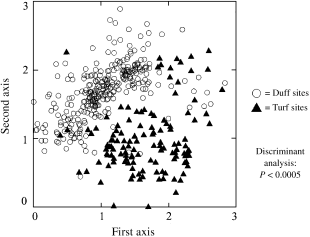
Location of duff and turf sites within the ordination diagram.
Discriminant analyses conducted separately on duff and turf sites demonstrated that disturbed duff sites were significantly (P < 0.0005) shifted to the right of undisturbed ones (Fig. 7). The classification summary for this analysis indicates that only 50 of the 295 duff sites (16.9%) were improperly classified when anthropogenic disturbance was used as the sole predictor variable. However, no significant differences (P = 0.758) were noted in the location of disturbed vs. undisturbed turf sites (Table 8).
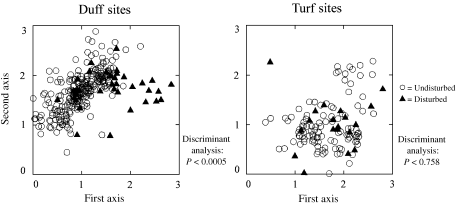
Location of disturbed and undisturbed sites in the ordination diagram for duff and turf sites.
Faunistic turnover between duff and turf soils
As differences in occurrence frequency between duff and turf sites were analysed for all 108 species, the significance threshold was lowered using a Bonferroni correction to P = 0.000463. Species with P-values ranging from 0.05 to 0.000463 were considered to possess statistically nonsignificant trends in their response to soil surface architecture. Species with P-values exceeding 0.05 were considered generalists.
Thirty-six species demonstrated no significant differences in their occurrence frequencies between duff and turf soils (Appendix I). Sixteen of these (Cochlicopa lubricella, Deroceras spp., Discus cronkhitei, Gastrocopta armifera, Gastrocopta contracta, Haplotrema concavum, Hawaiia minuscula, Helicodiscus parallelus, Helicodiscus singleyanus, Planogyra asteriscus, Striatura ferrea, Striatura milium, Triodopsis multilineata, Vallonia costata, Vertigo pygmaea, Vitrina limpida) were found in 10 or more sites, and clearly represent generalists. However, the remaining 20 (Carychium nannodes, Cepaea nemoralis, Discus patulus, Glyphyalinia wheatleyi, Helicodiscus inermis, Mesodon pennsylvanicus, Mesomphix cupreus, Mesomphix inornatus, Oxychylus cellarius, Oxychylus draparnaudi, Oxyloma peoriensis, Pomatiopsis lapidaria, Pupilla muscorum, Succinea indiana, Trichia striolata, Triodopsis denotata, Triodopsis fosteri, Vallonia excentrica, Vertigo modesta parietalis, Zonitoides limatulus) are known from fewer locations, making Type 2 errors a significant concern. Additional data will be needed to adequately assess the response of these species to duff vs. turf soils.
Eighteen species (Cochlicopa lubrica, Cochlicopa morseana, Discus macclintockii, Mesodon clausus clausus, Mesodon thyroidus, Punctum minutissimum, Punctum vitreum, Stenotrema barbatum, Strobilops aenea, Strobilops labyrinthica, Succinea ovalis, Triodopsis albolabris, Triodopsis alleni, Triodopsis tridentata, Vallonia perspectiva, Vertigo meramecensis, Vertigo modesta modesta, Vertigo tridentata) nonsignificantly favoured duff sites. Another eight (Gastrocopta procera, Gastrocopta rogersensis, Gastrocopta similis, Hawaiia n.sp., Helicodiscus n.sp., Striatura exigua, Vallonia pulchella, Zonitoides nitidus) nonsignificantly favoured turf sites. While a number of these (e.g. Discus macclintockii, Gastrocopta procera, Gastrocopta rogersensis, Vertigo meramecensis) demonstrated very strong absolute preferences, their few total occurrences in combination with the conservative Bonferroni correction prevented them from exhibiting significant responses.
The remaining 46 species demonstrated clear and significant soil surface preferences. Twenty-eight species (Allogona profunda, Anguispira alternata, Carychium exile, Catinella ‘gelida’, Columella simplex, Discus catskillensis, Euconulus fulvus, Euconulus polygyratus, Gastrocopta corticaria, Gastrocopta holzingeri, Gastrocopta pentodon, Glyphyalinia indentata, Glyphyalinia rhoadsi, Guppya sterkii, Helicodiscus shimeki, Hendersonia occulta, Nesovitrea binneyana, Paravitrea multidentata, Stenotrema fraternum, Vallonia gracilicosta, Vertigo bollesiana, Vertigo cristata, Vertigo gouldi, Vertigo hubrichti, Vertigo n.sp., Vertigo paradoxa, Zonitoides arboreus, Zoogenetes harpa) favoured duff soils, while another 18 (Carychium exiguum, Catinella avara, Catinella exile, Catinella ‘vermeta’, Euconulus alderi, Gastrocopta tappaniana, Nesovitrea electrina, Oxyloma retusa, Punctum n.sp., Pupoides albilabris, Stenotrema leai leai, Strobilops affinis, Vallonia parvula, Vertigo elatior, Vertigo milium, Vertigo morsei, Vertigo nylanderi, Vertigo ovata) favoured turf soils.
DISCUSSION
These data demonstrate clearly that at large environmental and spatial scales most land snail species possess pronounced ecological preferences. They thus represent a paradox, being generalists at small scales, yet responding to specific environmental factors at larger ones. At large scales, species tend to congregate into six major compositional clusters related to habitat type, soil surface architecture, geography, moisture levels and presence of anthropogenic disturbance.
Habitat type
The six compositional clusters significantly differ in their habitat representations. Clusters A–D generally consist of forested sites while clusters E–F generally consist of grasslands. These results are in agreement with previous studies from other regions, including north-western Spain (Ondina & Mato, 2001), southern France (Magnin et al., 1995), western Switzerland (Baur et al., 1996), Croatia (Stamol, 1991, 1993), Hungary (Bába, 1989) and north-eastern Nevada (Ports, 1996). The contrast between open-ground and forest faunas is not limited to terrestrial gastropods. Other soil invertebrate groups that demonstrate this pattern include fungus-eating microarthropods (Branquart et al., 1995), carabid beetles (McCracken, 1994), terrestrial amphipods (Taylor et al., 1995) and collembola (Greenslade, 1997). In an ordination of global earthworm communities, Lavelle et al. (1995) demonstrated that open-ground and forest assemblages were distinct from the warm-tropics to the arctic. The distinction between forest and grassland faunas thus appears to be a general driving factor in soil biota community composition.
Soil surface architecture
The greater similarity of most lowland forest faunas to lowland grasslands, as opposed to upland forests and rock outcrops (Fig. 2), suggests additional factors underlie observed land snail composition patterns. The potential importance of soil surface architecture is implied as many lowland forest sites (e.g. tamarack, white cedar and most black ash swamp forests), and all lowland grasslands, possess turf soils. Only 8% of sites were improperly classified when soil surface type was used as the sole predictor variable (Table 7). Even this rate may be exaggerated, as most misclassifications were limited to two specific instances. First, even though having turf soils, igneous shoreline habitats had faunas almost identical to surrounding rock outcrop sites. Snails in this habitat, however, were largely restricted to friable accumulations of organic matter under stunted white cedar trees. Secondly, almost all duff sites with faunas similar to upland grasslands had experienced severe levels of anthropogenic disturbance.
Striking differences exist between the species of duff and turf sites: 43% of taxa significantly favoured one soil surface type over the other (even with use of a conservative Bonferroni-corrected significance threshold), while only 15% of frequent taxa (10 + occurrences) showed no preference. Inspection of these data indicate that for eight groups of closely related taxa within the same genus (24 total), one or more significantly favour duff soils, while the other(s) significantly favour turf (Table 9). In another four groups (10 additional taxa), one or more taxa significantly favour one of these soil types, while the other(s) exhibit a nonsignificant preference (Table 9). These 12 groups represent a wide variety of phylogenetic stocks (representing nine families: Carychiidae, Helicarionidae, Polygyridae, Punctidae, Pupillidae, Strobilopsidae, Succineidae, Valloniidae, Zonitidae), shell shapes (five wider than tall, five taller than wide and two equally tall as wide), and maximum shell dimensions (0.8 mm– 12 mm). The presence of so many pairs of closely related duff- and turf-specialist taxa across such a wide range of phylogenies, shell shapes and dimensions suggests that very strong selective pressures between these soil surface types have extended over evolutionary time scales.
| Duff-specialist | Sign. level | Turf-specialist | Sign. level | Family | Shell shape | Shell size (mm) |
|---|---|---|---|---|---|---|
| Carychium exile | ** | Carychium exiguum | ** | Carychiidae | Tall | 1.5–2 |
| Catinella ‘gelida’ | ** | Catinella avara, C. exile, C. ‘vermeta’ | ** | Succineidae | Tall | 4–6 |
| Euconulus fulvus | ** | Euconulus alderi | ** | Helicarionidae | Equal | 3–4 |
| Gastrocopta pentodon | ** | Gastrocopta tappaniana | ** | Pupillidae | Tall | 2 |
| Nesovitrea binneyana | ** | Nesovitrea electrina | ** | Zonitidae | Wide | 4–7 |
| Punctum minutissimum, P. vitreum | * | Punctum n.sp. | ** | Punctidae | Wide | 0.8–1.5 |
| Stenotrema fraternum | ** | Stenotrema leai | ** | Polygyridae | Wide | 8–12 |
| Strobilops labyrinthica | * | Strobilops affinis | ** | Strobilopsidae | Equal | 2–3 |
| Vallonia perspectiva | * | Vallonia parvula | ** | Valloniidae | Wide | 2 |
| Vertigo gouldi ‘group’ | ** | Vertigo ovata ‘group’ | ** | Pupillidae | Tall | 1.5–3 |
| (V. bollesiana, V. cristata, V. gouldi) | (V. elatior, V. morsei, V. ovata) | |||||
| Vertigo hubrichti, V. paradoxa, V. n.sp. | ** | Vertigo nylanderi | ** | Pupillidae | Tall | 1.5–2 |
| Zonitoides arboreus | ** | Zonitoides nitidus | * | Zonitidae | Wide | 4–6 |
It is not possible via the current analyses to definitively identify what such factors might be. They must be limited to the detritusphere (Coleman & Crossley, 1996), as almost 90% of snails live within 5 cm of the soil surface (Hawkins et al., 1998). One possible mechanism is increased competition with living plant roots in turf soils for inorganic nutrients (Lavelle et al., 1995). Another may be the greater organic litter thickness in duff soils, as the abundance (Berry, 1973), diversity (Cain, 1983; Locasciulli & Boag, 1987) and composition (Cameron & Morgan-Huws, 1975; Baur et al., 1996; Barker & Mayhill, 1999) of land snail communities often correlates positively with litter depth. The architecture of organic litter (Burch, 1956; Cameron, 1986; Young & Evans, 1991; Alvarez & Willig, 1993), and the underlying soil (Cameron, 1982; Hermida et al., 2000) may also have strong impacts on land snail community structure.
Similarly, the composition and abundance of other soil taxa communities can be influenced by organic litter depth and architecture, including amphipods (Taylor et al., 1995), microarthropods (Borcard & Matthey, 1995; Branquart et al., 1995; Kay et al., 1999; Whitford & Sobhy, 1999), collembola (Kovac & Miklisova, 1997) and ground beetles (McCracken, 1994). Thus, like habitat type, upper soil layer architecture appears to be another vital factor driving soil biota composition.
Geography
The geographical location of sites also influences community composition, particularly in duff soils (Fig. 5). Each of the three duff clusters have an unique geographical affiliation, with cluster A being largely restricted to the most northern sites, cluster B to sites in the northern half of the Lake Michigan–Huron basin, and cluster C to sites in Iowa, Illinois, and southern Wisconsin.
While a significant correlation with both latitude and longitude was also observed in turf sites, this result is almost certainly an artefact of the limitation of upland grassland sites to the south-west of the study region. Fens and lowland forests, found throughout, exhibited little geographical trends inside of the ordination diagram. Geographical location was presumably less important for these sites due to the overriding importance of habitat type and soil moisture.
Soil moisture and temperature
Soil moisture and sunlight levels also appear to influence land snail community composition in turf sites, with the driest and sunniest habitats (upland grasslands) being most different in composition from wet, shaded lowland forests. However, temperature and relative humidity, not sunlight, are probably the important driving factors (Suominen, 1999), as in both duff and turf sites the coolest and wettest habitats (northern cliff, upland forest and lowland forest) were most different in composition from the hottest and driest sites (southern cliff, upland forest and upland grassland).
Disturbance and conservation
Anthropogenic disturbance influences snail composition differentially between duff and turf sites. While turf faunas were not impacted, the most disturbed duff sites had faunas more characteristic of upland grasslands. Typical species found on such disturbed sites include Cochlicopa lubrica, Pupilla muscorum, Vallonia costata, Vallonia excentrica, Vallonia pulchella and Vertigo pygmaea. These faunistic differences may be related to differential changes in soil surface architecture with disturbance. Because undisturbed turf soils usually have thinner and less structurally complex organic litter layers, they may be less susceptible to soil compaction (and changes in composition) as compared to duff sites.
As anthropogenic soil compaction negatively impacts soil invertebrates more severely than plants in the same communities (Duffey, 1975), conservation of duff-specialist land snails will likely require protection of the soil litter layer architecture, perhaps by limiting forestry and recreation activities in duff soil sites of conservation importance. While turf sites appear to be more tolerant of human disturbance, this should not indicate that their land snail communities are immune to human activity. For instance, heavy grazing can negatively impact grassland snails (Cameron & Morgan-Huws, 1975), while the use of fire-management can lead to significant reductions in both species richness and abundance (Nekola, 2002b).
ACKNOWLEDGMENTS
Robert Cameron and Peter White provided valuable comments on earlier drafts. Matt Barthel, Tracy Kuklinski, Pete Massart, Chela Moore, Eric North, J.J. Schiefelbein and Tamara Smith processed many soil litter samples and assisted in field collection. Assistance in litter sample processing was also provided by students participating in the Land Snail Ecology Practicum at the University of Wisconsin — Green Bay. Funding was provided by the Door County Office of the Wisconsin Chapter of The Nature Conservancy, a Louis Almon grant (administered by the Wisconsin Academy of Sciences, Arts and Letters), three Cofrin Arboretum grants (administered by the Cofrin Arboretum Committee at the University of Wisconsin — Green Bay), the U.S. Fish and Wildlife Service and the Small Grants Program of the Michigan Department of Natural Resources. Funding for the survey of Minnesota sites was received from the Minnesota Nongame Wildlife Tax Checkoff and Minnesota State Park Nature Store Sales through the Minnesota Department of Natural Resources Natural Heritage and Nongame Research Program.
SUPPLEMENTARY MATERIAL
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/DDI/DDI165/DDI165sm.htm
Table S1 Species occurrences and frequencies within the six main ordination clusters, and within sites with duff or turf organic horizons.
Appendix
APPENDIX I
| Species | Number of occurrences/frequency in cluster | Duff vs. turf | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | Duff | Turf | ||
| Allogona profunda (Say, 1821) | 0 | 8 | 32 | 0 | 0 | 0 | 40 | 0 | |
| 0.00 | 6.50 | 31.37 | 0.00 | 0.00 | 0.00 | 13.61 | 0.00 | 0.0000000 | |
| Anguispira alternata (Say, 1817) | 19 | 104 | 87 | 0 | 3 | 3 | 212 | 4 | |
| 27.54 | 84.55 | 85.29 | 0.00 | 6.38 | 11.54 | 71.86 | 3.17 | 0.0000000 | |
| Carychium exiguum (Say, 1822) | 1 | 5 | 9 | 48 | 40 | 2 | 12 | 93 | |
| 1.45 | 4.07 | 8.82 | 88.89 | 85.11 | 7.69 | 4.07 | 73.81 | 0.0000000 | |
| Carychium exile (H.C. Lea, 1842) | 12 | 60 | 90 | 17 | 5 | 3 | 158 | 29 | |
| 17.39 | 48.78 | 88.24 | 31.48 | 10.64 | 11.54 | 53.56 | 23.02 | 0.0000000 | |
| Carychium nannodes (Clapp, 1905) | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |
| 0.00 | 0.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.34 | 0.00 | 0.3986709 | |
| Catinella avara (Say, 1824) | 0 | 0 | 3 | 3 | 15 | 1 | 3 | 19 | |
| 0.00 | 0.00 | 2.94 | 5.56 | 31.91 | 3.85 | 1.02 | 15.08 | 0.0000000 | |
| Catinella exile (Leonard, 1972) | 0 | 0 | 0 | 2 | 14 | 0 | 0 | 16 | |
| 0.00 | 0.00 | 0.00 | 3.70 | 29.79 | 0.00 | 0.00 | 12.70 | 0.0000000 | |
| Catinella ‘gelida’ (F.C. Baker, 1927) | 0 | 1 | 26 | 0 | 0 | 0 | 27 | 0 | |
| 0.00 | 0.81 | 25.49 | 0.00 | 0.00 | 0.00 | 9.15 | 0.00 | 0.0000078 | |
| Catinella ‘vermeta’ | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 6 | |
| 0.00 | 0.00 | 2.94 | 0.00 | 0.00 | 11.54 | 0.00 | 4.76 | 0.0001273 | |
| Cepaea nemoralis (Linné, 1798) | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | |
| 0.00 | 0.00 | 0.00 | 1.85 | 0.00 | 3.85 | 0.34 | 0.79 | 0.5522717 | |
| Cochlicopa lubrica (Müller, 1774) | 7 | 23 | 34 | 7 | 9 | 12 | 72 | 20 | |
| 10.14 | 18.70 | 33.33 | 12.96 | 19.15 | 46.15 | 24.41 | 15.87 | 0.0471465 | |
| Cochlicopa lubricella (Porro, 1838) | 4 | 13 | 17 | 1 | 10 | 4 | 37 | 12 | |
| 5.80 | 10.57 | 16.67 | 1.85 | 21.28 | 15.38 | 12.54 | 9.52 | 0.3684043 | |
| Cochlicopa morseana (Doherty, 1878) | 3 | 23 | 9 | 2 | 1 | 0 | 35 | 3 | |
| 4.35 | 18.70 | 8.82 | 3.70 | 2.13 | 0.00 | 11.86 | 2.38 | 0.0005306 | |
| Columella simplex (Gould, 1841) | 25 | 103 | 67 | 26 | 5 | 2 | 187 | 41 | |
| 36.23 | 83.74 | 65.69 | 48.15 | 10.64 | 7.69 | 63.39 | 32.54 | 0.0000000 | |
| Deroceras spp. | 1 | 28 | 67 | 12 | 26 | 8 | 95 | 47 | |
| 1.45 | 22.76 | 65.69 | 22.22 | 55.32 | 30.77 | 32.20 | 37.30 | 0.3131198 | |
| Discus catskillensis (Pilsbry, 1898) | 57 | 115 | 37 | 13 | 1 | 1 | 204 | 20 | |
| 82.61 | 93.50 | 36.27 | 24.07 | 2.13 | 3.85 | 69.15 | 15.87 | 0.0000000 | |
| Discus cronkhitei (Newcomb, 1865) | 13 | 15 | 15 | 4 | 20 | 6 | 47 | 26 | |
| 18.84 | 2.20 | 14.71 | 7.41 | 42.55 | 23.08 | 15.93 | 20.63 | 0.2491709 | |
| Discus macclintockii (F.C. Baker, 1928) | 0 | 1 | 9 | 0 | 0 | 0 | 10 | 0 | |
| 0.00 | 0.81 | 8.82 | 0.00 | 0.00 | 0.00 | 3.39 | 0.00 | 0.0072231 | |
| Discus patulus (Deshayes, 1830) | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |
| 0.00 | 0.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.34 | 0.00 | 0.3986709 | |
| Euconulus alderi (Gray, 1840) | 0 | 1 | 1 | 26 | 33 | 0 | 1 | 60 | |
| 0.00 | 0.81 | 0.98 | 48.15 | 70.21 | 0.00 | 0.34 | 47.62 | 0.0000000 | |
| Euconulus fulvus (Müller, 1774) | 34 | 81 | 47 | 8 | 7 | 6 | 156 | 27 | |
| 49.28 | 65.85 | 46.08 | 14.81 | 14.89 | 23.08 | 52.88 | 21.43 | 0.0000000 | |
| Euconulus polygyratus (Pilsbry, 1899) | 2 | 58 | 42 | 3 | 2 | 0 | 98 | 9 | |
| 2.90 | 47.15 | 41.18 | 5.56 | 4.26 | 0.00 | 33.22 | 7.14 | 0.0000000 | |
| Gastrocopta armifera (Say, 1821) | 0 | 0 | 25 | 0 | 0 | 7 | 24 | 8 | |
| 0.00 | 0.00 | 24.51 | 0.00 | 0.00 | 26.92 | 8.14 | 6.35 | 0.5198906 | |
| Gastrocopta contracta (Say, 1822) | 0 | 46 | 90 | 7 | 27 | 11 | 133 | 48 | |
| 0.00 | 37.40 | 88.24 | 12.96 | 57.45 | 42.31 | 45.08 | 38.10 | 0.1831730 | |
| Gastrocopta corticaria (Say, 1816) | 0 | 9 | 58 | 0 | 0 | 2 | 65 | 4 | |
| 0.00 | 7.32 | 56.86 | 0.00 | 0.00 | 7.69 | 22.03 | 3.17 | 0.0000001 | |
| Gastrocopta holzingeri (Sterki, 1889) | 0 | 13 | 80 | 1 | 1 | 10 | 89 | 16 | |
| 0.00 | 10.57 | 78.43 | 1.85 | 2.13 | 38.46 | 30.17 | 12.70 | 0.0000716 | |
| Gastrocopta pentodon (Say, 1821) | 3 | 70 | 76 | 10 | 5 | 9 | 141 | 32 | |
| 4.35 | 56.91 | 74.51 | 18.52 | 10.64 | 34.62 | 47.80 | 25.40 | 0.0000130 | |
| Gastrocopta procera (Gould, 1840) | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 4 | |
| 0.00 | 0.00 | 1.96 | 0.00 | 0.00 | 7.69 | 0.00 | 3.17 | 0.0018020 | |
| Gastrocopta rogersensis (Nekola & Coles, 2001) | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 4 | |
| 0.00 | 0.00 | 0.98 | 0.00 | 0.00 | 11.54 | 0.00 | 3.17 | 0.0018020 | |
| Gastrocopta similis (Sterki, 1909) | 0 | 0 | 15 | 0 | 1 | 11 | 13 | 14 | |
| 0.00 | 0.00 | 14.71 | 0.00 | 2.13 | 42.31 | 4.41 | 11.11 | 0.0138256 | |
| Gastrocopta tappaniana (C.B. Adams, 1842) | 0 | 1 | 9 | 29 | 44 | 5 | 12 | 76 | |
| 0.00 | 0.81 | 8.82 | 53.70 | 93.62 | 19.23 | 4.07 | 60.32 | 0.0000000 | |
| Glyphyalinia indentata (Say, 1823) | 2 | 48 | 46 | 4 | 6 | 7 | 94 | 19 | |
| 2.90 | 39.02 | 45.10 | 7.41 | 12.77 | 26.92 | 31.86 | 15.08 | 0.0002203 | |
| Glyphyalinia rhoadsi (Pilsbry, 1899) | 0 | 21 | 3 | 0 | 0 | 0 | 24 | 0 | |
| 0.00 | 17.07 | 2.94 | 0.00 | 0.00 | 0.00 | 8.14 | 0.00 | 0.0000261 | |
| Glyphyalinia wheatleyi (Bland, 1883) | 0 | 1 | 0 | 1 | 0 | 1 | 3 | 0 | |
| 0.00 | 0.81 | 0.00 | 1.85 | 0.00 | 3.85 | 1.02 | 0.00 | 0.1423208 | |
| Guppya sterkii (Dall, 1888) | 0 | 5 | 19 | 0 | 0 | 0 | 24 | 0 | |
| 0.00 | 4.07 | 18.63 | 0.00 | 0.00 | 0.00 | 8.14 | 0.00 | 0.0000261 | |
| Haplotrema concavum (Say, 1821) | 0 | 6 | 23 | 1 | 2 | 0 | 27 | 5 | |
| 0.00 | 4.88 | 22.55 | 1.85 | 4.26 | 0.00 | 9.15 | 3.97 | 0.0516709 | |
| Hawaiia minuscula (A. Binney, 1840) | 0 | 12 | 80 | 1 | 28 | 15 | 91 | 45 | |
| 0.00 | 9.76 | 78.43 | 1.85 | 59.57 | 57.69 | 30.85 | 35.71 | 0.3304475 | |
| Hawaiia n.sp. | 0 | 0 | 1 | 0 | 6 | 0 | 1 | 6 | |
| 0.00 | 0.00 | 0.98 | 0.00 | 12.77 | 0.00 | 0.34 | 4.76 | 0.0019219 | |
| Helicodiscus inermis (H.B. Baker, 1929) | 0 | 0 | 6 | 0 | 0 | 2 | 4 | 4 | |
| 0.00 | 0.00 | 5.88 | 0.00 | 0.00 | 7.69 | 1.36 | 3.17 | 0.2308550 | |
| Helicodiscus n.sp. | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 10.64 | 0.00 | 0.00 | 3.97 | 0.0004766 | |
| Helicodiscus parallelus (Say, 1817) | 9 | 30 | 46 | 16 | 10 | 12 | 85 | 38 | |
| 13.04 | 24.39 | 45.10 | 29.63 | 21.28 | 46.15 | 28.81 | 30.16 | 0.7814013 | |
| Helicodiscus shimeki (Hubricht, 1962) | 15 | 82 | 68 | 11 | 6 | 2 | 162 | 22 | |
| 21.74 | 66.67 | 66.67 | 20.37 | 12.77 | 7.69 | 54.92 | 17.46 | 0.0000000 | |
| Helicodiscus singleyanus (Pilsbry, 1890) | 0 | 0 | 9 | 0 | 0 | 3 | 8 | 4 | |
| 0.00 | 0.00 | 8.82 | 0.00 | 0.00 | 11.54 | 2.71 | 3.17 | 0.7959002 | |
| Hendersonia occulta (Say, 1831) | 0 | 25 | 50 | 0 | 3 | 3 | 75 | 6 | |
| 0.00 | 20.33 | 49.02 | 0.00 | 6.38 | 11.54 | 25.42 | 4.76 | 0.0000001 | |
| Mesodon clausus clausus (Say, 1821) | 0 | 1 | 16 | 0 | 0 | 0 | 16 | 1 | |
| 0.00 | 0.81 | 15.69 | 0.00 | 0.00 | 0.00 | 5.42 | 0.79 | 0.0115199 | |
| Mesodon pennsylvanicus (Green, 1827) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| 0.00 | 0.00 | 0.98 | 0.00 | 0.00 | 0.00 | 0.34 | 0.00 | 0.3986709 | |
| Mesodon thyroidus (Say, 1816) | 0 | 4 | 8 | 0 | 0 | 0 | 12 | 0 | |
| 0.00 | 3.25 | 7.84 | 0.00 | 0.00 | 0.00 | 4.07 | 0.00 | 0.0032079 | |
| Mesomphix cupreus (Rafinesque, 1831) | 0 | 3 | 1 | 0 | 0 | 0 | 4 | 0 | |
| 0.00 | 2.44 | 0.98 | 0.00 | 0.00 | 0.00 | 1.36 | 0.00 | 0.0907153 | |
| Mesomphix inornatus (Say, 1821) | 0 | 5 | 0 | 0 | 0 | 0 | 5 | 0 | |
| 0.00 | 4.07 | 0.00 | 0.00 | 0.00 | 0.00 | 1.69 | 0.00 | 0.0584018 | |
| Nesovitrea binneyana (Morse, 1864) | 57 | 56 | 20 | 6 | 1 | 0 | 128 | 12 | |
| 82.61 | 45.53 | 19.61 | 11.11 | 2.13 | 0.00 | 43.39 | 9.52 | 0.0000000 | |
| Nesovitrea electrina (Gould, 1841) | 10 | 10 | 5 | 44 | 40 | 7 | 23 | 93 | |
| 14.49 | 8.13 | 4.90 | 81.48 | 85.11 | 26.92 | 7.80 | 73.81 | 0.0000000 | |
| Oxychylus cellarius (Müller, 1774) | 0 | 1 | 2 | 0 | 0 | 0 | 3 | 0 | |
| 0.00 | 0.81 | 1.96 | 0.00 | 0.00 | 0.00 | 1.02 | 0.00 | 0.1423208 | |
| Oxychylus draparnaudi (Beck, 1837) | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |
| 0.00 | 0.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.34 | 0.00 | 0.3986709 | |
| Oxyloma peoriensis (Wolf in Walker, 1892) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 2.13 | 0.00 | 0.00 | 0.79 | 0.1199264 | |
| Oxyloma retusa (I. Lea, 1834) | 0 | 0 | 5 | 2 | 26 | 1 | 4 | 30 | |
| 0.00 | 0.00 | 4.90 | 3.70 | 55.32 | 3.85 | 1.36 | 23.81 | 0.0000000 | |
| Paravitrea multidentata (A. Binney, 1840) | 4 | 72 | 12 | 0 | 0 | 1 | 89 | 0 | |
| 5.80 | 58.54 | 11.76 | 0.00 | 0.00 | 3.85 | 30.17 | 0.00 | 0.0000000 | |
| Planogyra asteriscus (Morse, 1857) | 7 | 4 | 0 | 7 | 0 | 0 | 9 | 9 | |
| 10.14 | 3.25 | 0.00 | 12.96 | 0.00 | 0.00 | 3.05 | 7.14 | 0.0685170 | |
| Pomatiopsis lapidaria (Say, 1817) | 0 | 0 | 3 | 1 | 3 | 1 | 3 | 5 | |
| 0.00 | 0.00 | 2.94 | 1.85 | 6.38 | 3.85 | 1.02 | 3.97 | 0.0547007 | |
| Punctum minutissimum (I. Lea, 1841) | 43 | 112 | 6 | 32 | 9 | 2 | 157 | 47 | |
| 63.32 | 91.06 | 5.88 | 59.26 | 19.15 | 7.69 | 53.22 | 37.30 | 0.0026417 | |
| Punctum n.sp. | 0 | 0 | 0 | 2 | 18 | 1 | 0 | 21 | |
| 0.00 | 0.00 | 0.00 | 3.70 | 38.30 | 3.85 | 0.00 | 16.67 | 0.0000000 | |
| Punctum vitreum (H.B. Baker, 1930) | 0 | 5 | 91 | 1 | 7 | 11 | 93 | 22 | |
| 0.00 | 4.07 | 89.22 | 1.85 | 14.89 | 42.31 | 31.53 | 17.46 | 0.0022757 | |
| Pupilla muscorum (Linné, 1758) | 0 | 3 | 0 | 2 | 0 | 1 | 4 | 2 | |
| 0.00 | 2.44 | 0.00 | 3.70 | 0.00 | 3.85 | 1.36 | 1.59 | 0.8558760 | |
| Pupoides albilabris (C.B. Adams, 1821) | 0 | 0 | 6 | 0 | 1 | 9 | 3 | 13 | |
| 0.00 | 0.00 | 5.88 | 0.00 | 2.13 | 34.62 | 1.02 | 10.32 | 0.0000141 | |
| Stenotrema barbatum (Clapp, 1904) | 0 | 1 | 21 | 1 | 2 | 0 | 22 | 3 | |
| 0.00 | 0.81 | 20.59 | 1.85 | 4.26 | 0.00 | 7.46 | 2.38 | 0.0287753 | |
| Stenotrema fraternum fraternum (Say, 1824) | 2 | 47 | 42 | 0 | 0 | 0 | 90 | 1 | |
| 2.90 | 38.21 | 41.18 | 0.00 | 0.00 | 0.00 | 30.51 | 0.79 | 0.0000000 | |
| Stenotrema leai leai (A. Binney) | 0 | 0 | 2 | 6 | 26 | 6 | 2 | 38 | |
| 0.00 | 0.00 | 1.96 | 11.11 | 55.32 | 23.08 | 0.68 | 30.16 | 0.0000000 | |
| Striatura exigua (Stimpson, 1847) | 32 | 45 | 2 | 39 | 4 | 0 | 76 | 46 | |
| 46.38 | 36.59 | 1.96 | 72.22 | 8.51 | 0.00 | 25.76 | 36.51 | 0.0278698 | |
| Striatura ferrea (Morse, 1864) | 4 | 35 | 1 | 13 | 3 | 0 | 38 | 18 | |
| 5.80 | 28.46 | 0.98 | 24.07 | 6.38 | 0.00 | 12.88 | 14.29 | 0.6992505 | |
| Striatura milium (Morse, 1859) | 49 | 91 | 32 | 45 | 10 | 0 | 165 | 62 | |
| 71.01 | 73.98 | 31.37 | 83.33 | 21.28 | 0.00 | 55.93 | 49.21 | 0.2052325 | |
| Strobilops aenea (Pilsbry, 1926) | 0 | 1 | 5 | 0 | 0 | 0 | 6 | 0 | |
| 0.00 | 0.81 | 4.90 | 0.00 | 0.00 | 0.00 | 2.03 | 0.00 | 0.0380037 | |
| Strobilops affinis (Pilsbry, 1893) | 0 | 0 | 1 | 5 | 23 | 0 | 1 | 28 | |
| 0.00 | 0.00 | 0.98 | 9.26 | 48.94 | 0.00 | 0.34 | 22.22 | 0.0000000 | |
| Strobilops labyrinthica (Say, 1817) | 24 | 108 | 81 | 39 | 16 | 3 | 203 | 68 | |
| 34.78 | 87.80 | 79.41 | 72.22 | 34.04 | 11.54 | 68.81 | 53.97 | 0.0038899 | |
| Succinea indiana (Pilsbry, 1905) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.85 | 0.00 | 0.79 | 0.1199264 | |
| Succinea ovalis (Say, 1817) | 9 | 26 | 24 | 4 | 9 | 2 | 60 | 14 | |
| 13.04 | 21.14 | 23.53 | 7.41 | 19.15 | 7.69 | 20.34 | 11.11 | 0.0182788 | |
| Trichia striolata (Pfeiffer) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| 0.00 | 0.00 | 0.98 | 0.00 | 0.00 | 0.00 | 0.34 | 0.00 | 0.3986709 | |
| Triodopsis albolabris (Say, 1816) | 0 | 14 | 1 | 0 | 0 | 0 | 15 | 0 | |
| 0.00 | 11.38 | 0.98 | 0.00 | 0.00 | 0.00 | 5.08 | 0.00 | 0.0009592 | |
| Triodopsis alleni (Wetherby in Sampson, 1883) | 0 | 1 | 14 | 0 | 0 | 0 | 14 | 1 | |
| 0.00 | 0.81 | 13.73 | 0.00 | 0.00 | 0.00 | 4.75 | 0.79 | 0.0230409 | |
| Triodopsis denotata (Férussac, 1821) | 0 | 2 | 1 | 0 | 0 | 0 | 3 | 0 | |
| 0.00 | 1.63 | 0.98 | 0.00 | 0.00 | 0.00 | 1.02 | 0.00 | 0.1432081 | |
| Triodopsis fosteri (F.C. Baker, 1932) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| 0.00 | 0.00 | 0.98 | 0.00 | 0.00 | 0.00 | 0.34 | 0.00 | 0.3986709 | |
| Triodopsis multilineata (Say, 1821) | 0 | 0 | 8 | 1 | 6 | 0 | 8 | 7 | |
| 0.00 | 0.00 | 7.84 | 1.85 | 12.77 | 0.00 | 2.71 | 5.56 | 0.1651915 | |
| Triodopsis tridentata (Say, 1816) | 0 | 11 | 1 | 0 | 0 | 1 | 13 | 0 | |
| 0.00 | 8.94 | 0.98 | 0.00 | 0.00 | 3.85 | 4.41 | 0.00 | 0.0021429 | |
| Vallonia costata (Müller, 1774) | 1 | 10 | 21 | 2 | 8 | 12 | 38 | 16 | |
| 1.45 | 8.13 | 20.59 | 3.70 | 17.02 | 46.15 | 12.88 | 12.70 | 0.9590560 | |
| Vallonia excentrica (Sterki, 1893) | 0 | 0 | 1 | 0 | 0 | 3 | 2 | 2 | |
| 0.00 | 0.00 | 0.98 | 0.00 | 0.00 | 11.54 | 0.68 | 1.59 | 0.3993604 | |
| Vallonia gracilicosta (Reinhardt, 1883) | 1 | 28 | 19 | 0 | 0 | 1 | 48 | 1 | |
| 1.45 | 22.76 | 18.63 | 0.00 | 0.00 | 3.85 | 16.27 | 0.79 | 0.0000001 | |
| Vallonia parvula (Sterki, 1892) | 0 | 1 | 4 | 0 | 0 | 9 | 3 | 11 | |
| 0.00 | 0.81 | 3.92 | 0.00 | 0.00 | 34.62 | 1.02 | 8.73 | 0.0001277 | |
| Vallonia perspectiva (Sterki, 1892) | 0 | 0 | 41 | 0 | 0 | 3 | 39 | 5 | |
| 0.00 | 0.00 | 40.20 | 0.00 | 0.00 | 11.54 | 13.22 | 3.97 | 0.0020759 | |
| Vallonia pulchella (Müller, 1774) | 0 | 2 | 13 | 3 | 17 | 10 | 21 | 24 | |
| 0.00 | 1.63 | 12.75 | 5.56 | 36.17 | 38.46 | 7.12 | 19.05 | 0.0005061 | |
| Vertigo bollesiana (Morse, 1865) | 5 | 76 | 28 | 3 | 2 | 0 | 108 | 6 | |
| 7.25 | 61.79 | 27.45 | 5.56 | 4.26 | 0.00 | 36.61 | 4.76 | 0.0000000 | |
| Vertigo cristata (Sterki, 1919) | 50 | 25 | 0 | 3 | 0 | 0 | 72 | 6 | |
| 72.46 | 20.33 | 0.00 | 5.56 | 0.00 | 0.00 | 24.41 | 4.76 | 0.0000002 | |
| Vertigo elatior (Sterki, 1894) | 1 | 2 | 4 | 32 | 35 | 1 | 4 | 71 | |
| 1.45 | 1.63 | 3.92 | 59.26 | 74.47 | 3.85 | 1.36 | 56.35 | 0.0000000 | |
| Vertigo gouldi (A. Binney, 1843) | 4 | 103 | 89 | 0 | 1 | 1 | 192 | 6 | |
| 5.80 | 83.74 | 87.25 | 0.00 | 2.13 | 3.85 | 65.08 | 4.76 | 0.0000000 | |
| Vertigo hubrichti (Pilsbry, 1934) | 2 | 52 | 31 | 0 | 1 | 1 | 86 | 1 | |
| 2.90 | 42.28 | 30.39 | 0.00 | 2.13 | 3.85 | 29.15 | 0.79 | 0.0000000 | |
| Vertigo meramecensis (Van Devender, 1979) | 0 | 0 | 16 | 0 | 0 | 0 | 16 | 0 | |
| 0.00 | 0.00 | 15.69 | 0.00 | 0.00 | 0.00 | 5.42 | 0.00 | 0.0006424 | |
| Vertigo milium (Gould, 1840) | 0 | 4 | 26 | 5 | 23 | 1 | 28 | 31 | |
| 0.00 | 3.25 | 25.49 | 9.26 | 48.94 | 3.85 | 9.49 | 24.60 | 0.0000835 | |
| Vertigo modesta modesta (Say, 1824) | 6 | 2 | 0 | 0 | 0 | 0 | 8 | 0 | |
| 8.70 | 1.63 | 0.00 | 0.00 | 0.00 | 0.00 | 2.71 | 0.00 | 0.0164278 | |
| Vertigo modesta parietalis (Ancey) | 3 | 1 | 0 | 0 | 0 | 0 | 4 | 0 | |
| 4.35 | 0.81 | 0.00 | 0.00 | 0.00 | 0.00 | 1.36 | 0.00 | 0.0907153 | |
| Vertigo morsei (Sterki, 1894) | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 6 | |
| 0.00 | 0.00 | 0.00 | 3.70 | 8.51 | 0.00 | 0.00 | 4.76 | 0.0001273 | |
| Vertigo n.sp. sensu (Frest, 1991) | 1 | 26 | 17 | 0 | 0 | 1 | 45 | 0 | |
| 1.45 | 21.14 | 16.67 | 0.00 | 0.00 | 3.85 | 15.25 | 0.00 | 0.0000000 | |
| Vertigo nylanderi (Sterki, 1909) | 0 | 3 | 0 | 10 | 4 | 0 | 1 | 16 | |
| 0.00 | 2.44 | 0.00 | 18.52 | 8.51 | 0.00 | 0.34 | 12.70 | 0.0000000 | |
| Vertigo ovata (Say, 1822) | 0 | 0 | 1 | 4 | 23 | 0 | 2 | 26 | |
| 0.00 | 0.00 | 0.98 | 7.41 | 48.94 | 0.00 | 0.68 | 20.63 | 0.0000000 | |
| Vertigo paradoxa (Sterki, 1900) | 34 | 28 | 0 | 3 | 0 | 0 | 60 | 5 | |
| 49.28 | 22.76 | 0.00 | 5.56 | 0.00 | 0.00 | 20.34 | 3.97 | 0.0000024 | |
| Vertigo pygmaea (Draparnaud, 1801) | 0 | 1 | 19 | 2 | 9 | 10 | 26 | 15 | |
| 0.00 | 0.81 | 18.63 | 3.70 | 19.15 | 38.46 | 8.81 | 11.90 | 0.3353210 | |
| Vertigo tridentata (Wolf, 1870) | 0 | 1 | 32 | 0 | 0 | 4 | 32 | 5 | |
| 0.00 | 0.81 | 31.37 | 0.00 | 0.00 | 15.38 | 10.85 | 3.97 | 0.0145287 | |
| Vitrina limpida (Gould, 1850) | 7 | 17 | 0 | 3 | 4 | 2 | 22 | 11 | |
| 10.14 | 13.82 | 0.00 | 5.56 | 8.51 | 7.69 | 7.46 | 8.73 | 0.6594118 | |
| Zonitoides arboreus (Say, 1816) | 57 | 111 | 76 | 37 | 19 | 8 | 240 | 68 | |
| 82.61 | 90.24 | 74.51 | 68.52 | 40.43 | 30.77 | 81.36 | 53.97 | 0.0000000 | |
| Zonitoides limatulus (W.G. Binney, 1840) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| 0.00 | 0.00 | 0.98 | 0.00 | 0.00 | 0.00 | 0.34 | 0.00 | 0.3986709 | |
| Zonitoides nitidus (Müller, 1774) | 0 | 3 | 10 | 6 | 10 | 10 | 21 | 18 | |
| 0.00 | 2.44 | 9.80 | 11.11 | 21.28 | 38.46 | 7.12 | 14.29 | 0.0248105 | |
| Zoogenetes harpa (Say, 1824) | 37 | 11 | 0 | 2 | 0 | 0 | 46 | 4 | |
| 53.62 | 8.94 | 0.00 | 3.70 | 0.00 | 0.00 | 15.59 | 3.17 | 0.0000629 | |




