Growth/differentiation factor 7 is preferentially expressed in the primary motor area of the monkey neocortex
Abstract
We applied a differential display PCR technique to isolate molecules that are area-specific in expression in the primate neocortex, and found that growth/differentiation factor 7 (GDF7), a member of the bone morphogenetic protein (BMP)/transforming growth factor (TGF) beta super-family, is preferentially expressed in the primary motor area of African green monkeys (Cercopithecus aethiops). We proved that GDF7 is 10 times more abundant in the motor cortex than in the visual cortex by northern blotting and quantitative RT-PCR. When we examined the neocortex of closely related rhesus monkeys (Macaca mulatta), GDF7 was also most abundant in the motor cortex, although the regional difference was reduced to 3-fold. This differential expression pattern was observed in both newborn and infant rhesus monkeys. We found that several type I/II receptors of BMP, candidates of the receptors for GDF7, are uniformly expressed in the mature neocortex. The unique expression pattern of GDF7 suggests that it may play an active role in the motor area of the primate neocortex.
Abbreviations used
-
- AGPC
-
- acid guanidinium thiocyanate-phenol-chloroform
-
- BMP
-
- bone morphogenetic protein
-
- DD-PCRGDF
-
- differential display PCR
-
- growth/differentiation
-
- factor
-
- MMLV
-
- Moloney murine leukaemia virus
-
- TGF
-
- transforming growth factor
The mammalian neocortex can be subdivided into multiple areas that are morphologically and functionally divergent. Classic histological studies have revealed that each cortical area has characteristic laminar structures, and researchers have used such morphological criteria to draw cytoarchitectonic maps that consist of numerous areas (Brodmann 1909; von Bonin and Bailey 1947). Such morphological subdivisions appear to coincide in part with functional subdivisions (Feinberg and Farah 1997). For example, the presence of giant pyramidal cells of Betz coincides with the primary motor cortex in primates and other species. However, we still do not know how the morphological diversity of the neocortex is related to the functional diversity.
Recent studies have shown that molecular and cellular components of different cortical regions are also different. For example, several genes are shown to have differential expression patterns within the mature neocortex. In some cases, the overall level of mRNA expression has been shown to differ by 2–3-fold among different regions (Travis et al. 1987; Chandrasekaran et al. 1992; Chowdhury et al. 1995; Oishi et al. 1998; Okuno et al. 1999), and in other cases a subset of neurones that express a certain molecular marker show differential distribution within the neocortex (Campbell et al. 1987; Arimatsu et al. 1992; Bruckner et al. 1994; Kondo et al. 1994; Reinoso et al. 1996; Kondo et al. 1999; Barone and Kennedy, 2000). Another case of cortical heterogeneity is the dopaminergic projection from the mid-brain to the neocortex: they are densest in the motor cortex but scarcely present in the visual cortex (Berger et al. 1991). These data suggest that the functional diversity of the neocortex may be attributable in part to the diversity of molecular and cellular components of various areas.
In this paper, we attempt to identify the difference in cortical areas at the molecular level. For this purpose, we have performed differential display PCR (DD-PCR) using the monkey neocortex. We selected the monkey cortex for the following three reasons. First, the neocortex of monkeys is highly evolved, with refined laminar structures (Marin-Padilla 1992) and areas not present in rodents (Northcutt and Kaas 1995). Secondly, many physiological studies using monkeys provide extensive information on functions of specific areas. Thirdly, the large surface area of the monkey cortex, together with its distinct convolutions, renders it easy to identify and separate specific areas. Our results reveal that GDF7, a member of the BMP/TGF-β super-family, is preferentially expressed in the primary motor area. Members of this family are known to function as intercellular signalling molecules (Mehler et al. 1997). They undergo proteolytic cleavage and their carboxy-terminal domains form dimers for secretion. GDF7 was originally identified by PCR using degenerate primers for conserved carboxy-terminal domains of the TGF-β super-family (Storm et al. 1994). Recently, it was found that GDF7 is expressed specifically in the roof plate of the mouse spinal cord and is required for differentiation of a subclass of commissural interneurones (Lee et al. 1998). It was also shown that GDF7 may be involved in differentiation of cerebellar granule neurones (Alder et al. 1999). While these studies show the importance of GDF7 in neuronal differentiation, its role in neocortical organization is not known. Thus, our finding of preferential expression of GDF7 in the motor cortex suggests, for the first time, that GDF7 may play a yet undetermined role in the primate neocortex.
Materials and methods
Experimental animals and tissue preparation
Adult African green monkeys (Cercopithecus aethiops) and rhesus monkeys (Macaca mulatta) as well as newborn and infant (4- or 5-month-old) rhesus monkeys were used. The African green monkeys were bred in the Japan Poliomyelitis Research Institute and the rhesus monkeys were bred in the Primate Research Institute, Kyoto University. Monkeys were deeply anaesthetized with an overdose of sodium pentobarbital (35 mg/kg i.p.: Nembutal, Abbot, North Chicago, IL, USA) and killed by bloodletting from the carotid artery in accordance with the guidelines on animal experiments in each institute.
Monkey brains were placed on crushed ice and dissected as quickly as possible into cortical areas determined from the sulcal patterns according to the classification by von Bonin and Bailey (Fig. 1). The landmarks for dissection are as follows: area FDΔ (area 46), the region in the frontal association area, was taken from the upper and lower banks of the principal sulcus. Area FA, the region that corresponds to the primary motor area, was taken from the anterior bank of the central sulcus. Area PC, the region that corresponds to the somatosensory area, was taken from the posterior bank of the central sulcus. Area TE, the region in the temporal association area, was taken from the inferior temporal gyrus anterior to the posterior middle temporal sulcus, posterior to the posterior end of the inferior temporal sulcus and dorsal to the anterior middle temporal sulcus. Area OC, the region that corresponds to the primary visual area, was taken from the exposed surface of the dorsolateral occipital cortex. Area FD corresponds to the prefrontal region anterior to the arcuate sulcus. Area PG corresponds to the parietal region, taken from the exposed convexity between the intraparietal sulcus, the superior temporal sulcus and the medial end of the Sylvian sulcus. Dissected tissues were immediately frozen in dry ice and stored at − 130°C until use.
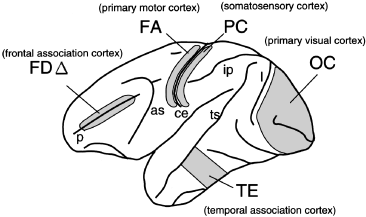
Neocortical areas of monkeys used in this study. Dissection was carried out according to the cytoarchitectonic map proposed by von Bonin and Bailey (von Bonin and Bailey 1947). Shown is the left hemisphere of the cerebral cortex. The anterior is to the left and the posterior is to the right. Each cytoarchitectonic area (FDΔ, FA, PC, OC and TE) corresponds to a functional area annotated in parentheses. Major sulci shown by the lower case letters are as follows: p, principle sulcus; as, arcuate sulcus; ce, central sulcus; ip, intraparietal sulcus; ts, superior temporal sulcus; and l, lunate sulcus.
RNA isolation and northern blotting
Total RNA was isolated from frozen tissues by the acid guanidinium thiocyanate-phenol-chloroform (AGPC) extraction method (Chomczynski and Sacchi 1987). Poly(A) RNA was purified from the total RNA using Oligotex-dT30 (Takara, Shiga, Japan) according to the manufacturer's recommended procedure. Northern blotting was carried out essentially as described previously (Sambrook et al. 1989): glyoxylated poly(A) RNA was electrophoresed in 1.2% agarose gel and transferred onto a Hybond N+ nylon membrane (Amersham-Pharmacia, Piscataway, NJ, USA). For the GDF7 probe, a 1.1-kb region in the 3′UTR (from 714 bp downstream of the stop codon to 1791 bp downstream) was 32P-labelled by random labelling. After 1 h of pre-hybridization in Rapid Hyb buffer (Amersham-Pharmacia), a 32P-labelled probe was added to the final concentration of 5 × 105 cpm/mL. After 5 h of hybridization, the membrane was washed in a solution of 0.2 × SSC and 0.1% SDS and autoradiographed.
Differential display PCR
Differential display PCR (DD-PCR) was performed using an RNA image Kit (GenHunter Corp., Nashville, TN, USA). Several modifications were made to improve the reproducibility and sensitivity of DD-PCR as follows. Because the quality of the sample RNA greatly affects the result of DD-PCR, we always treated the total RNA with RNase-free DNase (Promega Corp., Madison, WI, USA) followed by another round of extraction with AGPC. The cDNA synthesis was conducted in a relatively low dNTP concentration (400 µm each) using Moloney Murine Leukaemia Virus (MMLV) reverse transcriptase purchased from GenHunter. The anchor primer was labelled with [γ-32P] ATP using T4 polynucleotide kinase and the reaction mixture was used for PCR without further purification. Long-distance PCR was performed using Klentaq polymerase mix (Clontech Laboratories, Palo Alto, CA, USA). For a better resolution of bands in the range of 200–500 bp, electrophoresis was carried out using a 4% LongRanger (FMC Bioproducts, Rockland, ME, USA) gel. The band that showed differential expression among the areas was isolated, reamplified and cloned into the pBluescript with T-overhang. The amplified fragments usually contained several different clones. The selection of clones with differential expression was carried out as previously described (Consalez et al. 1996). The differential expression of a candidate clone was confirmed by semiquantitative RT-PCR before further characterization.
Library screening and 5′ RACE
The cDNA fragment that we obtained from DD-PCR was used to screen a cDNA library of the motor cortex of a cynomologous monkey (constructed by Dr Satoshi Koike). Screening 1.5 × 106 clones, we isolated one positive clone that turned out to have a partial coding sequence for GDF7 (the sequence for this clone is deposited in Genbank: AF254567). Using this sequence, 5′ RACE was carried out with the poly(A) RNA purified from the area FA of an African green monkey using the 5′/3′ RACE kit (Roche Molecular Biochemicals, Indianapolis, IN, USA). Because of the high GC content of the 5′ region, the 5′ RACE did not extend further than the putative initiator methionine. To confirm the initiator Met, we screened genomic libraries of the African green monkey (constructed by Dr Satoshi Koike) and mouse and obtained clones containing the upstream region of the putative initiator Met of GDF7 for both species (AF254568 and AF254569 for the African green monkey sequence, and AF254570 and AF254571 for the mouse sequence). The genomic sequences showed that the putative Met is most likely to be the initiator Met for the following reasons.
(i) There is a stop codon in frame upstream from the putative Met. RT-PCR confirmed that this UTR region was transcribed into mRNA (data not shown).
(ii) The translational products of GDF7 of the African green monkey and mouse are no longer homologous in the upstream region of the putative Met due to a sequence gap.
Transfection, antibody production and western blotting
The expected full-length coding sequence for monkey GDF7 was cloned into the pCG vector (Tanaka and Herr 1990) to express the gene under the control of the strong CMV promoter. A control plasmid that contains the cDNA of Xenopus BMP4 was also constructed. These plasmids were transfected into HEK 293T cells using Lipofectamine (Gibco, Rockville, MD, USA) according to the manufacturer's instruction. After the transfection, the cells were incubated for 24 h in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), then the culture medium was changed to one without serum and the cells were further incubated for 48 h. At this point, the culture medium was collected and concentrated 10-fold by drying using Speed Vac (Savant Instruments, Holbrook, NY, USA). In the case of the transfected cells, they were scraped and suspended in ice-cold phosphate-buffered saline (PBS) after washing with PBS. The cell suspension was centrifuged, and the cell pellets were sonicated in 1 × SDS buffer (Sambrook et al. 1989). The protein concentration was determined using a Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). 10 µL of the concentrated medium and 10 µg/lane of the cell extracts were loaded onto 15% SDS-PAGE followed by western blotting analysis.
The predicted mature domain of monkey GDF7 was cloned into pET-19b vector (Novagen Inc., Madison, WI, USA) to produce His-tagged recombinant proteins in E. coli. The recombinant GDF7 protein was purified using Ni-NTA resin (Qiagen, Valencia, CA, USA) under denaturation condition and further purified by excising the band corresponding to GDF7 after SDS-PAGE. The gel piece was crushed in PBS and used for immunizing rabbits. Western blotting analysis was performed using the ImmunoStar chemiluminescence reagent (Wako Pure Chemical Industries Ltd, Osaka, Japan) according to the manufacturer's instruction.
Quantitative RT-PCR
DNase-treated total RNA (2 µg) was converted to cDNA using random nonamer and MMLV reverse transcriptase (Gibco) in a 20-µL reaction mixture. After the reaction was terminated by heating at 70°C, the reaction mixture was diluted 2-fold with distilled water and stored at − 80°C (RT mix). PCR was performed in 10-µL reaction mixtures, containing 1 or 0.25 µL of RT mix (see below), 0.4 µm gene-specific primers, 1× ExTaq buffer, 8 µm dNTPs, 2 µCi [α-32P] dCTP, 0.5% DMSO and 0.5 unit of ExTaq polymerase (Takara). The PCR protocol was as follows: 1 cycle of (95°C for 5 min), 12–30 cycles of (94°C for 30 s; 60°C for 1 min; 72°C for 2 min). The PCR products were separated by 5% polyacrylamide gel electrophoresis and quantified using a BAS2000 phosphoimager (Fuji Photo Film Co. Ltd, Tokyo, Japan). The optimal cycle number for each gene was determined in the preliminary experiments so that the PCR products resulting from serial dilutions of 1–1/16 of RT mix fall in a linear range after amplification. These were: 15 cycles for GAPDH; 26 cycles for GDF7; 20 cycles for ActRI and BMPRII; and 23 cycles for ActRIIa, ActRIIb, BMPRIa and BMPRIb. RT-PCR was performed for samples from three different monkeys (Fig. 2), two monkeys (Fig. 3) and three monkeys (Fig. 4). For each monkey, two independent reactions were performed and averaged. Various areas of the brain from the same monkey were always tested in the same trial. We normalized the value of GDF7 and BMP receptors to the value of he GAPDH gene. The GAPDH gene is a housekeeping gene and is frequently used as an internal standard (Oishi et al. 1998). In addition, we observed similar expression levels of GAPDH gene among different cortical areas in northern blotting (Fig. 2a), which confirmed that the use of this gene as the standard was appropriate. Because we can only determine the relative values of GDF7 and BMP receptors in different areas, we normalized each value in the following manner to integrate the values of different individuals. For 2, 3, we calculated the relative value of each area so that the value of FA becomes unity. In Fig. 4, the relative value of each area was normalized so that the average becomes unity. After the normalization, the mean and the standard deviation (SD) for each area was calculated.
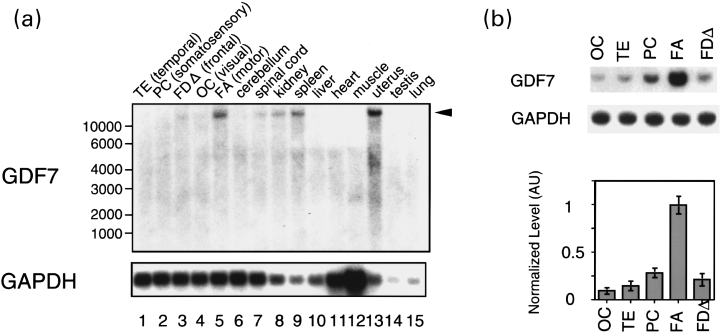
Expression of GDF7 in various tissues of the African green monkey. (a) Northern blotting using Poly(A) RNA (4 µg) from various tissues. Upper panel: the 3′ UTR fragment of GDF7 was used for northern blotting. The arrowhead on the right of the panel indicates the position of the band for GDF7. The positions of size marker RNA are indicated on the left. Lower panel: the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) fragment was used for northern blotting as a control. (b) Quantitative RT-PCR of GDF7 was performed to compare its expression in five neocortical areas of African green monkeys. Different neocortical areas of African green monkeys as shown in Fig. 1 were dissected and relative expression levels of GDF7 were examined. The top panel shows a typical example of RT-PCR for GDF7 and the middle panel for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The graph in the bottom panel indicates the means and the standard deviations calculated from experiments using samples from three different monkeys. The normalization was performed as described in Materials and methods.
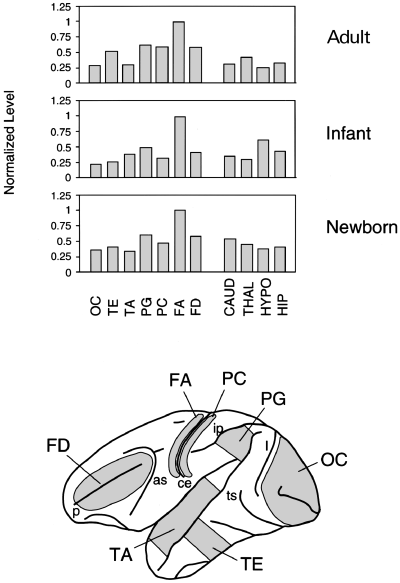
Expression of GDF7 in rhesus monkeys of various ages. GDF7 expression during cortical maturation was examined using adult, infant and newborn rhesus monkeys. Quantitative RT-PCR was performed using various cortical (lower panel) and subcortical regions of rhesus monkeys of different ages. The same brain regions were dissected from adult, infant (4 or 5 months) and newborn monkeys. The graph shows the mean of the results obtained from two different monkeys. The subcortical regions used are as follows: CAUD, caudate; THAL, thalamus; HYPO, hypothalamus; and HIP, hippocampus.
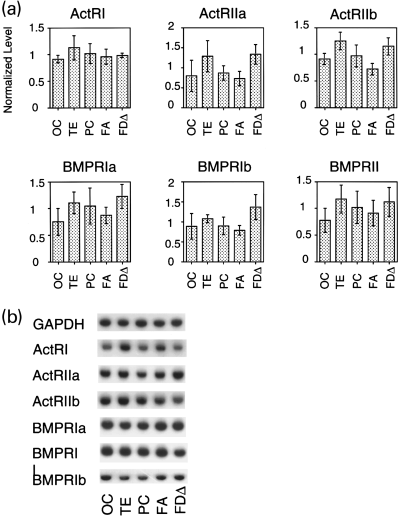
Expression of various BMP receptors in the neocortex of African green monkeys. Quantitative RT-PCR was performed to determine expression levels of various type I and type II serine/threonine kinase BMP receptors in different cortical areas: activin receptor type I (ActRI), activin receptor type IIa (ActRIIa), activin receptor type IIb (ActRIIb), bone morphogenetic protein receptor type Ia (BMPRIa), bone morphogenetic protein receptor type Ib (BMPRIb), and bone morphogenetic protein receptor type II (BMPRII). The graph in (a) shows the relative expression level of each receptor in the five cortical areas in Fig. 1. The shaded boxes and the error bars indicate the means and the standard deviations calculated from the results obtained from three different monkeys. All of the receptors that we tested showed similar expression levels across different cortical areas. (b) A typical example of RT-PCR. GAP, glyceraldehyde-3-phosphate dehydrogenase.
Results
Identification of GDF7 as the mRNA preferentially expressed in the motor cortex
To search for molecules that are differentially expressed within the monkey neocortex, we carried out differential display PCR (DD-PCR) using RNA samples obtained from various cortical areas of African green monkeys. In the initial screening, we selected three areas for comparison, areas FA, OC and TE, which correspond to the primary motor cortex, the primary visual cortex, and the association area in the inferior temporal cortex, respectively (Fig. 1; see Materials and methods for a detailed description of the areas used in the current study). These three areas were selected because they are distinct in position and function, and have characteristic cytoarchitectures.
In this screening, we found a DD band specific to area FA (Fig. 5, asterisks). The reproducibility of this band was confirmed by carrying out DD-PCR using RNAs derived from two different monkeys (Fig. 5). The cDNA fragment corresponding to this band (denoted as DD FG in Fig. 6a) was cloned from the gel and used to screen the monkey cDNA library. By this screening, a longer cDNA clone was obtained from the monkey cDNA library and the nucleotide sequence was determined. A database search revealed that the clone we obtained was the monkey homologue of GDF7, a member of the BMP/TGF-β super-family.
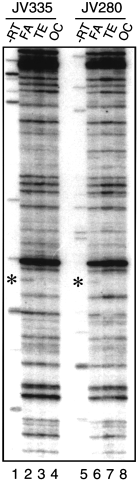
Differential display PCR of three areas of the monkey neocortex. Areas FA (primary motor cortex), TE (temporal association cortex) and OC (primary visual cortex) were dissected from two African green monkeys (JV335 and JV280). DD-PCR was performed to compare the gene expression in various neocortical areas of each individual monkey. We obtained similar band patterns for the two monkeys. The FA-specific bands observed in both monkeys are indicated by asterisks. -RT: DD-PCR in the absence of reverse transcriptase confirms that the FA-specific band is not derived from contamination of genomic DNA.
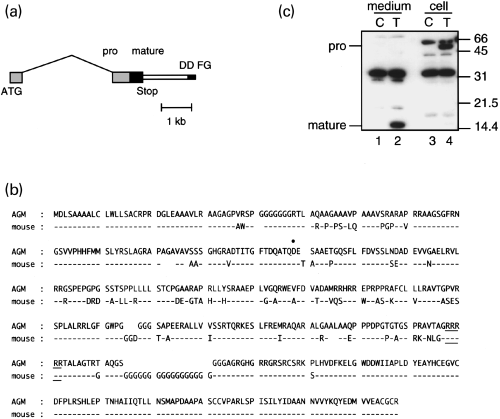
The FA-specific band is the monkey homologue of GDF7. The FA-specific band in Fig. 5 was identified as the cDNA fragment derived from the monkey GDF7 gene. (a) Gene structure of the monkey GDF7, which was determined by cDNA screening, genomic library screening, 5′-RACE, RNase protection and RT-PCR. Light grey boxes, exons for the pro-domain; dark box, exon for the mature domain; thin box, 3′UTR; thin filled box, cDNA fragment (FG) obtained from the DD gel (DD FG); and thin black line, intron. The scale bar under the gene is 1 kb. (b) Comparison of the African green monkey and the mouse GDF7 amino acid sequences deduced from genomic sequences. The putative proteolytic site is underlined. The boundary site of the first and the second exons is indicated by the filled circle: the boundary exists between the first and second nucleotides for the amino acid D. (c) Western blotting of GDF7-transfected HEK293T cells. HEK293T cells were transfected with either the control (C: control) or GDF7 (T: transfected) cDNA and the culture media (medium) and the cell extracts (cell) were analysed by western blotting using the anti-GDF7 antiserum. The molecular weight standard (kDa) is shown at the right side of the panel. Note the presence of a 15-kDa band in lane 2 but not in lane 1, and a 55-kDa band in lane 4 but not in lane 3, which correspond to the predicted size for the GDF7 mature peptide (mature) and proprotein (pro), respectively.
The members of the BMP/TGF-β family are synthesized as large precursors, and proteolytically cleaved to yield carboxy-terminal mature protein dimers. Whereas the amino acid sequence of GDF7 for the highly conserved carboxy-terminal domain have been previously reported for zebrafish (Davidson et al. 1999), mice (Storm et al. 1994), chickens (Lee et al. 1998) and humans (Wolfman et al. 1997), the entire coding sequence including the pro-region has not been determined for any of the species. Therefore, we carried out 5′ RACE as well as genomic library screening to extend our partial cDNA clone (see Materials and methods for details). The results are summarized in Fig. 6. The coding region of GDF7 was encoded by two separate exons (Fig. 6a). The mature domain, predicted from homology to other BMP/TGF-β family members, was located in the 3′ exon. This exon also contained a part of the pro-domain. The rest of the pro-domain was located in the 5′ exon approximately 3 kb upstream from the 3′ exon. For comparison, we also isolated genomic clones of the mouse GDF7. The genomic structure of the mouse and the monkey GDF7 was well conserved. The deduced amino acid sequences for the two species are compared in Fig. 6(b). The sequence of the mature domain of GDF7 was highly conserved except for the characteristic G-stretch in the mouse GDF7. The N-terminus region of the pro-domain was also conserved, containing a stretch of hydrophobic amino acids that was considered to encode signal peptides.
To investigate if the full-length GDF7 that we determined undergoes proper processing and secretion, we transfected HEK293T cells with GDF7 cDNA. The conditioned medium and the cells of control and GDF7-transfected samples were harvested and analysed by western blotting, using a rabbit antiserum raised against the mature domain of the GDF7 (Fig. 6c). In this experiment, we detected a 15-kDa band, the predicted size for the GDF7 mature peptide, in the culture medium of GDF7-transfected sample (Fig. 6c, lane 2). This result shows that the GDF7 can be secreted as predicted from its structure.
GDF7 expression in the neocortex of the African green monkey
To confirm the FA-specific expression of GDF7, we carried out northern blotting. As shown in Fig. 2(a), a single band of more than 10 kb was detected in area FA (lane 5). This band was not detected in area OC (lane 4) or area TE (lane 1), which is consistent with the result of DD-PCR. In this series of experiments, two more cortical areas, areas PC and FDΔ, which correspond to the somatosensory cortex and the frontal association cortex, respectively, were investigated in addition to areas FA, OC and TE. In areas PC and FDΔ, the band for GDF7 was barely detectable (lanes 2 and 3). GDF7 was not detected in the cerebellum (lane 6) and detected at low level in the spinal cord (lane 7). Outside the brain, a strong expression was observed in the uterus (lane 13) and the expression was observed also in the kidney (lane 8) and spleen (lane 9). GDF7 was also strongly expressed in the mouse uterus (data not shown), suggesting its role in this organ.
We also carried out quantitative RT-PCR to determine the expression level of GDF7 in various cortical areas in more detail. To compare the expression level in different cortical areas, RT-PCR with the GAPDH gene was performed in parallel and used for normalization. Consistent with the results of DD-PCR and northern blotting, GDF7 was most abundant in area FA (Fig. 2b). In area PC, which is located next to area FA across the central sulcus, GDF7 expression was about one-third of that in area FA. GDF7 expression was lowest in area OC, where GDF7 expression was about 1/10 of that in area FA.
GDF7 expression pattern is already established at birth in rhesus monkeys
Because macaque monkeys have been used most often as an experimental model among monkeys, we examined GDF7 expression in the motor cortex of macaque monkeys. For this experiment various cortical and subcortical brain regions of adult rhesus monkeys were tested for GDF7 expression by quantitative RT-PCR. As shown in Fig. 3, the GDF7 expression was highest in the motor cortex, which was similar to the results obtained using African green monkeys. In rhesus monkeys, however, the difference in the expression levels between the motor cortex and the visual cortex was only approximately 3-fold, compared with the 10-fold difference in African green monkeys. This was rather surprising because the sulcus-pattern of the neocortexes are indistinguishable between these two species. When we tested another macaque monkey (crab-eating monkeys; Macaca fascicularis), we also obtained results similar to those obtained from rhesus monkeys (data not shown). Thus, we conclude that the GDF7 expression is higher in the motor cortex than in other areas in the neocortexes of various monkeys, although the extent of difference varies among species. We also found that the GDF7 expression in the subcortical regions was lower than that in the motor cortex in rhesus monkeys (Fig. 3).
Next we examined GDF7 expression during the post-natal maturation of the neocortex. Cortical areas corresponding to those used in the above experiment were collected from two newborn and two infant (aged 4–5 months) rhesus monkeys and analysed by RT-PCR. The expression pattern was similar to that of adult rhesus monkeys (Fig. 3). Thus, the differential expression of GDF7 within the neocortex is already observed at birth.
Expression of BMP receptors in neocortical areas
The differential expression of GDF7 led us to examine the expression patterns of candidate receptors for GDF7 within the mature neocortex. The receptors for the BMP family members are classified into type I and type II serine/threonine kinase receptors (Mehler et al. 1997). BMPs require both types of receptors for high-affinity binding (Ebendal et al. 1998). Although the specific combination of receptors for GDF7 has not been determined, GDF5, which is 80% identical to GDF7 in the mature domain, binds to BMP receptor type Ib (BMPR-Ib) and BMP receptor type II (BMPR-II)/activin receptor type IIa (ActR-IIa) (Nishitoh et al. 1996). To determine if the known BMP and activin receptors are expressed in the adult monkey neocortex, we carried out quantitative RT-PCR of three type I receptors (ActR-I, BMPR-Ia and BMPR-Ib) and three type II receptors (ActR-IIa, ActR-IIb and BMPR-II). We found that all of the receptors were expressed in the cortical areas examined (Fig. 4). We did not observe the area-specific expression of these receptors. In all cases, the differences between the areas were no more than 1.5-fold.
Discussion
In this paper, we reported the identification of GDF7 mRNA as a molecule preferentially expressed in the motor cortex. Previous studies reported that several genes are expressed differentially within the mature neocortex of monkeys. To our knowledge, however, no genes other than GDF7 are known to be expressed preferentially in the primary motor cortex. For example, synaptotagmin I is more abundant in the visual cortex than in the motor cortex (Travis et al. 1987; Chowdhury et al. 1995). In the case of the trkB truncated isoform, which is less abundant in the visual cortex than in other areas, its expression level in the primary motor cortex was approximately equal to that in the temporal cortex (TE) (Okuno et al. 1999). Thus, GDF7 represents a new and relatively rare class of genes that is differentially expressed within the primate neocortex.
DD-PCR method has been used successfully to screen differentially expressed genes in many systems (Liang and Pardee 1997). Although it has been known that there are many parameters that affect sensitivity, reproducibility and resolution, in this study we have carefully examined these factors and arranged a highly sensitive and reproducible protocol (as shown in Materials and methods). The method is very sensitive, as we could detect a rare transcript such as GDF7, which we estimated to be present at the approximate abundance of 1 in 500 000 copy mRNA molecules (or 0.001% of mRNA) by competitive RT-PCR (data not shown). The method is highly reproducible and the independent reactions using RNAs from different monkeys could generally produce identical patterns (see Fig. 5 as an example). We have also optimized the resolution of the gel pattern by choosing appropriate enzymes, labelling and electrophoretic methods. We usually obtained about 100 bands for one primer set. In the current experiment, we used 24 primer sets for screening. Thus, in rough estimation we have screened 2000 to 3000 different bands and found only one type of transcript showing significantly different expression in neocortical areas through the series of experiments reported in this paper.
It is difficult to estimate how many different genes are expressed among distinct neocortical areas from the DD-PCR result, because of the uncertainty of the bias of the current method. We have recently carried out DNA macroarray analyses of human cortical areas and found that no gene showed a difference in area of more than 4-fold among the 1088 genes tested (Watakabe et al. 2001). Furthermore, in microarray analyses of mouse brains clear region-specific expression is observed for only a small number of genes even when more clearly defined structures are compared, such as cortex, cerebellum, hippocampus, mid-brain, amygdala and entorhinal cortex (Sandberg et al. 2000). Although DNA array analyses also have some bias, it seems likely that there are relatively few genes that exhibit great difference in expression level among different areas in mature neocortex, probably in the range of 1/1000–1/10 000. Further study is needed to confirm how exact this estimate is.
GDF7 belongs to the BMP subclass of TGF-β super-family. The BMP family was first identified by its ability to induce bone formation (Wozney et al. 1988). But it is now clear that proteins that belong to this family play important roles in regulating diverse developmental processes (Hogan 1996). Especially, it is notable that the BMPs play multiple roles in the formation of neural tissues (Mehler et al. 1997). The BMP signalling, which is mediated by binding of BMP ligands to two types of serine/threonin kinase receptors, are modified in many ways. For example, secreted molecules such as noggin, chordin and follistatin can antagonize the BMP effect by direct binding (Cho and Blitz 1998). Furthermore, the opposing effect of other growth factors, such as EGFs and FGFs, are thought to interfere with the intracellular BMP signalling pathway (Kretzschmar et al. 1997; Neubuser et al. 1997). The concerted actions of these regulator molecules exert various effect on neural and non-neural cells. An in vitro study using BMP2 and noggin typically demonstrate complex functions of BMP signalling (Mabie et al. 1999). The BMPs promoted cell death and inhibited proliferation of cortical ventricular zone cells of E13 embryonic culture. On the other hand, at E16, a low dose of BMP2 promoted neuronal and astroglial differentiation and inhibited oligodendroglial differentiation, whereas a high dose of BMP2 promoted cell death and inhibited proliferation. Addition of noggin promoted oligodendrogliogenesis. In addition to the roles of BMPs in fate determination of neural progenitor cells, a recent report suggests a role of BMPs in axon guidance (Augsburger et al. 1999). While many BMP molecules have similar activity and overlapping expression patterns (Mehler et al. 1997), there seems to be a unique function for each member. A unique role of GDF7 is reported in neural differentiation of the spinal cord of mice. Among several BMP family members tested, GDF7 was the only BMP family member that was selectively expressed by the roof plate cells at the dorsal mid-line of the neural tube in mice embryos (Lee et al. 1998). Disruption of GDF7 resulted in the loss of D1A neurones, a subclass of commissural interneurone in the spinal cord, leaving D1B neurones, another subclass, intact. Because BMP6 and 7 are still expressed by the roof plate cells in GDF7-KO mice, this result shows a non-redundant function of GDF7. In addition, it is also shown that GDF7 as well as BMP6 and BMP7 can induce specification of cerebellar granule neurones (Alder et al. 1999). Considering that the differential expression of GDF7 is already observed at birth, one possibility is that GDF7 may play a role in neuronal differentiation in the motor area during development and may contribute to the generation of neuronal types abundant in the motor area. Other than roof plate cells, GDF7 is expressed in the choroid plexus (Lee et al. 1998), and in the region of the developing shoulder in mice embryos (Wolfman et al. 1997). GDF7 expression is also reported in developing bovine teeth (Morotome et al. 1998). Although there has been no report that any neurones express GDF7, other BMP members are expressed in both neuronal and non-neuronal cells. Further study is needed to know what types of cells produce GDF7 to elucidate its role in the monkey motor cortex.
The observations that GDF7 is expressed in the adult cortex and that its potential receptors are also expressed in adult brains (Ebendal et al. 1998; Zhang et al. 1998; this study) suggest another possibility that GDF7 has a functional role other than developmental roles. It is reported that GDF5, a close family member of GDF7, exerts trophic and protective effects on mid-brain dopaminergic neurones (Krieglstein et al. 1995). Interestingly, dopaminergic innervation in the macaque neocortex shows a distribution pattern that is intriguingly similar to that of GDF7: abundant in the motor area and scarcely present in the visual area (Berger et al. 1991). It is tempting to speculate that GDF7 might be involved in maintaining the region-specific innervation of dopaminergic terminals. Although dopaminergic innervation is investigated in squirrel monkeys (Lewis et al. 1987) and in humans (Gaspar et al. 1989) by tyrosine hydroxylase staining, there is no report on it in African green monkeys. It thus remains to be further studied whether GDF7 expression is indeed related to dopaminergic innervation in African green monkeys.
Comparative studies of the mammalian neocortex show that there is a great variation in its structure and function, although core sensory areas are similar in many species (Krubitzer 1995; Northcutt and Kaas 1995). As shown in 2, 3, the area-specific difference in expression levels of GDF7 was smaller in rhesus monkeys than in African green monkeys. Although the reason for such a difference is unclear, it might reflect the adaptive evolution of these two species to different environments (rhesus monkeys inhabit forests, while African green monkeys inhabit savanna). In addition, we have preliminary data showing that GDF7 expression in the rodent cerebral cortex is much weaker than that in the primates (AW, unpublished observation), which might be consistent with the phenotype of GDF7-knockout mice showing grossly normal cellular architectures of their cerebral cortices (Lee et al. 1998). Thus, investigating GDF7 expression across various species may provide an interesting clue to understand the neocortical evolution of mammals. We believe that the systematic approach that we used in this study to identify area-specific molecules will further reveal unknown features of the neocortex and will aid to the understanding of the structure and functional organization of the primate neocortex.
Acknowledgements
We thank Drs Hitoshi Horie, Shinobu Abe and Sou Hashizume of the Japan Poliomyelitis Research Institute for providing monkey brains. We also thank the technical staff of their institute, particularly Mr Shinobu Fujita, for help in dissection. This work would not have been possible without their cooperation. We thank Dr Satoshi Koike of Tokyo Metropolitan Institute for Neuroscience for valuable advice while we executed the project as well as for providing us with monkey cDNA and genomic libraries. We thank Dr Takeshi Yagi of the National Institute for Physiological Science for providing the mouse genomic library, and Dr Naoto Ueno of the National Institute for Basic Biology for Xenopus BMP4 cDNA. We thank Dr Takuya Iwasaki of the National Institute of Infectious Diseases for helping us with histological staining of the monkey cortex. We thank Dr Yuriko Komine for critical reading of the manuscript. This work was supported by Grants-in-Aid for Scientific Research (A) (to TY) and Grant-in-Aid for Encouragement of Young Scientists (to AW) from the Ministry of Education, Science, Sports and Culture and a grant from Mitsubishi Foundation (to TY).




