Inhibition of tumour necrosis factor-α (TNFα)-induced NF-κB p52 converts the metabolic effects of microglial-derived TNFα on mouse cerebellar neurones to neurotoxicity
Abstract
Activated microglia are implicated in the injury of neurones and macroglia both in vitro and in vivo. Here, we demonstrate that media conditioned by interferon-γ treated microglia initially impair the metabolism of mouse cerebellar neurones grown in serum-free conditions without inducing cell death. Metabolic effects include inhibition of the ability of mitochondria to reduce 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) and cytochrome oxidase activity. These effects are blocked by antibodies to tumour necrosis factor-α (TNFα), a cytokine produced by microglial activation, and they are not reproduced by media conditioned by resting microglia. The metabolic effects are evident for up to 24 h in vitro. More prolonged exposure, up to 48 h, results in TNFα dependent neuronal death as previously observed. Between 2 and 48 h TNFα present in media conditioned by interferon-γ treated but not resting microglia is associated with nuclear factor kappa B (NF-κB) consensus sequence binding in paired mouse cerebellar neuronal cultures without affecting activation of the signal transducer and activator of transcription (STAT) transcription factor. Neuronal death can be accelerated by peptide blockade of the nuclear transport of NF-κB p52 subunit during exposure of cerebellar neurones to medium from interferon-γ treated microglia. This toxicity is blocked by anti-TNFα antibody. Soluble factors released by activated microglia therefore contribute to neuronal dysfunction that is initially reversible but may culminate in neurotoxicity. Characterizing and manipulating these events in vivo theoretically provides an opportunity for neuroprotection in selected diseases affecting the central nervous system.
Abbreviations used
-
- EMSA
-
- electrophoretic mobility shift assay
-
- LDH
-
- lactate dehydrogenase
-
- MTT
-
- 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
-
- NF-κB
-
- nuclear factor kappa B
-
- PBS
-
- phosphate-buffered saline
-
- STAT
-
- signal transducer and activator of transcription
-
- TNFα
-
- tumour necrosis factor-α
Microglia are toxic in vitro for neurones and oligodendrocytes through cell–cell contact-dependent mechanisms (Zajicek et al. 1992; Frade and Barde 1998). In contrast, soluble factors released by microglia show inconsistent toxicity (Toku et al. 1998) often requiring more prolonged exposure to the target (Roque et al. 1999; Tanabe et al. 1999). However, the interactions of microglia with other cell components of the central nervous system are more complex. Recent work has demonstrated effects of microglia on cell survival and maturation (Lazarov-Spiegler et al. 1996; Zeev-Brann et al. 1998; Zietlow et al. 1999). Soluble factors released by microglia in vitro promote oligodendrocyte precursor survival and maturation, protect mature oligodendrocytes from cytokine-mediated injury and stimulate astrocyte proliferation (Giulian et al. 1986; Nicholas et al. 2000). Tumour necrosis factor-α (TNFα) produced by microglia is one candidate for influencing neuronal survival both through direct and indirect actions. TNFα has variable effects on neuronal survival (de Bock et al. 1998; Sullivan et al. 1999) and these responses may relate to differential activation of the transcription factor nuclear factor kappa B (NF-κB). Originally identified in association with the immune system, NF-κB is induced by multiple stimuli within stressed cells of many different origins to form part of a protective response (Baichwal and Baeuerle 1997). In the PC12 neuronal cell line (Taglialatela et al. 1997) and in primary sympathetic neurones (Maggirwar et al. 1998), activated NF-κB has been found to mediate the antiapoptotic effect of nerve growth factor (NGF). Despite the implication of NF-κB in cell survival and maturation (Baeuerle and Baltimore 1996), cell fate is not wholly determined by NF-κB (Behl et al. 1994; Grilli et al. 1996). Here we show that media conditioned by interferon-γ treated activated microglia contain factors that initially down regulate neuronal metabolism whereas prolonged exposure of neurones to conditioned medium is also associated with cell death through non-TNFα dependent mechanisms. Toxicity is accelerated if the TNFα NF-κB p52 response in neurones is blocked.
Materials and methods
Cell culture: microglia
Strain 129 mice were prepared as previously described (McCarthy and de Vellis 1980). Cultures were incubated in Dulbecco's modified Eagle's medium (DMEM, Gibco, Paisley, UK) + 10% fetal calf serum (Gibco) + 1% penicillin and streptomycin (Gibco) (DFP) at 37°C in 7% CO2. The medium was changed after 24 h. Microglia were prepared from 6 day mixed glial cultures after 2-h shaking at 200 r.p.m. After centrifugation, the cell pellet was resuspended in DFP, counted and plated onto sterile glass coverslips or plastic for 15 min. The nonadherent cells were then removed by washing twice in Hank’s balanced salt solution (HBSS). The remaining microglia were > 95% CD11b positive (Ox42, Serotec, Oxford, UK). These microglia were cultured in N2B3 medium for 20 h alone or with 100 units/mL interferon-γ (Serotec). The media collected from the non-activated microglia was used as conditioned medium from resting microglia and the media from interferon-γ treated microglia was used as conditioned medium from activated microglia. N2B3 consisted of DMEM ± 0.5% fetal calf serum, 1 mm glutamine, 50 U/mL penicillin and streptomycin and 1 mL Sato mix per 100 mL basal medium leading to a final concentration of 100 µg/mL bovine serum albumin, 60 ng/mL progesterone water soluble, 16 µg/mL putrescine, 40 ng/mL sodium selenite, 40 ng/mL thyroxine, 40 ng/mL triiodothyronine, 5 µg/mL insulin and 10 µg/mL transferrin (all Sigma). Conditioned media were collected from microglial cultures, filtered (0.2 µm) and incubated with neurones at a 1 : 2 dilution unless otherwise stated, or tested using the L929 bioassay for TNFα as described previously (Zajicek et al. 1992). The TNFα (Santa Cruz, Santa Cruz, CA, USA) antibody was a goat polyclonal purified IgG raised against mouse TNFα with no cross-reactivity to TNFβ. TNFα was from Serotec.
Cell culture: neurones
Preparation of cerebellar cells from 6-day-old mice (P6) was as previously described (Brown et al. 1996). Briefly, the cerebella were dissociated in Hank's medium (Gibco) containing 0.5% trypsin (Sigma) and plated at 1–2 × 106 cells/cm2 in 24 well trays (Falcon, Becton-Dickinson, Franklin Lanes, NJ, USA) coated with poly-d-lysine (50 µg/mL, Sigma). Cultures were maintained in DMEM (Gibco) supplemented with 10% fetal calf serum, 2 mm glutamine and 1% antibiotics (penicillin, streptomycin, fungizone) (Gibco). Cultures were maintained at 37°C with 6% CO2. After 24-h culture, the cerebellar cells were transferred to serum-free medium consisting of DMEM with 1% penicillin/streptomycin and addition of the TCM® supplement (ICN, Irvine, CA, USA). Cells were maintained in this medium for 1–2 days before being used for experiments.
MTT, LDH and Hoechst assays
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma, Poole, UK) was diluted to 200 µm in Hanks' solution (Gibco) and added to cultures for 2 h at 37°C. After incubation the substrate was removed. The cells were then washed briefly with Hanks' solution. The MTT formazan product was released from cells by addition of 700 µL of dimethylsulfoxide (Sigma) and measured at 570 nm in a Unicam UV2 spectrophotometer (ATI Unicam, Cambridge, UK). Survival of treated cerebellar cells in comparison to untreated controls could then be determined. The relative values were expressed as a percentage of control values with untreated control values in the MTT assay being equate to 100%. The assay for total lactate dehydrogenase (LDH) activity was the Sigma commercial assay. This was performed according the manufacturer's instructions. Briefly, cerebellar neurones were extracted in a buffer containing phosphate-buffered saline (PBS) and 0.1% NP-40 (Sigma). The extracted cells (in 100 µL) were centrifuged and the supernatant added to 2 mL of the LDH assay reagent. After 20 min incubation at room temperature the samples were measured spectrophotometrically at 340 nm. Numbers of apoptotic cells were determined using the Hoechst staining method. The Hoechst reagent (Sigma) was applied to live cells at 50 µm for 10 min. Stained cells were examined using a Leitz DMRB fluorescence microscope. Fields at 20 times magnification were examined and the number of cells with fragmented or condensed nuclei counted.
ATPase and cytochrome oxidase assays
The assay for total ATPase activity was according to the method of Lowry (1957). Briefly, cells were extracted in 0.03 m Tris pH.8.4 and then diluted in buffer (0.03 m Tris, 0.03 m HCl, 2 mm MgCl2, 0.03 m 2-amino-2-methyl-1,3-propandiol, pH 8.4) until the protein concentration was 20 µg/mL. Samples are incubated on ice for 10 min after which time 3% trichloroacetic acid was added. The reactants were then centrifuged and the supernatant collected. After further incubation on ice for 30 min 100 µL of sample was added to 1 mL of 2.5% ammonium molybdate in 0.1 m acetic acid buffer. After 15 min the colour was measured spectrophotometrically at 870 nm. Values were compared with a standard curve based on bovine ATPase (Sigma) and expressed as units per/mg protein. The assay for cytochrome oxidase was carried out as previously described (Wharton and Tzagoloff 1967). Briefly, the assay was based on whole cell extracts prepared from homogenized cells in PBS and 1% NP-40. 0.8 mL of 1% ferrocytochrome c prepared in 0.01 m potassium phosphate buffer pH 7. The protein sample was diluted to 100 µg/mL in 0.01 m potassium phosphate buffer and 100 µL added to the reaction. A blank with 0.1 m potassium ferricyanide instead of ferrocytochrome C was subtracted from the values. Increased absorbance was measured over 15 min at 550 nm. Protein content of extracts was determined using the BCA assay (Sigma).
Analysis of NF-κB and STAT transcription factors
Nuclear extracts were prepared and incubated with 32P-αATP radio-labelled double stranded oligonucleotides containing consensus binding sequences for STAT or NF-κB as previously described (Girdlestone and Wing 1996). Cells were plated at 1–2 × 106 cells/well for up to 24 h after washing twice in serum-free plating media, test media was added. Nuclear extracts were made after washing the cells twice in PBS before transferring them to a microfuge tube where they were counted and pelleted (4500 r.p.m., 4 min). On ice 30 µL of buffer A (10 mm HEPES, pH 7.9, 1.5 mm MgCl2, 10 mm KCl, 0.5 mm DTT, 0.5 mm PMSF, 0.5% NP40, Aproteinin 10 µg/mL in distilled water) was added to the cell pellet for 1 min. After centrifugation (14 000 r.p.m., 2 min) 15 µL of buffer C (20 mm HEPES, pH 7.9, 25% glycerol, 0.42 mm NaCl, 1.5 mm MgCl2, 0.2 mm EDTA, 0.5 mm DTT, 0.5 mm PMSF, aproteinin 10 µg/mL in distilled water) was added to the pellet for a total of 10 min and vortexed regularly. After a further centrifugation the DNA pellet was removed and the nuclear extract was frozen at − 70°C. At room temperature 2 µL nuclear extract, 5 µL distilled water and 1 µL nonspecific DNA (poly-dI-dC) were incubated for 10 min in 96-well plate. Where appropriate 1 µL STAT-1 antibody (a rabbit anti-STAT-1 human antibody (Santa Cruz) without cross-reactivity to other STATs), was added for a further 10 min 2 µL 32P-labelled consensus sequence was added for 20 min, then 2 µL loading buffer (50% glycerol, 0.25% bromophenol blue in sterile water) before loading onto a nondenaturing 6% acrylamide gel in 0.5 × TBE and running at 200 V, 12 mA for 90 min (5 mL acryl stock [50% 37.5 : 1 acrylamide, 37.5% 19 : 1 acrylamide made up with sterile water], 10 × TBE 0.65 mL [0.045 m Tris-borate, 0.001 m EDTA], TEMED 50 µL, 25% AMPS 50 µL). The gel was dried and autoradiographed for up to 10 days. Optical density was calculated using NIH image software (NIH, Washington, WA, USA) and statistical analysis carried out using the paired t-test.
Results
Soluble factors secreted by interferon-γ treated microglia impair neuronal reduction of MTT
Cultured cerebellar neurones from 6-day-old mice were grown first in serum-free medium and then treated with conditioned medium either from resting or interferon-γ treated microglia. As further controls neurones were treated with unconditioned medium with and without interferon-γ. Cerebellar neurones were incubated for 2 h in increasing concentrations of conditioned media (Fig. 1a) and an MTT assay was performed directly. Unconditioned media, interferon-γ alone and media conditioned by resting microglia did not alter measured values of the MTT assay at 2 h. However, a 2-h treatment with medium from interferon-γ treated microglia caused a reduction (∼30%) in MTT values measured for cerebellar neurones (Fig. 1a). MTT assays directly measure the reducing activity of cells. The majority of this activity is contained in cells. Therefore MTT assays are a measure of mitochondrial activity. As reduced mitochondrial activity can also correspond to a decreased number of cells further assays were carried out. Parallel cultures were assayed for survival with a standard LDH assay to identify cell loss at 24 h (Fig. 1b). Neurones were treated for 2 h with conditioned media and then restored to unconditioned medium before assaying survival. LDH assays carried out at 24 h following a 2-h treatment with medium conditioned from interferon-γ treated microglia showed no significant difference (t-test, p > 0.05) implying that the medium was not toxic (Fig. 1b). MTT assays carried out at 24 h in parallel with the LDH assay also showed no significant change (p > 0.05) indicating that the change in MTT assay value for neurones treated with conditioned medium from interferon-γ treated microglia was transitory. Additionally we also examined neurones treated with conditioned medium from interferon-γ treated microglia using the Hoechst reagent. Directly after the 2 h treatment there was no significant increase in cells with condensed or fragment nuclei (treated = 4.2 ± 0.5 cells per field; control, 4.7 ± 0.6 cells per field, 10 fields, n = 3). Similarly after 24 h (cells returned to unconditioned medium) there was also no difference in the number of apoptotic nuclei (treated = 5.6 ± 0.8 cells per field; control, 6.7 ± 0.6 cells per field, 10 fields, n = 3). These results suggests that the measured reduction of MTT assay value after 2 h treatment of neurones with conditioned medium from interferon-γ treated microglia is not a result of cell death but of a transitory effect on mitochondrial ability to reduce MTT.
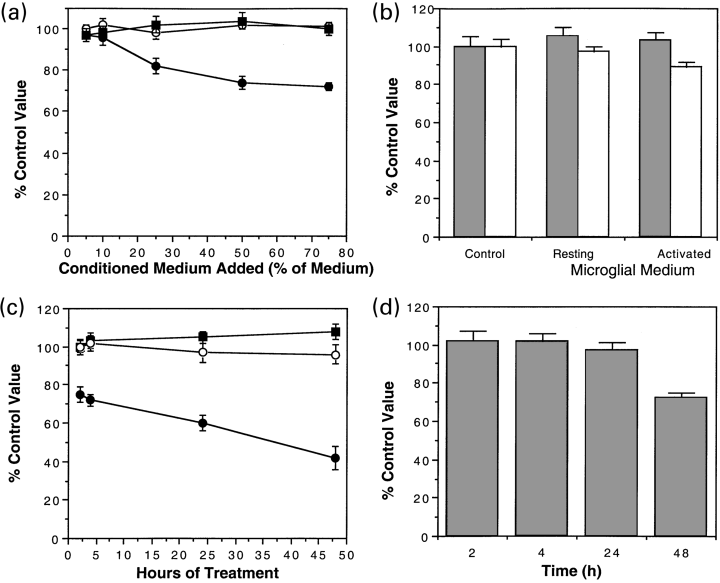
Conditioned medium from resting microglia (O), microglia treated with interferon-γ (●) or medium to which interferon-γ was added at 100 units/mL (▪) was applied to cerebellar cells for 2 h (a). MTT was measured after the 2-h treatment. Values for the MTT assay were compared with that of untreated cultures as a percentage (untreated = 100%). LDH (grey bars) and MTT (open bars) was also measured on parallel samples of cerebellar neurones 24 h after the 2 h treatment under the same conditions. (b) Cerebellar neurones (c, d) were treated with medium containing 50% conditioned medium from resting microglia (O), microglia treated with interferon-γ (●) or medium to which interferon-γ was added at 100 units/mL (▪). Cells were incubated for 2, 4, 24 or 48 h and then returned to serum-free medium. At 48 h, all cells were assayed for MTT (c) and LDH (d) (media conditioned by interferon-γ treated microglia only). The mean and SEM of four experiments each is illustrated.
Prolonged culture with media conditioned by interferon-γ treated microglia is toxic for mouse cerebellar neurones
Cerebellar neurones were given a longer exposure to medium consisting of microglial conditioned medium diluted 1 in 2 with unconditioned medium. At each time point (2, 4, 24 and 48 h), cells were either assayed with the MTT assay directly (Fig. 1c) or transferred to unconditioned medium and maintained in culture before carrying out an MTT assay at 48 h. The MTT assay values measured directly were reduced for cells treated with medium from interferon-γ treated microglia even after brief exposure (2 h, Student's t-test, p < 0.05). Further exposure led to a progressive reduction in MTT at 24 or 48 h (Student's t-test, p < 0.05). Medium from resting microglia, or interferon-γ added with medium from resting microglia had no effect on MTT assay values. The changes in MTT assay values measured directly after 2 and 4 h treatment were transitory and could not be detected at 24 (Fig. 1b) or 48 h (2 h, 97 ± 3%; 4 h, 96 ± 2% compared with control value 100 ± 2% n = 4) suggesting that removing the medium activated microglial medium allowed the neurones to recover from the effect causing the reduction in MTT assay value. However, the value from 24 h treated neurones did not return to control values when measured 24 h after returning the cells to the unconditioned medium, i.e. at 48 h (24 h, 88 ± 3% compared with control value 100 ± 2%). This suggests that the effect after 24 h of continuous treatment is no longer transitory. To determine if these changes in MTT reflect cell loss, LDH assay was carried out at 48 h after initial exposure for 2, 4, 24 and 48 h with test media and then maintained with unconditioned medium up to 48 h (Fig. 3d). Only the 48-h incubation with medium conditioned by interferon-γ treated microglia led to a reduction in LDH implying that the onset of cell death was at ≥ 24 h. This result was confirmed using Hoechst staining of neurones treated with medium conditioned from interferon-γ treated microglia for 48 h. There was a significant difference (p < 0.05) in the number of apoptotic nuclei (treated = 3.6 ± 0.8 cells per field; control, 27.7 ± 0.6 cells per field, 10 fields, n = 3).
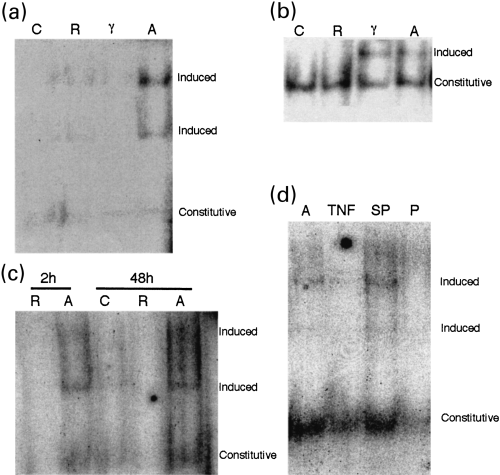
Mouse cerebellar neurone nuclear extracts were run on an EMSA gel. At 2 h, exposure to media conditioned by interferon-γ treated microglia induced binding to a NF-κB consensus sequence (a, p < 0.02, three experiments) whereas interferon-γ alone and media conditioned by interferon-γ treated microglia induced binding to a STAT consensus sequence (b, three experiments). The NF-κB consensus sequence binding was evident both at 2 and 48 h (c, three experiments). C, untreated control neurones; R, neurones treated with conditioned medium from resting microglia; A, neurones treated with conditioned medium from interferon-γ treated microglia; γ, neurones treated with interferon-γ. (d) The high molecular weight NF-κB consensus sequence binding seen after 2 h in the presence of medium conditioned by interferon-γ treated microglia (lane A) was blocked by anti-TNFα antibody (lane TNF) whereas constitutive activity was less affected. Addition of a scrambled peptide (lane SP) had no effect on the high molecular weight NF-κB consensus sequence binding whereas it was inhibited by anti-NF-κB p52 peptide (lane p). Constitutive, bands not induced by treatment; induced, bands induced by treatment
Soluble factors secreted by interferon-γ treated microglia impair neuronal mitochondrial activity
To confirm the impairment of mitochondrial metabolism, extracts from cells treated for 2 h with microglial conditioned medium were assayed for total ATPase and cytochrome oxidase activity (Fig. 2). The activity of these enzymes was significantly (p < 0.05, n = 7 both assays) reduced in cerebellar neurones treated with medium conditioned by interferon-γ treated but not resting microglia. This suggests that cells treated with this extract have reduced metabolic capacity due to impaired mitochondrial activity.
Interferon-γ treated microglia stimulate NF-κB expression in mouse cerebellar neurones
We wished to determine if NF-κB activation was associated with the changes in the cerebellar neurones as it has been suggested that such activation might be protective against neuronal death. We cultured cerebellar neurones from 6-day-old mice for up to 24 h with media conditioned by interferon-γ treated and resting microglia and with unconditioned medium with and without interferon-γ added. Nuclear extracts were made from these cultures and run in an electrophoretic mobility shift assay (EMSA) with NF-κB and STAT consensus sequences. In the presence of interferon-γ treated but not resting microglia conditioned media or controls, an additional band bound to the NF-κB consensus sequence after 2 h (Fig. 3a) and this was still present after 24 h incubation (Fig. 3c). Controls were also carried out using cold NF-κB consensus sequence peptides. These abolished the bands in the extracts induced by conditioned medium from interferon-γ microglia (data not shown).
A single band was observed in the presence of the STAT consensus sequence after a 2-h incubation with interferon-γ treated microglia conditioned medium (Fig. 3b). The STAT band was also present on incubation with interferon-γ alone (data not shown) and could be super-shifted with anti-STAT-1 antibody (data not shown), compatible with a direct effect of interferon-γ ligation on its receptor within neuronal membranes.
Anti-TNFα antibody inhibits cerebellar responses to interferon-γ treated microglial medium
Using the L929 TNFα bioassay, we demonstrated a rise in TNFα secretion from 1.05 ± 0.5 in resting to 5.46 ± 1.2* U/mL in interferon-γ treated microglia conditioned medium (four experiments, mean ± SEM, *p < 0.05) confirming microglial activation. TNFα is known to have deleterious effects on neurones. Therefore, cerebellar cells were treated with conditioned medium from interferon-γ activated microglia for 2 h in the presence of increasing concentrations of an antibody to TNFα (Fig. 4a). This abolished the transient decrease in MTT suggesting that either TNFα or TNFα acting synergistically with other factors is responsible for the observed reduction in MTT caused by medium from interferon-γ treated microglia. NF-κB induction seen with medium conditioned by interferon-γ treated microglia was blocked in the presence of anti-TNFα antibody (5 ng/mL) confirming that the effect is TNFα dependent (Fig. 3d, lanes 1 and 2).
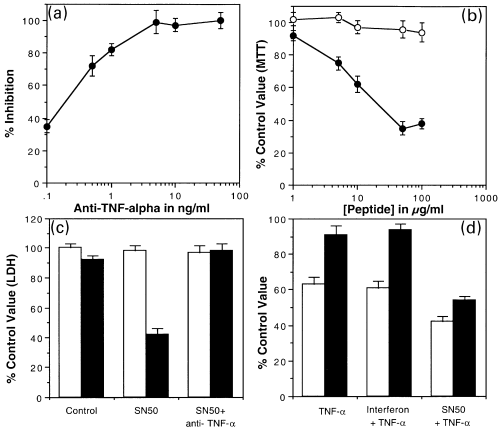
Cerebellar cells were treated for 2 h with conditioned medium from interferon-γ treated microglia (50% v/v). (a) At the same time they were treated with an antibody against TNFα. Cerebellar cells were assayed for MTT after 2 h. Results were normalized to the difference between untreated and conditioned medium from interferon-γ treated microglia and expressed as a percentage inhibition of the effect of conditioned medium. The mean and SEM for a minimum of three experiments each are shown. (b) After this time the cells were returned to unconditioned medium until 24 h. MTT was assayed 24 h after the initial application. From beginning of the treatment until the MTT assay at 24 h the cells were maintained in increasing concentrations of either SN50 (●) or SN50M (O). Results are expressed as a percentage of untreated cells. (c) LDH was assayed on parallel samples. Cells were treated without conditioned medium (open bars) and either SN50 or both SN50 and anti-TNFα (5 ng/mL). Further samples were treated with conditioned medium from interferon-γ treated microglia and either SN50 or both SN50 and anti-TNFα (5 ng/mL). Results are expressed as a percentage of LDH in untreated cultures. (d) Cerebellar cells were treated for 24 h with either TNFα or TNFα in combination with interferon-γ or SN50. Cultures were assayed with both the MTT assay (open bars) and the LDH (black bars) assay. The results are expressed as a percentage of the MTT assay value or the LDH value for untreated cultures. The mean and SEM for a minimum of three experiments each are shown.
Interferon-γ treated microglia stimulated NF-κB expression is inhibited by SN50
We attempted to block the NF-κB activation seen in cerebellar cells on treatment with interferon-γ treated microglia conditioned medium. Experiments were carried out using SN50 (Maggirwar et al. 1998; a peptide which inhibits entry of the p52 subunit of NF-κB into the nucleus) and a mutated control peptide (SN50M; which has a similar sequence but no effect on NF-κB transport). The peptides were added to cerebellar neurone cultures together with conditioned media from interferon-γ treated microglia for 2 h before making nuclear extracts. These extracts were run on an EMSA gel run and analysed in coded assays (i.e. blind to treatment) on three separate occasions. The SN50 peptide but not the SN50M peptide inhibited entry of NF-κB subunit p52 into the nucleus (Fig. 3d, lanes 3 and 4).
SN50 causes medium conditioned by interferon-γ treated microglia to be neurotoxic
Cerebellar neurones treated with medium conditioned by interferon-γ treated microglia for 2 h were cotreated with SN50 and SN50M at increasing concentration. After 2 h the neurones were returned to unconditioned medium but were maintained in the same concentration of the peptides until MTT assays carried out at 24 h. Treatment of cerebellar cells with SN50 or SN50M alone had no effect on MTT assay values. MTT assay values of conditioned media treated cerebellar neurones were reduced by SN50 but not by SN50M (Fig. 4b).
The effects of SN50 on cerebellar was also assessed using LDH assay (Fig. 4c). SN50 that was not toxic to cerebellar cells at 50 µg/mL. SN50 was significantly toxic to cerebellar neurones treated for 2 h with medium from interferon-γ treated microglia (50% of total medium) (p < 0.05). Parallel cultures were treated with SN50 and anti-TNFα antibody. The toxic effect of SN50 was blocked by the anti-TNFα antibody.
In another set of experiments cerebellar neurones were treated for 24 h with TNFα at 10 units/mL. Neurones were also treated with TNFα with addition of from 100 units/mL interferon-γ or 50 µg/mL SN50 peptide. At 24 h an assay showed that TNFα reduced the assay values of MTT but not LDH. Interferon-γ did not reduce the effect further (Fig. 4d). Addition of the SN50 peptide enhanced the effect of TNFα on the MTT value and caused a reduction in the LDH assay value indicating a toxic effect. These results indicate that NF-κB protects against the toxic effects of TNFα in medium conditioned by interferon-γ treated microglia and SN50 blocks this effect of NF-κB by preventing its entry into the nucleus.
Discussion
Microglia are the major immunological cell within the CNS. Activation leads to the release of TNFα– one of a wide range of cytokines and growth factors that could potentially elaborate toxic and regenerative aspects of the immune response (Kollias et al. 1999). TNFα has major effects on cellular metabolism and can result either in death or survival depending on the cell target and environment (Rath and Aggarwal 1999), acting directly and indirectly through cell recruitment, further elaboration of the immune response and the release of soluble mediators. We demonstrate that media conditioned by interferon-γ treated microglia rapidly and reversibly inhibit mouse neuronal metabolism through TNFα release. Neuronal toxicity before 24 h of treatment is only seen if TNFα-induced NF-κB activation is blocked.
The lack of NF-κB in neurones has been linked to an increased susceptibility to excitotoxic damage (Yu et al. 1999), amyloid beta peptide toxicity (Kaltschmidt et al. 1999), and cell death in traumatic brain injury (Sullivan et al. 1999). Here, we demonstrate that NF-κB p52 fulfils a protective role in mouse cerebellar neurones in response to physiological levels of TNFα present in media conditioned by interferon-γ treated microglia. Blockade of the TNFα-induced NF-κB p52 subunit allows TNFα to induce rapid neuronal toxicity, previously only seen in vitro with cell–cell contact. However TNFα-induced NF-κB activation does not prevent the late neurotoxicity induced by activated microglia. The extent of NF-κB activation may have a role in determining the variable in vitro results on early neuronal survival reported using soluble microglia derived factors (de Bock et al. 1998; Toku et al. 1998; Downen et al. 1999; Warchol and Kaplan 1999). NF-κB has a similar role in NGF signalling through the related TNF family receptor p75NTR. A survival response is seen when the high affinity NGF receptor (TrkA) is present. This receptor activates NF-κB in addition to signalling through the p75NTR but cell toxicity is seen when TrkA and thus NF-κB activation, are absent (Casaccia-Bonnefil et al. 1999). Extending this earlier work, we demonstrate that activated microglia are not directly neurotoxic but reversibly modulate neuronal mitochondrial function in neurones after 2 h in vitro. This inhibition of neuronal metabolism is through the action of TNFα and is unlikely to be NF-κB dependent. Mitochondria are essential to cellular function and survival (Skulachev 1999) but previous work has confirmed that the TNFα-mediated effects on mitochondrial function do not inevitably lead to cell death (Prins et al. 1998).
Microglia, the macrophage/monocyte lineage cell within the CNS have been implicated in the neuronal toxicity seen in many CNS diseases (Zielasek and Hartung 1996). Evidence is however, emerging that microglia also exert beneficial effects on neurones and other cells within the CNS (Lazarov-Spiegler et al. 1996; Zeev-Brann et al. 1998; Zietlow et al. 1999; Nicholas et al. 2000). In vivo studies show that microglia become activated in response to minor changes in the CNS environment yet this does not necessarily result in cell death and may in fact promote cell survival and regeneration (Kreutzberg 1996). Therefore these cells play a complex role within the CNS. By reversibly inhibiting neuronal metabolism but not inducing toxicity, activated microglia could transiently switch off neuronal function restricting secondary excitotoxic injury until the activating stimulus subsides or its persistence ultimately leads to cell death. In diseases such as multiple sclerosis, the clinical deficits are initially reversible (Smith and McDonald 1999); and in stroke neuronal function can recover in the penumbra where microglial activation has been demonstrated (Sette et al. 1993). Our in vitro study suggests that microglia play a homeostatic role in CNS function (Nicholas et al. 2000). Limiting neuronal function in the presence of an activating stimulus might therefore serve a physiological role in vivo by suspending energy expenditure and transiently impairing function but in the interests of long-term survival.
Acknowledgement
This work was supported in part by a grant from SmithKline Beecham.




