Increased Expression of Apolipoprotein D Following Experimental Traumatic Brain Injury
Abbreviations used : apoD, apolipoprotein D ; apoE, apolipoprotein E ; GAPDH, glyceraldehyde-3-phosphate dehydrogenase ; NeuN, neuron-specific nuclear protein (neuronal nuclei) ; PBS, phosphate-buffered saline ; TBI, traumatic brain injury.
Abstract
Abstract : Increasing evidence suggests that apolipoprotein D (apoD) could play a major role in mediating neuronal degeneration and regeneration in the CNS and the PNS. To investigate further the temporal pattern of apoD expression after experimental traumatic brain injury in the rat, male Sprague-Dawley rats were subjected to unilateral cortical impact injury. The animals were killed and examined for apoD mRNA and protein expression and for immunohistological analysis at intervals from 15 min to 14 days after injury. Increased apoD mRNA and protein levels were seen in the cortex and hippocampus ipsilateral to the injury site from 48 h to 14 days after the trauma. Immunohistological investigation demonstrated a differential pattern of apoD expression in the cortex and hippocampus, respectively : Increased apoD immunoreactivity in glial cells was detected from 2 to 3 days after the injury in cortex and hippocampus. In contrast, increased expression of apoD was seen in cortical and hippocampal neurons at later time points following impact injury. Concurrent histopathological examination using hematoxylin and eosin demonstrated dark, shrunken neurons in the cortex ipsilateral to the injury site. In contrast, no evidence of cell death was observed in the hippocampus ipsilateral to the injury site up to 14 days after the trauma. No evidence of increased apoD mRNA or protein expression or neuronal pathology by hematoxylin and eosin staining was detected in the contralateral cortex and hippocampus. Our results reveal induction of apoD expression in the cortex and hippocampus following traumatic brain injury in the rat. Our data also suggest that increased apoD expression may play an important role in cortical neuronal degeneration after brain injury in vivo. However, increased expression of apoD in the hippocampus may not necessarily be indicative of neuronal death.
Apolipoprotein D (apoD) is a 169-amino acid glycoprotein of apparent Mr 29,000 that was originally identified as a component of high-density plasma lipoproteins (McConathy and Alaupovic, 1973). ApoD is not a typical apolipoprotein, however, but a member of the lipocalin superfamily, a large and diverse family of transporter proteins that carry various small hydrophobic ligands (Flower, 1996).
ApoD is widely expressed in both neural and peripheral tissues (Boyles et al., 1990a ; Provost et al., 1990 ; Seguin et al., 1995 ; Navarro et al., 1998). In the CNS immunocytochemical studies have shown that apoD is expressed in neurons and glial cells (Boyles et al., 1990a). In the PNS, neurolemmal cells and fibroblasts are immunoreactive for apoD (Boyles et al., 1990a).
ApoD has been shown to bind several hydrophobic molecules, including cholesterol, progesterone, pregnenolone, heme-related molecules such as biliverdin and porphyrins, and arachidonic acid (Lea, 1988 ; Peitsch and Boguski, 1990 ; Boyles et al., 1990a ; Cabral et al., 1995 ; Patel et al., 1997). The diversity of ligands transported by apoD suggests that it may have wide-ranging physiological cellular functions. In contrast, the function of apoD in pathological conditions has not been clearly identified. However, there is increasing evidence that apoD may be involved in the steroid-mediated growth of some tumor cell lines (Labrie et al., 1990 ; Simard et al., 1990, 1991). Moreover, recent reports suggest that apoD may also be involved in the neurochemical cascade associated with chronic CNS degeneration. For instance, recent data indicate that apoD is overexpressed in neural tissues in Niemann-Pick's type C disease, an inherited neurovisceral lipid storage disorder that is associated with defects in cellular cholesterol homeostasis and progressive neurodegeneration (Higashi et al., 1991 ; Loftus et al., 1997 ; Suresh et al., 1998 ; Patel et al., 1999). In addition, recent work has begun to implicate overexpression of apoD as an important component of the neurodegeneration in Alzheimer's disease. For example, increased levels of apoD have been found in the CSF and hippocampus of Alzheimer's patients (Terrisse et al., 1998). Also, apoD is thought to play an important role in lipid redistribution after experimental peripheral nerve injury (Boyles et al., 1990b ; Spreyer et al., 1990).
Recent data also suggest that another glycoprotein, apolipoprotein E (apoE), plays a central role in the CNS response to injury. ApoE exists as three isoforms in humans encoded by three different alleles (ε2, ε3, and ε4). The ε4 allele of the apoE gene is overexpressed in patients with Alzheimer's disease and is associated with an earlier age of disease onset and a higher cortical amyloid burden (Rebeck et al., 1993 ; Strittmatter et al., 1993 ; Hyman et al., 1996). Moreover, the consequences of acute injury on the expression of apoE have been studied recently. For example, in the PNS, apoE accumulates at the site of axonal damage in response to sciatic nerve crush (Skene and Shooter, 1983 ; Boyles et al., 1985 ; Mahley, 1988 ; LeBlanc and Poduslo, 1990). In the CNS, entorhinal cortex lesions that deafferent the hippocampus result in a similar up-regulation of apoE by astrocytes within the lesion bed (Poirier, 1991 ; Poirier et al., 1991). Moreover, following transient ischemic injury in the rat, there is evidence that the apoE level is increased in astrocytes and then apoE taken up in neurons that are destined to die (Horsburgh and Nicoll, 1996 a, b). Furthermore, a report by Sheng et al. (1998) suggested that apoE-specific isoforms may differentially influence the outcome following focal cerebral ischemia in transgenic mice. In addition, marked alterations in the levels of apoE have been observed following experimental traumatic brain injury (TBI) (Horsburgh et al., 1997). Also, a recent report provided evidence that apoE-deficient mice exhibit an impaired ability to recover from head injury, suggesting that apoE may play an important role in neuronal repair (Chen et al., 1997).
Despite increasing evidence that apoD and apoE may have a potential role in CNS and PNS injury, little is known, however, about the functional role of apoD in acute brain injuries, including cerebral ischemia and TBI. To investigate further potential changes in apoD following TBI in vivo, rodents were subjected to a widely used model of experimental mechanical brain injury—lateral cortical impact injury (Dixon et al., 1991). The present study used RT-PCR and western blot analyses of cortical and hippocampal samples to determine the temporal profile of changes in apoD mRNA and protein levels from 15 min to 14 days after TBI. Furthermore, immunohistochemical investigations were performed to investigate the cellular distribution of apoD expression, and concurrent hematoxylin and eosin investigations assessed histopathological changes in cortical and hippocampal tissue after TBI in vivo.
MATERIALS AND METHODS
Rat model of TBI
A controlled cortical impact device was used to induce a moderate level of TBI as previously described (Dixon et al., 1991). In brief, adult male Sprague-Dawley rats (weighing 250-350g) were intubated and anesthetized with 2% halothane in a 2:1 mixture of N2O/O2. Core body temperature was monitored continuously by a rectal thermistor probe and maintained at 37-38°C. Animals were mounted in a stereotaxic frame on the injury device in a supine position secured by ear and incisor bars. The head was held in a horizontal plane with respect to the interaural lines. A midline incision was made, the soft tissues were reflected, and two 7-mm craniotomies were made between lambda and bregma and centered 5 mm laterally on either side of the central suture. The dura was kept intact over the cortex. Injury was induced by impacting the right cortex (ipsilateral cortex) with a 6-mm-diameter tip at a rate of 4 m/s. The injury device was set to produce a tissue deformation of 2 mm. Velocity was measured directly by the linear velocity displacement transducer (model 500 HR ; Shaevitz, Detroit, MI, U.S.A.), which produces an analog signal that was recorded by a PC-based data acquisition system for analysis of time/displacement parameters of the impactor. Following cortical impact, animals were extubated and immediately assessed for recovery of reflexes (Dixon et al., 1991). Sham-injured animals underwent identical surgical procedures but did not receive impact injury. Naive animals were not exposed to any injury-related surgical procedures. In total, 128 animals were used in this study (naive, n = 8 ; sham-injured rats, n = 8 ; injured rats, n = 112). All animal studies carefully conformed to the guidelines outlined in the Guide for the Care and Use of Laboratory Animals from the Austrian Department of Health and Science and were approved by the University of Innsbruck Medical School Animal Welfare Committee.
Sample preparation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblotting, and quantification
Before they were killed, all animals (n = 32) were given a lethal dose of phenobarbital (20 mg/kg i.p.). The animals were killed by decapitation 15 min, 6 h, 24 h, 48 h, 72 h, 7 days, and 14 days after TBI (n = 4 for each time point after injury ; n = 2 for sham and naive animals), and both cortices and hippocampi (ipsilateral and contralateral to the injury site) were removed. Excision of both cortices beneath the craniotomies extended ~4 mm laterally and ~7 mm rostrocaudally and to a depth extending to the white matter. All samples were frozen immediately in liquid N2. The microdissected tissue was homogenized at 4°C in ice-cold homogenization buffer containing 20 mM piperazine-N,N'-bis(2-ethanesulfonic acid) (PIPES ; pH 7.1), 2 mM EGTA, 1 mM EDTA, 1 mM dithiothreitol, 0.3 mM phenylmethylsulfonyl fluoride, and 0.1 mM leupeptin. Protein concentrations were determined by bicinchoninic acid microprotein assays (BCA ; Sigma, U.S.A) with albumin standards. Protein-balanced samples were prepared for polyacrylamide gel electrophoresis in twofold loading buffer containing 0.25 M Tris (pH 6.8), 0.2 M dithiothreitol, 2% sodium dodecyl sulfate, 0.005% bromophenol blue, and 5% glycerol in distilled water. Samples were heated for 5 min at 95°C. Sixty micrograms of protein was routinely resolved in each lane. We used a vertical electrophoresis chamber with a 4% acrylamide stacking gel over a 13% acrylamide resolving gel. Following separation, proteins were immediately transferred to nitrocellulose membranes using western blotting. Lateral transfer was employed using transfer buffer made up of 0.192 M glycine and 0.025 M Tris (pH 8.3) with 10% methanol at a constant voltage of 25 V for 90 min at room temperature. Blots were immediately blocked for immunolabeling by overnight incubation using 2% nonfat milk in phosphate-buffered saline (PBS) and 0.05% Tween 20 at 4°C. Coomassie Blue (Bio-Rad, U.S.A.) and Ponceau Red (Sigma, U.S.A.) stainings were routinely performed to confirm that equal amounts of protein were loaded in each lane. Following incubation with a rabbit polyclonal apoD antibody (Ong et al., 1997) (dilution of 1 : 4,000) for 2 h at room temperature, nitrocellulose membranes were incubated for 45 min at room temperature with a secondary antibody linked to alkaline phosphatase (dilution of 1 : 5,000 ; Accurate Chemical and Scientific Corp., U.S.A.). Visualization was performed with a p-nitro blue tetrazolium chloride (Boehringer-Mannheim, Germany) and 5-bromo-4-chloro-3-indolyl phosphate (Boehringer-Mannheim, Germany) alkaline phosphatase substrate.
Semiquantitative RT-PCR analysis
Total RNA was isolated from frozen ipsilateral and contralateral cortex and hippocampus of all previously described groups (n = 32) with Trizol reagent (GIBCO, U.S.A.). The cDNAs were synthesized from 10 μg of total RNA per cDNA reaction using Superscript reverse transcriptase (GIBCO, U.S.A.) with oligo(dT) as a primer. The resulting cDNAs were diluted to 100 μl and used for PCR analysis. Each PCR mixture contained equal amounts of diluted cDNA corresponding to 200 ng of total RNA, 100 pmol of each primer, 10 pmol of deoxynucleotide triphosphates, 1 × Ampli-Taq reaction buffer, and 2.5 U of Ampli-Taq-Gold DNA polymerase (Perkin-Elmer Cetus, U.S.A.). All cDNAs were amplified with glyceraldehyde-3-phosphate dehydrogenase (GAPDH ; GenBank accession no. X02231)-specific primers (5′-CCCACGGCAAGTTCAACGG, 5′-CTTTCCAGAGGGGCCATCCA ; size of the PCR product is 430 bp) together with apoD (GenBank accession no. X55572)-specific primers (5′-TCTTGGGAAATGCCCATCTCC, 5′-CGGTGGCATCAACGAGAAGAAC ; size of the PCR product is 280 bp). PCR amplification was carried out for 25 cycles of 45 s at 95°C, 45 s at 60°C, and 45 s at 72°C, followed by a final step of 10 min at 72°C. PCR products were analyzed by agarose gel electrophoresis in 2% NuSieve agarose gels (FMC, U.S.A.). The identity of the PCR products obtained was confirmed by Southern blot analysis using an internal oligonucleotide as hybridization probe.
Immunohistochemistry
Animals from all treatment groups (n = 32) were given a lethal injection of phenobarbital (20 mg/kg i.p.) before perfusion. Rats were transcardially perfused through the left ventricle (120 ml of 0.9% NaCl and 200 ml of 4% paraformaldehyde) at 15 min, 6 h, 24 h, 48 h, 72 h, 7 days, and 14 days after TBI (n = 4 for each time point after injury ; n = 2 for sham and naive animals). The brains were then removed and incubated in 30% sucrose overnight for cryoprotection. The brains were then grossly sectioned, frozen, and mounted in a Hacker-Bright cryostat. Coronal sections 30-40 μm thick were cut at - 15°C and immediately placed into wells containing PBS. Sections were washed for 3 h in PBS to remove any traces of fixative and immershed for 1 h in a solution of 1% defatted dry milk in PBS to block nonspecific binding of the antibody. After blocking of the endogenous peroxidase using 0.3% H2O2, sections were then incubated overnight at 4°C with a rabbit polyclonal antiserum against rat apoD (Ong et al., 1997), diluted 1 : 10,000 in blocking solution. The sections were washed twice in PBS and incubated for 1 h at room temperature in a 1 : 200 dilution of a biotinylated goat anti-rabbit IgG (Vector Laboratories, U.S.A.). This was followed by three changes of PBS to remove unreached secondary antibody. Sections were then reacted for 90 min with an avidin-biotinylated horseradish peroxidase complex. The reaction was visualized by treatment for 3 min with 0.05% 3,3′-diaminobenzidine tetrahydrochloride solution in PBS containing 0.05% H2O2. The color reaction was stopped with several washes of PBS. Sections were mounted on glass slides and allowed to dry. Dried sections were then converslipped with Elvanol. Sections without primary antibody did not stain. For double immunostaining using bright-field chromagens, sections were incubated with a pooled solution of rabbit anti-rat apoD (Ong et al., 1997) (1 : 10,000) and mouse anti-neuron-specific nuclear protein (neuronal nuclei) (NeuN) (Mullen et al., 1992 ; Wolf et al., 1996 ; Eriksson et al., 1998) (Chemicon, U.S.A. ; 1 : 500) antisera. After being rinsed and blocked, sections were incubated first with a biotinylated horse antimouse antibody (Vector Laboratories, U.S.A.) followed by incubation with an alkaline phosphatase avidin-biotin substrate and then reaction with blue chromagen (Vector Blue ; Vector Laboratories, U.S.A.). After being rinsed and blocked further, the sections were then incubated with a biotinylated horse anti-rabbit antibody (Vector Laboratories, U.S.A.) followed by incubation with a peroxidase avidin-biotin substrate. The reaction was visualized by treatment for 3 min with 0.05% 3,3′-diaminobenzidine tetrahydrochloride solution in PBS containing 0.05% H2O2. Sections were mounted and converslipped with an aqueous mounting medium (Sigma, U.S.A.).
Histopathological assessments
Animals from all treatment groups (n = 32) were given a lethal injection of phenobarbital (20 mg/kg i.p.) before perfusion. Rats were transcardially perfused through the left ventricle (120 ml of 0.9% NaCl and 200 ml of 10% buffered formalin) at 15 min, 6 h, 24 h, 48 h, 72 h, 7 days, and 14 days after TBI (n = 4 for each time point after injury ; n = 2 for sham and naive animals). Brains were sectioned at 2-3-mm intervals, embedded in paraffin, and cut at 4-5 μm. Paraffin sections from regions +0.2 mm bregma through -3.8 mm bregma were mounted on glass slides and stained with hematoxylin and eosin.
Statistical analysis
Western blotting and RT-PCR band densities were quantified via computer-assisted two-dimensional densitometric scanning on a Macintosh computer using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Relative band densities on western blots (n = 1 per blot) were expressed as arbitrary densitometric units for each time point. This procedure was repeated up to four times for a total of n = 4. Data acquired in arbitrary densitometric units were transformed to percentages of the densitometric levels observed on scans from sham-operated animals on the same blot. RT-PCR data were acquired in densitometric units and were transformed to percentages of the densitometric units from GAPDH bands visualized on the same agarose gel. This procedure was repeated four times for a total of n = 4. Group differences were determined by ANOVA and post hoc Tukey's HSD test. Data are mean ± SEM values. Differences were considered significant when p≤ 0.05.
RESULTS
ApoD mRNA expression
ApoD mRNA was detected by semiquantitative RT-PCR analysis in cortical and hippocampal samples of sham-operated and injured animals, respectively (Fig. 1). Cortical impact injury resulted in an increase of apoD mRNA levels in the ipsilateral cortex (Fig. 1A). Starting at 48 h after injury, a significant increase in apoD mRNA levels was observed. Rising rapidly, the apoD mRNA level packed at least sixfold above control (sham-operated) levels by day 3 after the trauma and remained thereafter to a steady-state level of approximately five-fold above control at 14 days after TBI.
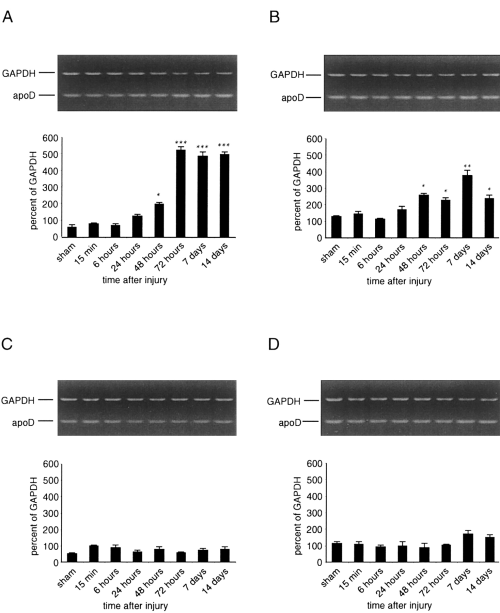
RT-PCR analysis of apoD mRNA following TBI. Cortical and hippocampal samples of single control animals (sham) and single injured animals were prepared for RT-PCR at the indicated times after TBI in vivo. Sizes of the PCR products were 430 bp for GAPDH and 280 bp for apoD, respectively. Data are mean ± SEM (bars) values, presented as percentages of the GAPDH value, of four independent experiments. A : Ipsilateral cortex. Levels of mRNA increased at 48 h after injury as compared with controls (*p < 0.05) and reached a steady-state levels of approximately sixfold above controls from 72 h to 14 days postinjury (***p < 0.001). B : Ipsilateral hippocampus. Levels of apoD mRNA increased 48 h postinjury and reached a maximal level of approximately threefold above controls at 7 days after the trauma (**p < 0.01). C and D : No significant changes in apoD mRNA levels were seen in, respectively, cortex and hippocampus contralateral to the injury site from 15 min to 14 days after injury.
In the ipsilateral hippocampus, injured animals manifested a significant increase in apoD mRNA levels from 48 h to 14 days posttrauma (Fig. 1B). ApoD mRNA levels peaked at 7 days after injury, where a maximal level of at least threefold above values in sham animals was detected. ApoD mRNA levels declined thereafter to a level of approximately twofold above controls at 14 days after the impact. No statistically significant differences in apoD mRNA levels were seen between naive and sham-injured animals (data not shown). In addition, no significant increases in apoD mRNA levels were seen in cortical and hippocampal samples contralateral to the injury site from 15 min to 14 days after the impact (Fig. 1C and D).
Western blotting
ApoD appeared as heterogeneous immunoreactive bands at 27-31 kDa (Fig. 2), probably due to differentially glycosylated isoforms (Suresh et al., 1998). Cortical impact injury resulted in an increase of apoD protein levels in the ipsilateral cortex. Ipsilateral apoD protein levels from injured animals increased by 5,000% relative to sham levels at 72 h after TBI (Fig. 2A). The increase in apoD immunoreactivity peaked by day 7 after TBI (9,100% increase relative to sham animals) and declined to a 2,000% increase relative to sham values at 14 days after TBI.
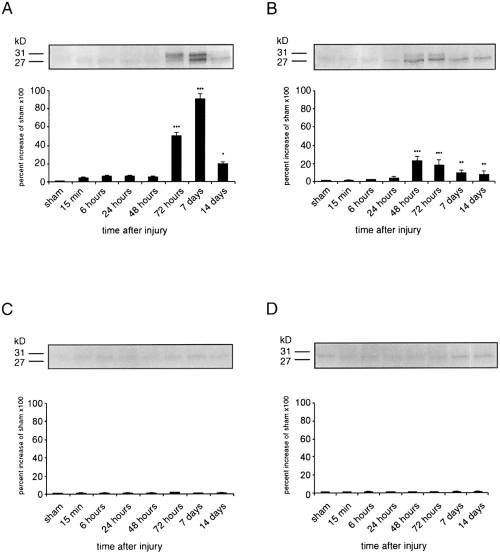
Time course of apoD protein expression after TBI. Samples from single control (sham) and single injured animals were prepared for western blotting at 15 min to 14 days after TBI. Levels of apoD protein are expressed as arbitrary densitometric units. Data were transformed to percentages of the densitometric levels observed on scans from sham animals visualized on the same blot and are mean ± SEM (bars) values of four independent experiments. A : Ipsilateral cortex. ApoD protein levels increased significantly at 72 h (***p <0.001) after TBI and peaked at 7 days postinjury as compared with controls. ApoD protein levels were still significantly increased up to 14 days posttrauma (*p < 0.05 compared with controls). B : Ipsilateral hippocampus. A large significant increase in apoD protein content was seen at 48 h postinjury (***p <0.001) followed by smaller significant increases from 72 h (***p <0.001) to 14 days after injury (**p <0.01). C and D : Cortical impact injury did not induce an increase of apoD protein levels in, respectively, cortical and hippocampal samples contralateral to the injury site.
In the ipsilateral hippocampus apoD levels were significantly higher from 48 h to 14 days after injury as compared with control (sham-operated) animals (Fig. 2B). ApoD immunoreactivity in the hippocampus peaked at 48 h postinjury with an increase of 2,230% relative to sham-operated animals and slowly decreased thereafter to a level of 760% relative to controls at 14 days postrauma. Only very little apoD immunoreactivity was observed in cortical and hippocampal samples obtained from naive (data not shown) and sham-injured animals (Fig. 2). No significant differences were observed in apoD protein levels between naive and sham-injured animals (data not shown). In addition, no significant changes in apoD were seen in cortical and hippocampal samples contralateral to the injury site at 15 min to 14 days after TBI (Fig. 2C and D).
Immunohistochemical studies
Ipsilateral and contralateral cortical and hippocampal tissues were examined rostrocaudally from +0.2 to -3.8 mm bregma. No localized apoD immunoreactivity was present in the tissue from sham-injured (Fig. 3A) or naive (data not shown) control rats. ApoD immunoreactivity was observed in the ipsilateral cortex at the primary injury zone (-1.5 to -3.4 mm bregma) from 2 to 14 days after the trauma. At 48 h after the injury, apoD immunoreactivity was seen primarily in glial cells in the cortical layers 3-5 (Fig. 3B-D). Seven days after the impact, prominent apoD labeling was seen in cortical pyramidal neurons at the area of contusion (Fig. 3E and F). ApoD immunoreactivity was mainly detected in neuronal somata and along extending apical dendrites of cortical layers 3-5. ApoD labeling of glia cells and neurons corresponded to regions showing morphopathological characteristics of injured neurons (see Hematoxylin and eosin staining).
In the ipsilateral hippocampus, apoD immunoreactivity was present in glial cells at 48 h after TBI (Fig. 3H and I). Similar to the ipsilateral cortex, apoD immunoreactivity was seen primarily in neurons at later time points after the trauma (Fig. 3J and K). Dense apoD immunostaining of cell bodies and apical dendrites was seen from 72 h to 7 days after the injury, primarily involving the CA1 field of the hippocampus. Only very little apoD immunoreactivity was observed in hippocampal neurons of CA2 and CA3 fields (data not shown). No immunoreactive neurons and glial cells were detected in cortical and hippocampal samples contralateral to the injury site (data not shown).
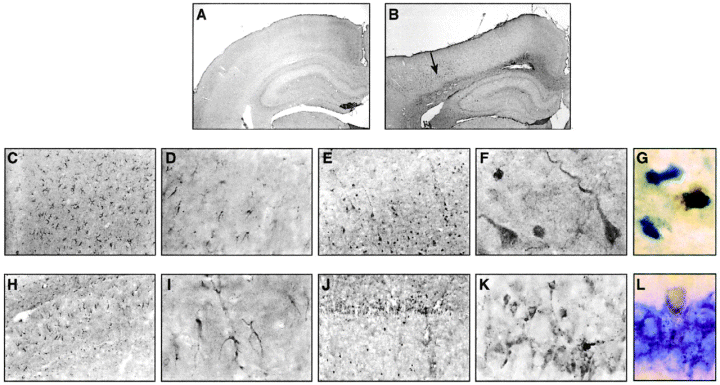
Sections (-3.4 mm bregma) immunolabeled with apoD antibody (A-F and H-K), apoD (G), and NeuN (L) following cortical impact injury. Sham-injured brains (A) and cortex and hippocampus contralateral to the injury site (data not shown) showed no specific immunolabeling. Low- (B, arrow) and high-magnification (C and D) micrographs revealed specific immunolabeling of glial cells in the ipsilateral cortex 48 h post-TBI. At 7 days postinjury, neuronal cell bodies and apical dendrites were labeled specifically for apoD (E and F). In the ipsilateral hippocampus (CA1 field), apoD immunoreactivity was seen in glial cells at 48 h posttrauma (H and I). At 7 days after TBI, dense apoD labeling was seen in a large number of neurons in the CA1 field of the hippocampus (J and K). Double immunostaining experiments with apoD (brown color) and the neuronal marker NeuN (shown in blue) provided further evidence that apoD is expressed in cortical (G) and hippocampal CA1 (L) neurons at 7 days after TBI. Cortical neurons appeared rather shrunken and irregular-shaped as compared with hippocampal neurons. A and B, ×20 ; C, E, H, and J, ×100 ; D, F, G, I, K, and L, ×1,000.
To confirm further that apoD is expressed in hippocampal and cortical neurons at late time points after TBI, we performed double-labeling experiments for apoD and the neuronal cell-specific marker NeuN. These immunohistochemical analyses of apoD-positive cells at 7 days (Fig. 3G and L) and 14 days (data not shown) after TBI unambiguously identified labeling with NeuN and demonstrated the expression of apoD in cortical (Fig. 3G) and hippocampal (Fig. 3L) neurons. In contrast to 7 days after trauma, only very few apoD-immunopositive neurons were detected in the ipsilateral cortex and hippocampus at 14 days post-TBI, respectively (data not shown).
Hematoxylin and eosin staining
Sham-operated (Fig. 4A and B) and naive (data not shown) brains (-3.4 mm bregma) revealed no detectable histopathological cortical alterations in either hemisphere. Similar to our previous studies (Kampfl et al., 1996 ; Newcomb et al., 1997 ; Posmantur et al., 1997), cortices from injured animals at the impact zone (-1.5 to -3.8 mm bregma) at 15 min after TBI were characterized by focal intracortical hemorrhage and the appearance of a few darkened neurons, i.e., eosinophilia, throughout cortical layers 1-3 (Fig. 4D and E). At 48 h after TBI, superficial cortical contusion ipsilateral to the injury site overlaid an area of cortical damage manifesting dark, shrunken, and pyknotic neurons in cortical layers 1-5, characteristic of impending cell death (Fig. 4G and H). By (Fig. 4J and K) and 14 days (data not post-TBI, the cortical area at the impact site appeared overtly necrotic in cortical layers 1-6. At all time points examined this primary injury zone revealed a gradual progression to normal cortex on either side with decreasing numbers of pyknotic neurons. Photomicrographs of the hippocampus of sham-operated animals (CA1 shown ; Fig. 4A and C) and of the ipsilateral hippocampus of injured animals at 15 min (Fig. 4D and F), 48 h (Fig. 4G and I), (Fig. 4J and L), and 14 days (data not shown) after TBI revealed no discernible histological alterations. Moreover, cortical and hippocampal tissue contralateral to the injury site showed no detectable histopathological changes from 15 min to 14 days after TBI (data not shown).
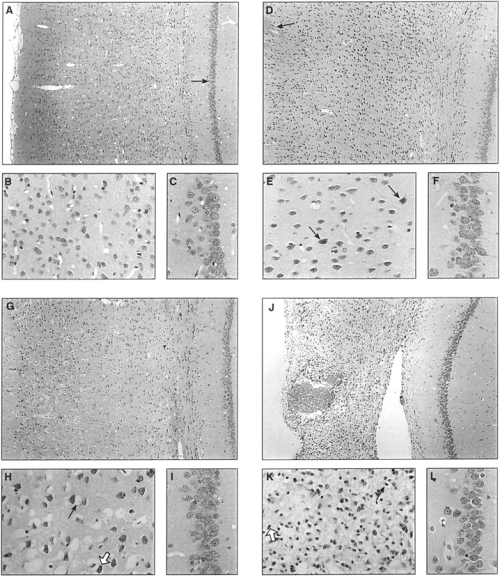
Sections (-3.4 mm bregma) stained with hematoxylin and eosin to assess morphopathology at the site of impact. Sham-injured brains revealed no detectable histopathological alterations in cortex (A and B). Injured rat cortex ipsilateral to the site of injury at 15 min, 48 h, and 7 days after injury revealed evolutionary cortical changes in the morphology of the contusion site. Photomicrographs of ipsilateral cortex at 15 min after TBI showed focal intracortical hemorrhage (D ; straight arrow) and the appearance of few eosinophilic neurons (E ; straight arrows). Photomicrographs at 48 h (G and H) and 7 days (J and K) after the injury revealed a progressive increase in pyknotic, shrunken neurons (H and K ; white arrows) that was associated with cytoplasmic retractions (H and K ; straight arrows). No evidence of histopathological alterations was seen in the CA1 field of the hippocampus of sham animals (A, arrow ; and C) and in the ipsilateral CA1 field of the hippocampus at 15 min (D and F), 48 h (G and I), and 7 days (J and L) after unilateral cortical impact. A, D, G, and J, X100 ; B, C, E, F, H, I, K, and L, X500.
DISCUSSION
The results of our study provide the first evidence that apoD is overexpressed in the cortex and hippocampus following TBI in the rat. In the cortex ipsilateral to the injury site, increased apoD mRNA and protein levels were detected from 48 h to 14 days after TBI. ApoD immunoreactivity appeared at 48 h posttrauma in cortical glial cells, and increased apoD immunlabeling was observed at later time points in the cell bodies and dendrites of neurons. Hippocampal samples ipsilateral to the injury site also revealed a significant increase in apoD mRNA and protein levels from 48 h up to 14 days after trauma. Similar to the immunohistochemical results in the cortex, apoD-immunopositive glial and neuronal cells were seen at early and late time points after the impact, respectively. Concurrent hematoxylin and eosin staining provided evidence that apoD expression in cortex is found in degenerating cells. In contrast, hippocampal cells revealed no evidence of cell death up to 14 days after injury.
Several lines of evidence suggest that apoD may play an important role in neuronal degeneration after acute CNS injury. For example, apoD expression has been implicated in excitotoxic damage of CNS neurons induced by kainic acid (Ong et al., 1997). Excessive excitatory amino acid release with subsequent neurotoxicity has been described in experimental models of TBI (for review, see Faden et al., 1989 ; Katayama et al., 1990 ; Nilsson et al., 1990 ; Hayes et al., 1992 ; Palmer et al., 1993 ; Globus et al., 1995) and cerebral ischemia (for review, see Rothman and Olney, 1989). In addition, it is noteworthy that cortical impact injury may produce focal ischemia in the cortex ipsilateral to the injury site (Bryan et al., 1995). Thus, reduced cerebral blood flow may have also contributed to increased excitotoxicity and increased apoD expression in our experiments. Moreover, recent evidence suggests that in addition to sterols and steroid hormones, apoD also binds arachidonic acid (Cabral et al., 1995 ; Patel et al., 1997). Increases in the levels of free fatty acids, particularly stearic and arachidonic acids, with subsequent histopathological changes consistent with cortical and hippocampal damage have been observed after TBI (Dhillon et al., 1997) and cerebral ischemia (Keller et al., 1998) in vivo. Therefore, it seems possible that apoD may indeed have a functional role in binding one or more of its ligands during CNS degeneration following acute experimental brain injury.
The increase in apoD immunoreactivity in the ipsilateral cortex was observed in glial cells and neurons at early and late time points after TBI, respectively. Similar to our results, increased apoD immunoreactivity has also been observed in CNS glia and neurons after excitatory amino acid injury (Ong et al., 1997). Our data suggest that neuronal apoD immunoreactivity is mainly found in cell bodies and apical dendrites of neurons. It is interesting that a similar distribution of apoD immunoreactivity has been described in excitotoxin-induced hippocampal injury in the rat (Ong et al., 1997). Preferential damage of apical dendrites has been described in cerebral ischemia in vivo (Matesic and Lin, 1994), and recent reports have shown that TBI can produce a significant loss of dendritic microtubule-associated protein 2 in the absence of marked axonal changes, especially within the acute and subacute stage after injury (Taft et al., 1992, 1993 ; Hicks et al., 1995 ; Posmantur et al., 1996 a, b ; Kampfl et al., 1997 ; Saatman et al., 1998). Although the precise role of apoD in CNS degeneration is not fully understood, some evidence suggests that during excitotoxin-induced injury, apoD immunoreactivity is differentially expressed in neurons that are ultimately destined to die (Ong et al., 1997). Moreover, a recent report has shown increased levels of apoD in the hippocampus and CSF in Alzheimer's disease (Terrisse et al., 1998). Our concurrent hematoxylin and eosin staining investigations of the ipsilateral cortex following TBI also reveal apoD immunoreactivity at late time points after injury in dark, shrunken, and pyknotic neurons, indicating neuronal death.
Our current data indicate that apoD is also up-regulated in the hippocampus ipsilateral to the injury site. Similar to the immunohistochemical results of the ipsilateral cortex, apoD-immunoreactive hippocampal glial cells and neurons were found at early and late time points after the injury, respectively. In addition, apoD immunoreactivity in the hippocampus was mainly detected in cell bodies and apical dendrites of pyramidal neurons. However, and in contrast to the ipsilateral cortex, our data and previous reports suggest that there is no clear evidence of hippocampal cell death at early and late time points following unilateral cortical impact injury (Newcomb et al., 1997). Therefore, the appearance of apoD-immunopositive cells in an area not associated with cell death suggests that apoD up-regulation may occur in the absence of overt hippocampal neuronal necrosis. Although higher magnitudes of cortical impact injury (Colicos and Dash, 1996 ; Colicos et al., 1996) or lateral fluid percussion injury (McIntosh et al., 1989) have been reported to produce hippocampal cell loss, the injury parameters in the present study did not result in overt hippocampal necrosis at any of the time points examined. It is important that previous studies using central fluid percussion-induced TBI have also shown that neuronal somatodendritic cell damage can occur in the absence of neuronal cell death (Taft et al., 1992, 1993). Moreover, a recent report has also indicated that cortical cells following cortical impact injury eventually die, whereas hippocampal cells, although probably functionally impaired as indicated by increased proteolysis, appear morphologically intact even at later time points following lateral cortical impact injury in vivo (Newcomb et al., 1997). In this regard it is noteworthy that there is some evidence that increased apoD expression may not always be associated with definite neuronal cell death. For example, a recent report by Ong et al. (1999) indicated that a maturation-associated induction of apoD gene expression in CNS neurons had features of viable and not apoptotic or degenerating necrotic neurons. In addition, after a crush injury of rat sciatic nerve there is a moderate increase in apoD expression that appears to be the result of the experimental lesion (Boyles et al., 1990b ; Spreyer et al., 1990). This, however, is followed by an up-regulation of apoD gene expression that is restricted to sites undergoing regeneration, suggesting the possibility that apoD may have a specific role in peripheral nerve regeneration and possibly in CNS maintenance and repair. It is important that it has been shown that apoD binds bilirubin and other heme-related molecules, raising the possibility that apoD may be part of the antioxidant defense system in which the conversion of heme to biliverdin and then to bilirubin serves to protect cells from oxidant damage (Peitsch and Boguski, 1990). A recent report suggested that also apoE has antioxidant capacity and may thus share common mechanistic pathways with apoD (Miyata and Smith, 1996). Oxidative stress may indeed play a role in acute and chronic neurodegeneration in several CNS disorders. For example, some reports suggest that oxygen free radicals and nitric oxide may lead to neuronal degeneration following acute cerebral ischemia and TBI in vivo (Chan et al., 1995 ; Simonian and Coyle, 1996 ; Bidmon et al., 1998). Therefore, one could hypothesize that increased expression of apoD in the hippocampus may be essential for neuroprotection after cortical impact injury.
Our current study did not definitely determine if the enhanced apoD observed immunocytochemically reflects increased biosynthesis and/or impaired cellular trafficking of apoD in glia and neurons following TBI in vivo. In this regard it is noteworthy that in the normal nervous system, apoD is mainly found in glia and in perivascular fibroblasts that surround penetrating leptomeningeal vessels. In contrast, very little apoD is normally found in neurons (Yoshida et al., 1996). ApoD is also a secreted protein from cultured glia, and its secretion is markedly stimulated by oxysterols (Patel et al., 1995). Its role as a transporter for various small hydrophobic ligands (Patel et al., 1997) and the observation that apoD accumulates in neurons during excitotoxic insults (Ong et al., 1997) and in glia and neurons at early and late time points after TBI (present study), respectively, raise the possibility that apoD may traffic between glia and neurons and that an impairment of such trafficking is a marker of neuronal injury.
In summary, our data indicate that apoD may have a differential functional role in the cortex and hippocampus following TBI in the rat. Our data suggest that apoD expression in the cortex may be a marker for neuronal degeneration. In contrast, our results also demonstrate that apoD expression in the hippocampus may not necessarily be indicative of neuronal cell death. However, future studies have to investigate further the molecular mechanisms of apoD expression and the specific role of apoD in CNS degeneration and regeneration following brain injury. This also implicates the need for further studies to determine the functional role of apoD in neuronal injury at late time points following TBI in vivo.
Acknowledgements
This study was supported by grants from the Austrian Science Foundation (FWF ; P12287 MED to A.K.) and the National Institutes of Health (NS-34339 to S.C.P.).




