Suppression of Heme Oxygenase-1 mRNA Expression by Interferon-γ in Human Glioblastoma Cells
Lippincott Williams & Wilkins, Inc., Philadelphia
The present address of Dr. H. Fujita is Department of Hygiene and Preventive Medicine, Hokkaido University School of Medicine, North-15, West-7, Kita-ku, Sapporo 060-8638 Japan.
Abbreviations used: CO, carbon monoxide; HSP-70, heat shock protein-70; IFN-γ, interferon-γ; IL-1β, interleukin-1β; NO, nitric oxide; TNF-α, tumor necrosis factor-α.
Abstract
Abstract: Heme oxygenase is a rate-limiting enzyme in heme catabolism that cleaves heme to form biliverdin, carbon monoxide, and iron. Heme oxygenase-1 is an inducible isozyme and is expressed in many types of cells and tissues. Large amounts of these heme degradation products may be noxious to the host, especially in the brain. We therefore searched for the factors that suppress the expression of heme oxygenase-1. Northern blot analysis showed that treatment with interferon-γ and with interleukin-1β for 24 h decreased the expression levels of heme oxygenase-1 mRNA to ∼20 and ∼50% of the control levels, respectively, in a human glioblastoma cell line, T98G. Treatment with a combination of these two cytokines additively decreased the expression levels of heme oxygenase-1 mRNA. Western blot analysis showed that the expression level of heme oxygenase-1 protein was also decreased by treatment with interferon-γ, but not with interleukin-1β. Moreover, pretreatment with interferon-γ partially suppressed the induction of heme oxygenase-1 mRNA expression caused by either sodium nitroprusside, cadmium, or hemin. These findings raise the possibility that the expression of heme oxygenase-1 is down-regulated by interferon-γ in the nervous system.
Heme oxygenase (EC 1.14.99.3) is a rate-limiting enzyme in heme catabolism that cleaves heme to form biliverdin, carbon monoxide (CO), and iron (Tenhunen et al., 1968). There are two isozymes of heme oxygenase: heme oxygenase-1 and heme oxygenase-2 (Cruse and Maines, 1988; McCoubrey et al., 1992; Shibahara et al., 1993; Shibahara, 1994). Heme oxygenase-1 is an inducible form, and heme oxygenase-2 is a noninducible form. These two isozymes are the products of distinct genes. Recently, a protein that shares ∼90% amino acid identity with heme oxygenase-2 has been identified and termed heme oxygenase-3 (McCoubrey et al., 1997), but its function remains to be investigated. Biliverdin is reduced to bilirubin by biliverdin reductase, and bilirubin may contribute to cellular defense mechanisms against oxidative stresses (Stocker et al., 1987).
Heme oxygenase-1 is expressed ubiquitously in various tissues, and is induced by its own substrate heme and by other various stimulants (for review, see Shibahara, 1994), such as oxidative stress (Keyse and Tyrrell, 1989), nitric oxide (NO) donors (Hara et al., 1996; Takahashi et al., 1996, 1997a), cadmium (K. Takeda et al., 1994), and phorbol ester (Muraosa and Shibahara, 1993). Accumulating evidence indicates the importance of heme oxygenase-1 in the physiology of brain and the pathophysiology of some disorders of the brain. We have shown the expression of heme oxygenase-1 and -2 mRNAs in the human brain and primary brain tumors (Hara et al., 1996; Takahashi et al., 1996). CO, a heme degradation product, has been proposed to act as a neurotransmitter in the brain (Verma et al., 1993; Zhuo et al., 1993). Heme oxygenase-1 was induced in the rat brain by hyperthermia (Ewing and Maines, 1991) and transient forebrain ischemia (A. Takeda et al., 1994). Treatment with NO donors induced the expression of heme oxygenase-1 in human glioblastoma cells (Hara et al., 1996; Takahashi et al., 1996). Finally, recent studies have shown that the expression of heme oxygenase-1 mRNA and protein was increased in the cerebral cortex and cerebral vessels in patients with Alzheimer's disease (Premkumar et al., 1995; Schipper et al., 1995).
The heme degradation products, CO, iron, and bilirubin, have potential cell toxicity if present at high concentrations (Shibahara, 1994). It is therefore likely that increased expression of heme oxygenase-1 is not necessarily beneficial for the host. In contrast to a number of reports showing the induction of heme oxygenase-1, information on the suppression of the expression of heme oxygenase-1 is limited. It has been reported recently that angiotensin II decreased heme oxygenase-1 mRNA levels in a calcium-dependent manner in rat vascular smooth muscle cells (Ishizaka and Griendling, 1997).
During the course of the search for the factors that affect the expression of heme oxygenase-1 in the brain, we have found that treatment with interferon-γ (IFN-γ) decreases the expression levels of heme oxygenase-1 mRNA in cultured T98G human glioblastoma cells. We have also studied the effects of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) on the expression levels of heme oxygenase-1 in T98G cells.
MATERIALS AND METHODS
Materials
Recombinant human IFN-γ (IFN-γ-1a, Imunomax-γ) was a gift from Shionogi Co. (Osaka, Japan). It consists of 146 amino acids without glycosylation, and its specific activity was ∼5 × 106 U/mg of protein. Human IL-1β was a gift from Otsuka Pharmaceutical Co. (Tokushima, Japan). The following were obtained as indicated: human TNF-α from Boehringer Mannheim Biochemica (Mannheim, Germany); a human glioblastoma cell line, T98G, from the American Type Culture Collection (Rockville, MD, U.S.A.); [α-32P]dCTP, ECL Plus western blotting system, and monoclonal anti-72kDa heat shock protein antibody from Amersham Pharmacia Biotech (Tokyo, Japan); Cell Counting Kit-8 from Dojindo (Kumamoto, Japan); restriction endonucleases from Takara Shuzo (Otsu, Japan), Boehringer Mannheim Biochemica, and New England BioLabs (Beverly, MA, U.S.A.).
Cell culture
A human glioblastoma cell line, T98G, was cultivated in minimum essential medium supplemented with 5% fetal bovine serum at 37°C under 5% CO2. To examine the effects of cytokines on the expression of heme oxygenase-1 mRNA, T98G cells were cultivated in fresh medium for 24 h and then exposed to the following three cytokines: IFN-γ (10-300 U/ml), TNF-α (1-30 ng/ml), and IL-1β (1-30 ng/ml). The cells were incubated at 37°C for 3-48 h and were harvested for RNA extraction. In some experiments, the effects of the pretreatment of cytokines on the induction of heme oxygenase-1 by sodium nitroprusside, cadmium, or hemin were examined in T98G cells. T98G cells were exposed to IFN-γ (100 U/ml), TNF-α (20 ng/ml), or IL-1β (10 ng/ml) for 19 h and then treated with sodium nitroprusside (1 mM), cadmium (50 μM), or hemin (50 μM) for 5 h.
Cell viability was assessed using Cell Counting Kit-8. T98G cells were cultivated in fresh medium for 24 h and then exposed to IFN-γ (100 U/ml), TNF-α (20 ng/ml), IL-1β (10 ng/ml), or combinations of two or three of these cytokines. After a 24-h incubation with cytokines, the number of cells was determined according to the manufacturer's protocols.
Northern blot analysis
Total RNA was extracted from cultured cells by the guanidinium thiocyanate-cesium chloride method and was subjected to the northern blot analysis, as previously reported (Takahashi et al., 1996). The northern probe for heme oxygenase-1 mRNA was the XhoI/XbaI fragment (-64/923) derived from the human heme oxygenase-1 cDNA, pHHO1 (Yoshida et al., 1988). The probe for β-actin mRNA was the SmaI/ScaI fragment (124/1,050) derived from a full-length human β-actin cDNA provided by Dr. T. Yamamoto (Gene Research Center, Tohoku University). These probes were labeled with [α-32P]dCTP by the random priming method. Radioactive signals were detected by exposing the filters to x-ray films (X-AR5, Kodak, Rochester, NY, U.S.A.) or with a Bioimage analyzer (BAS 2000, Fuji Film Co., Ltd., Tokyo, Japan).
Western blot analysis
Proteins in T98G cells were extracted and subjected to western blot analysis, as previously reported (Takahashi et al., 1996). Expression of heme oxygenase-1 protein was detected with a rabbit anti-human heme oxygenase-1 antibody (Shibahara et al., 1993) and ECL Plus western blotting system. Expression of heat shock protein-70 (HSP-70) was examined as an internal control using monoclonal anti-72-kDa heat shock protein antibody.
Statistics
The relative expression levels of heme oxygenase-1 mRNA are given as means ± SEM of four independent experiments. The expression levels were compared using one-way analysis of variance or Student's t test, where appropriate. p < 0.05 was considered to be significant.
RESULTS
The dose-response effects of treatment with IFN-γ (10-300 U/ml), TNF-α (1-30 ng/ml), or IL-1β (1-30 ng/ml) for 24 h on the expression of heme oxygenase-1 mRNA are shown in Fig. 1A and B. The expression levels of heme oxygenase-1 mRNA were decreased by treatment with either IFN-γ or IL-1β in T98G glioblastoma cells. The expression levels of heme oxygenase-1 mRNA were decreased by 100 or 300 U/ml IFN-γ to ∼20% of the control and by 3-30 ng/ml IL-1β to ∼50% of the control. In contrast, the expression levels of heme oxygenase-1 mRNA were not significantly changed by treatment with TNF-α. Western blot analysis also showed the decreased expression level of heme oxygenase-1 protein by 24-h treatment with 100 U/ml IFN-γ (∼30% of the control; Fig. 1C), whereas treatment with 20 ng/ml TNF-α or 10 ng/ml IL-1β caused no noticeable change in the expression levels of heme oxygenase-1 protein. Moreover, none of these cytokines affected the expression level of HSP-70 protein.
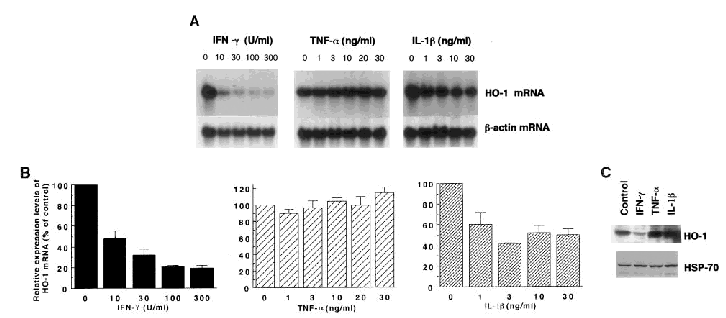
Effects of IFN-γ, TNF-α, and IL-1β on the expression levels of heme oxygenase-1 (HO-1) mRNA (A and B) and protein (C) in T98G glioblastoma cells. A: Northern blot analysis. T98G cells were treated for 24 h with one of these cytokines at the indicated concentrations and harvested. Each lane contained 15 μg of total RNA. The lanes labeled with 0 contained RNA prepared from the control cells treated with distilled water (10 μl per each dish containing 10 ml of medium). The blot for heme oxygenase-1 mRNA was exposed to an x-ray film for 10 days to detect the low levels of mRNA expression. The bottom panels show the expression of β-actin mRNA as an internal control. The data shown are from one of four independent experiments with similar results. B: The relative expression levels of heme oxygenase-1 mRNA (means ± SEM, n = 4). The intensity of hybridization signals in A was quantified with a Bioimage analyzer, and the intensity representing heme oxygenase-1 mRNA was normalized with respect to the intensity for β-actin mRNA in each experiment. The ratio of each normalized value to that of the control (indicated with 0) is shown as the relative expression levels of heme oxygenase-1 mRNA. C: Western blot analysis. Cells were treated for 24 h with distilled water (Control), 100 U/ml IFN-γ, 20 ng/ml TNF-α, or 10 ng/ml IL-1β. Each lane contained 50 μg of protein. The bottom panel shows the expression of HSP-70 as an internal control. The data shown are from one of three independent experiments with similar results.
The expression levels of heme oxygenase-1 mRNA were decreased in a time-dependent manner and reached the nadir ∼24 h after the addition of IFN-γ (100 U/ml) (Fig. 2, left panels). In contrast, the expression levels of heme oxygenase-1 were transiently decreased after the addition of the same volume of distilled water (10 μl of H2O to each dish containing 10 ml of medium) instead of IFN-γ, but returned to the levels of the 0 time control by 15 h of incubation (Fig. 2, right panels).
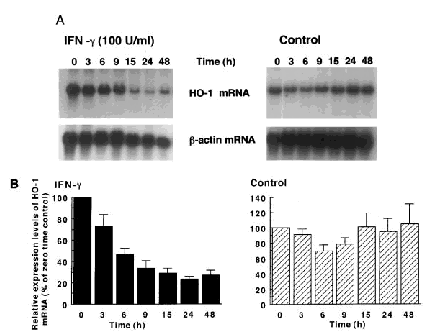
Time course of the effect of IFN-γ on the expression of heme oxygenase-1 (HO-1) mRNA in T98G glioblastoma cells. Cells were treated with IFN-γ (100 U/ml) for the indicated hours (left panels). In the control study (right panels), cells were treated with the same volume of distilled water (10 μl) instead of IFN-γ. A: Northern blot analysis. Each lane contained 15 μg of total RNA. The data shown are from one of four independent experiments with similar results. The blot for heme oxygenase-1 mRNA was exposed to an x-ray film for 3 days. The bottom panels show β-actin mRNA as an internal control. B: The relative expression levels of heme oxygenase-1 mRNA (means ± SEM, n = 4).
A combination of IFN-γ and IL-1β decreased additively the expression levels of heme oxygenase-1 mRNA (Fig. 3A and B). TNF-α remarkably enhanced the suppressive effect of IL-1β on the expression levels of heme oxygenase-1 mRNA, although TNF-α alone showed negligible effects. The addition of TNF-α to IFN-γ or to a combination of IFN-γ and IL-1β caused a further decrease in the expression levels of heme oxygenase-1 mRNA, but these changes were not statistically significant. A low concentration of IFN-γ (3 U/ml) decreased the expression level of heme oxygenase-1 mRNA to ∼60% of the concurrent control, and additively decreased it with TNF-α and/or IL-1β (data not shown). To assess the cell toxicity of cytokines, we then evaluated the viability of T98G cells treated with cytokines. Treatment with IFN-γ (100 U/ml) or TNF-α (20 ng/ml) caused a small decrease in cell number, whereas that with IL-1β (10 ng/ml) caused a small increase in it (Fig. 3C). In contrast, combinations of two or three cytokines caused no noticeable changes in the number of cells, indicating that the suppression of heme oxygenase-1 mRNA expression by cytokines is not likely to be caused by the toxic effects of cytokines.
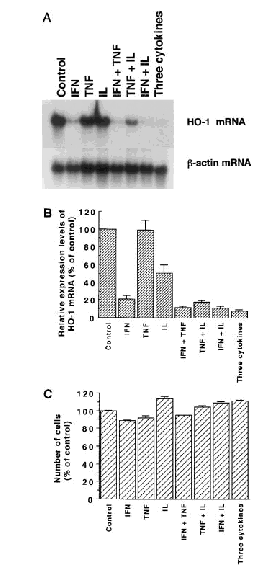
Effects of treatment with various combinations of cytokines on the expression levels of heme oxygenase-1 (HO-1) mRNA (A and B) and on the viability (C) in T98G cells. A: Northern blot analysis. Each lane contained 15 μg of total RNA. The cytokines used were IFN-γ (100 U/ml), TNF-α (20 ng/ml), and IL-1β (10 ng/ml). The blot for heme oxygenase-1 mRNA was exposed to an x-ray film for 10 days. The data shown are from one of four independent experiments with similar results. The bottom panel shows the expression of β-actin mRNA as an internal control. B: The relative expression levels of heme oxygenase-1 mRNA (means ± SEM). C: Viability of T98G cells treated with various combinations of cytokines for 24 h (means ± SEM, n = 4). The data shown represent percentages of the control level (viability of untreated cells).
Pretreatment with IFN-γ reduced the magnitude of induction of heme oxygenase-1 mRNA by sodium nitroprusside, cadmium, or hemin (Fig. 4). Especially, IFN-γ caused a 60% reduction in the sodium nitroprusside-mediated induction of heme oxygenase-1 mRNA expression. Pretreatment with IL-1β also suppressed the induction of heme oxygenase-1 mRNA by sodium nitroprusside, but did not suppress the induction by cadmium or hemin. TNF-α showed no significant effects on the induction of heme oxygenase-1 mRNA.
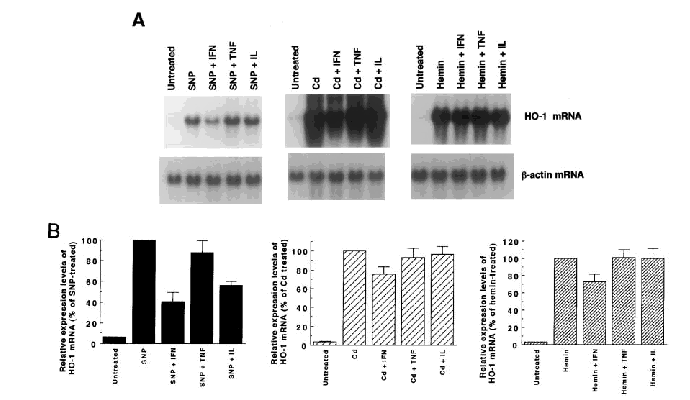
Effects of pretreatment with cytokines on the expression of heme oxygenase-1 (HO-1) mRNA induced by sodium nitroprusside (SNP), cadmium (Cd), and hemin. A: Northern blot analysis. Each lane contained 15 μg of total RNA. T98G cells were pretreated with distilled water (10 μl), IFN-γ (100 U/ml), TNF-α (20 ng/ml), or IL-1β (10 ng/ml). After 19 h of pretreatment, the cells were exposed to 1 mM SNP, 50 μM Cd, or 50 μM hemin for 5 h and then harvested. Heme oxygenase-1 mRNA was hardly detectable in untreated cells, because of the short exposure time to the x-ray film (14-24 h) in this series of experiments. The data shown are from one of four independent experiments with similar results. The bottom panels show the expression of β-actin mRNA as an internal control. B: The relative expression levels of heme oxygenase-1 mRNA (means ± SEM, n = 4).
DISCUSSION
The present study has shown for the first time the suppression of heme oxygenase-1 mRNA expression by IFN-γ or IL-1β in T98G glioblastoma cells. In particular, IFN-γ showed a potent suppressive effect on the expression of heme oxygenase-1 mRNA, even at low concentrations that are comparable to the levels in cerebrospinal fluid from patients with encephalitis (Lebon et al., 1988). These observations were initially unexpected, because the treatment with a combination of IFN-γ and lipopolysaccharide remarkably increased heme oxygenase-1 mRNA levels in some myelomonocytic leukemia cell lines, such as a human monocytic cell line, THP-1 (Muraosa and Shibahara, 1993). This increased expression of heme oxygenase-1 mRNA was accompanied by the functional differentiation of THP-1 cells into macrophage-like cells. In T98G glioblastoma cells, however, lipopolysaccharide (100 ng/ml) did not noticeably change the expression levels of heme oxygenase-1 mRNA and the IFN-γ-mediated reduction of heme oxygenase-1 mRNA expression (data not shown). In addition, no morphological changes were observed in T98G cells by treatment with lipopolysaccharide, IFN-γ, or IL-1β (data not shown). There appears to be a difference in the induction of heme oxygenase-1 by cytokines among various cell types. In this regard, IFN-γ (100 U/ml) decreased the expression levels of heme oxygenase-1 mRNA to ∼70% of concurrent control in the primary culture of normal human astrocytes (unpublished observations). The number of T98G cells was not greatly changed by treatment with these cytokines. It is therefore unlikely that the suppression caused by these cytokines represents the cytotoxic effects of IFN-γ, TNF-α, or IL-1β. In fact, our previous study has shown that IFN-γ increased the secretion of adrenomedullin, a potent vasodilator peptide, and the expression of its mRNA in T98G cells (Takahashi et al., 1997b).
Heme oxygenase-1 has been established as a stress protein (for review, see Shibahara, 1994). Especially, rat heme oxygenase-1 is a heat shock protein (Shibahara et al., 1987). On the other hand, high concentrations of the heme degradation products, CO, iron, and bilirubin, have potential cell toxicity (Shibahara, 1994). We therefore hypothesize the presence of regulatory systems that down-regulate the expression of heme oxygenase-1. In this context, we have reported recently that human heme oxygenase-1 is not induced by heat shock in contrast to the rat counterpart, possibly due to the silencing effect of a certain sequence, located downstream from the heat shock element in the human heme oxygenase-1 gene (Okinaga et al., 1996). This may represent a regulatory mechanism that prevents the noxious induction of heme oxygenase-1 by high fever in the brain. The suppression of heme oxygenase-1 expression by IFN-γ and IL-1β shown in this study may represent another type of regulatory system that modulates the heme oxygenase-1 expression.
It has been reported recently that peripheral administration of endotoxin induced the expression of IL-1β, inducible NO synthase, and heme oxygenase-1 mRNAs in the liver of rats (Jacobs et al., 1997; Satta et al., 1998). In contrast, peripheral administration of endotoxin at high doses could not induce the expression of heme oxygenase-1 mRNA in rat hypothalamus, although it induced the expression of IL-1β and inducible NO synthase mRNAs there (Satta et al., 1998). It is therefore difficult to explain the lack of induction of heme oxygenase-1 in the hypothalamus even after the highest dose of endotoxin. The findings in the present study raise the possibility that IFN-γ and/or IL-1β may suppress the induction of heme oxygenase-1 after endotoxin administration in the hypothalamus. It is also noteworthy that either IFN-γ or IL-1β suppressed partially the induction of heme oxygenase-1 by sodium nitroprusside in T98G cells.
IFN-γ is produced by T lymphocytes and natural killer cells. In addition, both neuronal and glial sources of IFN-γ have been proposed (Kuchinke et al., 1995). IFN-γ is known to connect the immune system functionally with the CNS. For example, IFN-γ induced the production of TNF-α by microglial cells (Meda et al., 1995). TNF-α and IL-1β are produced not only by activated macrophages, but also by astrocytes and microglial cells in the brain (Lieberman et al., 1989). TNF-α and IL-1β have various biological actions in the CNS, such as raising temperature and inducing anorexia (Dascombe et al., 1989; Kapas et al., 1992). Moreover, IFN-γ and other cytokines increased the expression of inducible NO synthase in microglial cells and the production of NO (Boje and Arora, 1992; Chao et al., 1992). NO is known to cause neuronal cell death (Chao et al., 1992) and may induce heme oxygenase-1 expression (Hara et al., 1996; Takahashi et al., 1996, 1997a). Thus, IFN-γ may play an important role in regulating the expression of inducible NO synthase and heme oxygenase-1.
In summary, we have shown the suppression of heme oxygenase-1 mRNA expression by IFN-γ or IL-1β in T98G glioblastoma cells. This may constitute an important part of the regulatory network that prevents the noxious induction of heme oxygenase-1, in particular, in the human brain.




