Up-Regulation of Protein Chaperones Preserves Viability of Cells Expressing Toxic Cu/Zn-Superoxide Dismutase Mutants Associated with Amyotrophic Lateral Sclerosis
The present address of Dr. W. Bruening is Fox Chase Cancer Center, Philadelphia, PA, U.S.A.
The present address of Dr. B. Giasson is Center for Neurodegenerative Research, Department of Pathology and Laboratory Medicine, Univcersity of Pennsylvania School of Medicine, Philadelphia, PA, U.S.A
Abbreviations used : FALS, familial amyotrophic lateral sclerosis ; FITC, flourescein isothiocyanate ; HSP, heat shock protein ; SOD-1, Cu/Zn-superoxide dismutase.
Abstract
Abstract : Mutations in the Cu/Zn-superoxidedismutase (SOD-1) gene underlie some familial cases of amytotrophic lateral sclerosis, a neurodegenerative disorder charactreized by loss of cortical, brainstem, and spinal motor nrurons. We present evidence that SOD-1 mutants alter the activity of molecular chaperones that aid in proper protein folding and targeting of abnormal proteins for degradation. In a cultured cell line (NOH 3T3), resistance to mutant SOD-1 toxicity correlated with increased overall chaperoning activity (measured by the ability of cytosolic extracts to prevent heat denaturation of catalase) as well as with up-regulation of individual chaperones/stress proteins. In transgenic mice expressing human SOD-1 with the G93A mutation, chaperoning activity was decreased in lumbar spinal cord but increased or unchanged in clinically unaffected tissues. Increasing the level of the stress-inducible chaperone 70-kDa heat shock protein by gene transfer reduced formation of mutant SOD-containing proteinaceous aggregates in cultured primary motor neurons expressing G93A SOG-1 and prolonged their survival. We propose that insufficiency of molecular chaperones may be directly involved in loss of motor neurons in this disease.
Cu/Zn-superoxide dimutase (SOD-1) is a solubl cytoplasmic enzyme that catalyzes the dismutation of the toxic superioxide anion (O2-), generated as a byproduct of normal metabolism, to yield hydrogen peroxide (H2O2) and molecular oxygen. Over 60 different mutations of SOD-1 have been identified in chromosome 21-linked familial amyotroiphic lateral sclerosis (FALS) (Rosen et al., 1993 ; Pramatarova et al., 1994, 1995 ; Orrell et al., 1997 ; Shaw et al., 1998). Although varying decreases in SOD-1 enzyme activity have been measured in cells derived from these patients (for review, see de Belleroche et al., 1996), most evidence indicates that a toxic gain of function rather than decreased dismutase activity is responsible for motor neuron loss. Mice lacking expression of SOD-1 as a result of targeted gene disruption are healthy (Reaume et al., 1996) ; however, mice expressing mutant SOD-1 develop ALS-like pathological changes and die in midlife despite a 5- to 10-fold increase in dismutase activity in tissue extracts from many of these animals (Gurney et al., 1994 ; Wong et al., 1995).
The mechanism of this toxic gain of function and how it causes preferential loss of motor neurons are uncertain. In cell-free systems, a number of SOD-1 mutants exhibited enhanced peroxidative activity relative to wild-type enzyme in two studies (Wiedau-Pazos et al., 1996 ; Yim et al., 1996 ; Singh et al., 1998) and altered metal-binding properties (Carri et al., 1994 ; Lyons et al., 1996 ; Crow et al., 1997 ; Ogawa et al., 1997 ; Hart et al., 1998), suggesting that production of damaging free radicals may be responsible for the toxicity. Supporting a pathogenic role for free radicals are the following observations : Vitamin E slightly delayed the onset of symptoms in G93A SOD-1 transgenic mice (Gurney et al., 1996) ; free radical scavengers reduced the toxicity of V148G murine- SOD-1 in PC12 cells (Ghadge et al., 1997) ; fibroblasts from FALS patients are more sensitive to free radical-generating agents in culture (Aguirre et al., 1998) ; some oxidative DNA damage and increased nitrotyrosine have been detected in spinal cord of FALS patients (Abe et al., 1997 ; Ferrante et al., 1997) ; and free, but not protein, nitrotyrosine was increased in G37R transgenic mice (Bruijn et al., 1997a ; Ghadge et al., 1997). On the other hand, it remains to be established that generalized free radical-induced damage is primarily responsible for motor neuron loss in FALS. Significant generation of hydroxyl radicals was not evident in tissue from G85R SOD-1 transgenic mice (Bruijn et al., 1997a) ; no significant increase in protein carbonyls has been detected in FALS patients (Bowling et al., 1993 ; Shaw et al., 1995 ; Ferrante et al., 1997) ; and free radical scavengers failed to protect cultured motor neurons expressing mutant SOD-1 (Roy et al., 1998).
There is evidence that mutations lead to misfolding and destabilization of the protein. X-ray crystallographic studies indicate that several mutations alter conserved interactions within the protein that are critical to the conformation of the active channel (Deng et al., 1993). Studies in vitro and in cultured cell lines have indicated that several mutated SOD-1 proteins are less stable than wild-type SOD-1 : (a) They denature under milder conditions and have shorter half-lives than does wild-type protein, particularly mutants that are associated with a severe disease phenotype (Borchelt et al., 1994 ; Nakano et al., 1996 ; Marklund et al., 1997 ; Ogawa et al., 1997). (b) Proteinaceous aggregates of SOD-1 have been found in the cytoplasm of cultured primary motor neurons expressing several different SOD-1 mutants (Durham et al., 1997) as well as in motor neurons and astrocytes of mice expressing the G85R SOD-1 transgene (Bruijn et al., 1997b) and FALS patients at autopsy (Shibata et al., 1996). Cultured motor neurons expressing SOD-1 mutants that are positive for markers of apoptosis are those that contain aggregates, indicating that aggregate formation is an important event in mutant SOD-1 toxicity (Durham et al., 1997). (c) Inhibiting the activity of the 26S proteasome (the ubiquitin and ATP-dependent proteinase complex) resulted in accumulation of mutated SOD-1 (Hoffman et al., 1996) ; thus, mutant SOD-1 enzyme is degraded by a pathway generally involved in proteolysis of denatured or misfolded protein, a pathway that in part is regulated by molecular chaperones (for review, see Parsell and Lindquist, 1994 ; Sherman and Goldberg, 1996).
We postulated that the availability of molecular chaperones to facilitate refolding or proteolysis of mutant or damaged protein would be a key factor in the ability of cells to defend themselves against the toxic properties of mutant SOD-1. The studies reported here demonstrate that expression of individual chaperone proteins and chaperoning activity is modified in cells and tissues expressing mutant SOD-1 and that increasing expression of 70-kDa heat shock protein (HSP70) by gene transfer protects cultured motor neurons from mutant SOD-1 toxicity.
MATERIALS AND METHODS
Plasmid constructs
Isolation of wild-type human SOD-1 cDNA, introduction of FALS-associated point mutations by site-specific mutagenesis, and cloning into the episomal expression vector pCEP4 have been described previously (Durham et al., 1997). For studies in NIH 3T3 cells, cDNAs were subcloned into the BamHI/HindIII restriction sites of the vector pcDNA-3 (Invitrogen, Carlsbad, CA, U.S.A.). This vector contains the neomycin resistance gene for selection of G418-resistant stable cell lines. In both pCEP4 and pcDNA-3, transcription is driven by the cytomegalovirus enhancer/promoter. The cDNA encoding the major murine inducible HSP70 was a kind gift of Dr. Simon Wing (McGill University). The StuI fragment, containing the entire coding region of HSP70, was subcloned into the expression vector pcDNA3.
Transfection of NIH 3T3 cells
NIH 3T3 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (GibcoBRL, Burlington, Ontario, Canada). Cells were transfected with the indicated plasmids using Lipofectamine (GibcoBRL) ; efficency of transient transfection was ~80%. Stable cell lines were selected with G418 (Geneticin ; Gibco-BRL), and individual clones were isolated and amplified using standard techniques. For mock transfections, cells were transfected under the same conditions but using empty pcDNA3 expression vector.
Transgenic mice
SOD-1 transgenic mice were purchased from the Jackson Laboratory (Bar Harbor, ME, U.S.A.). Wild-type SOD-1-expressing mice are strain B6SJL-TgN(SOD1)2Gur ; G93A SOD-1-expressing mice are strain B6SJL-TgN(SOD1-G93A)1Gurdl ; the nontransgenic mice used were noncarrier littermates of the transgenic mice. All procedures with experimental animals were approved by the McGill University Animal Care Committee and were carried out according to the guidelines of the Canadian Council on Animal Care.
Western blots
Forty-eight hours after transfection, cells were lysed in RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Nonidet 40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate). Insoluble debris was pelleted, and the protein concentration of the supernatant was determined using a bicinchoninic acid/copper assay (Bio-Rad, Mississauga, Ontario, Canada). Twenty-microgram samples were electrophoresed on standard sodium dodecyl sulfate polyacrylamide gels and transferred to Immobilon-P (MIllipore, Mississauga, Ontario, Canada). Blots were probed with the indicated antibodies using standard techniques and developed with enhanced chemiluminescence reagents (Amersham, Oakville, Ontario, Canada).
Chaperoning activity assays
Assays were performed essentially as described by Hook and Harding (1997), based on the ability of chaperones present in cytosolic extracts to prevent heat denaturation of an exogenously added protein (catalase in these experiments). In brief, cells or tissues were lysed in 50 mM HEPES (pH 7.4), 100 mM KCl, 5% glycerol, 1 mM MgCl2, and 0.1% NP40. Twenty micrograms of extract was mixed with 1 ml of a solution of 200 μg/ml catalase (Sigma) dissolved in phosphate-buffered saline. The reaction mix was heated at 55°C, and aggregation of the denatured proteins was followed by measuring light scattering at 360 nm with a spectrophotometer.
Primary culture model
Primary cultures of dissociated spinal cord dorsal root ganglia were prepared from E13 mouse embryos as previously described (Doroudchi and Durham, 1996 ; Durham et al., 1997). Following dissociation in trypsin, cells were plated at a density of 180,000 or 200,000/well in four-well Nunclon culture dishes containing round glass 13-mm coverslips (Fisher Scientific, Montreal, Quebec, Canada) coated with poly-D-lysine (Sigma Chemical Co., St. Louis, MO, U.S.A.) plus Matrigel basement membrane matrix (Collaborative Research, Bedford, MA, U.S.A.). The culture medium was minimal essential medium enriched with 5 g glucose and supplemented with 2% horse serum, 10 μg/ml bovine serum albumin, 26 ng/ml selenium, 20 μg/ml triiodothyronine, 10 μg/ml insulin, 200 μg/ml transferrin, 32 μg/ml putrescine, 9.1 ng/ml hydrocortisone, 13 ng/ml progesterone, and 10 ng/ml nerve growth factor. Triiodothyronine was purchased from Calbiochem (San Diego, CA, U.S.A.) ; all other growth factors and hormones were purchased from Sigma. On day 4-6, cultures were treated with 1.4 μg/ml cytosine-β-D-arabinoside (Calbiochem) to minimize growth of nonneuronal cells. The cultures were maintained at 37°C in 5% CO2. Cultures were used in experiments 4-7 weeks following dissociation to allow for differentiation of the motor neuronal phenotype. Plasmid constructs (in concentrations yielding expression of transgenic protein in >90% of cells) were microinjected into motor neuronal nuclei along with the fluorescent marker dextran-fluorescein isothiocyanate (dextran-FITC ; 15 mg/ml ; Molecular Probes, Eugene, OR, (U.S.A.). In preliminary experiments, expression of transgenic protein was verified on days 3 and 12 following microinjection by immunolabeling with mouse antibody specific for human SOD-1 (clone SD-G6 ; Sigma) as previously described (Durham et al., 1997). Viability was assessed microscopically each day by counting the number of motor neurons containing the marker. The number of neurons was normalized to the number present on day 1 following microinjection to exclude any neurons dying from the injection procedure. previous experiments demonstrated that viability of neurons injected with “empty” expression vector was similar to that of dextran-FITC-injected and noninjected neurons. Expression of transgenic protein in remaining neurons was verified at the end of the experiment (day 9) by immunolabeling with anti-SOD1 or with mouse anti-HSP70 (W27 ; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). To determine the percentage of motor neurons in which mutant SOD-1 had formed cytoplasmic aggregates, cultures were fixed on day 3 following microinjection and labeled with Sigma anti-human SOD-1.
RESULTS AND DISCUSSION
Wild-type human SOD-1 cDNA and cDNAs encoding human SOD-1 with FALS-associated point mutations [glycine 93 to alanine (G93A) and glycine 41 to serine (G41S)] were cloned into the expression vector pcDNA3 under control of the cytomegalovirus enhancer/promoter (see Materials and Methods). When NIH 3T3 cells were transiently transfected with these expression vectors, high levels of human SOD-1 were obtained (Fig. 1A). Cultures transiently transfected with plasmid encoding wild-type SOD-1 appeared similar to mock-transfected cultures, but NIH 3T3 cells expressing mutant SOD-1 failed to proliferate and slowly disintegrated over the course of a few days (Fig. 1B). This toxicity made it difficult to obtain clones that had stably integrated the plasmid ; however, a few G418-resistant clones were isolated that stably expressed mutant SOD-1 at levels two- to threefold more abundant than the endogenous murine SOD-1 (Fig. 1C). Thus, although mutant SOD-1 is toxic to naive cells, NIH 3T3 cells can adapt to its presence.
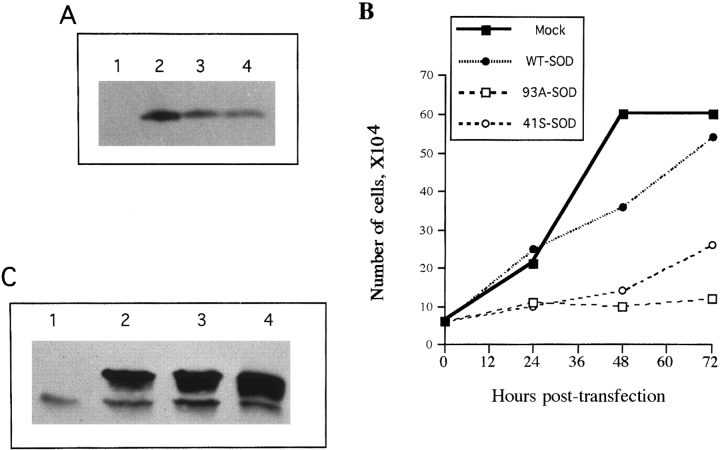
Adaptation of NIH 3T3 cells to expression of mutant SOD-1. A : Western blot demonstrating human SOD-1 expression resulting from transient transfection of NIH 3T3 cells with the following : lane 1, mock transfection ; lane 2, wild-type human SOD-1 ; lane 3, SOD-1 with the G93A mutation ; lane 4, SOD-1 with the G41S mutation. The blot was probed with anti-SOD-1 antibody (Binding Site) ; the murine SOD-1 band is not visible at this exposure level. B : Mutant SOD-1 is toxic. At various times after transfection, the cells were trypsinized and viable cells were counted (based on trypan blue dye exclusion). The inset legend indicates with which expression vector the cells were transfected. Efficiency of transient transfection was 80%. Shown is a typical experiment. Each point represents the average of duplicate cell counts in the same culture. The experiment was repeated in three independent trials. That reduction in the number of cells was due to cell death rather than decreased proliferation was verified by quantitating the number of nonviable cells (trypan blue positive) and cells entering S phase (5-bromo-2′-deoxyuridine staining kit ; Calbiochem). C : NIH 3T3 cells can adapt to the presence of mutant SOD-1. Western blot showing SOD-1 expression in three G418-resistant clones stably expressing similar amounts of human SOD-1. Lane 1, NIH 3T3 cells ; lane 2, clone expressing wild-type SOD-1 (WT-1) ; lane 3, clone expressing G93A SOD-1 (93A-1) ; lane 4, clone expressing 41S SOD-1 (41S-1). The upper band is human SOD-1 and the lower band is mouse SOD-1, labeled by anti-SOD-1 from the Binding Site.
NIH 3T3 cells surviving expression of mutant SOD-1 had altered chaperone activity ; i.e., cytosolic extracts prepared from clones that stably expressed mutant SOD-1 had a greater ability to prevent protein aggregation resulting from heat-induced denaturation in vitro than did untransfected NIH 3T3 cells or clones stably expressing wild-type SOD-1 (Fig. 2A). Complementing the increase in total chaperoning activity, clones that stably expressed mutant SOD-1 had chronically elevated levels of HSP70, HSP27, and αB-crystallin (Fig. 2B), key proteins with chaperoning function whose synthesis is induced as part of the stress response in cells (for review, see Parsell and Lindquist, 1994, Buchner, 1996).

Chaperoning activity is increased in NIH 3T3 cells that stably express mutant SOD-1. A: Extracts were prepared from clones of NIH 3T3 cells that stably express mutant SOD-1. The extracts were assayed for their ability to prevent aggregation of heat-denatured proteins (catalase); protein aggregation is pro-portional to optical density (O.D.) measured at 360 nm. Shown are means 6 SD of results from at least three experiments. B: Clones of NIH 3T3 cells that stably express mutant SOD-1 have elevated levels of HSPs, as demonstrated on these western blots. Strips were probed with an antibody that recognizes only the inducible form of HSP70 (K20; Santa Cruz Biotechnology), antibody to HSP27 (M20; Santa Cruz Biotechnology), and anti-aB-crystallin (SPA223; StressGen). Lane 1, NIH 3T3 cells; lane 2, clone WT-1 stably expressing wild-type SOD-1; lane 3, clone 93A-1 stably expressing G93A SOD-1; lane 4, clone 41S-1 sta-bly expressing G41S SOD-1.
Chaperoning activity also was measured in tissues from G93A mutant SOD-1 transgenic mice at 5 months of age, just prior to the expected onset of clinical symptoms. Extracts of liver showed elevated activity in comparison with liver from nontransgenic or wild-type SOD-1-expressing mice (Fig. 3A). Other tissues not affected clinically in ALS (kidney, testes, mucles) also had elevated or unchanged chaperoning activity (not shown). On the other hand, extracts of lumbar spinal cord (containing the motor neurons innervating the lower limbs) from G93A SOD-1 transgenic animals were deficient in total chaperoning activity as compared with spinal cord extracts from nontransgenic and wild-type SOD-1-expressing mice (Fig. 3A). This difference was not secondary to cell loss because similar results were obtained using tissues from very young animals (4 weeks old) before the period of neuronal loss (Dal Canto and Gurney, 1995), nor was it due to differences in SOD-1 levels, which were similar in both liver and spinal cord (Fig. 3C).
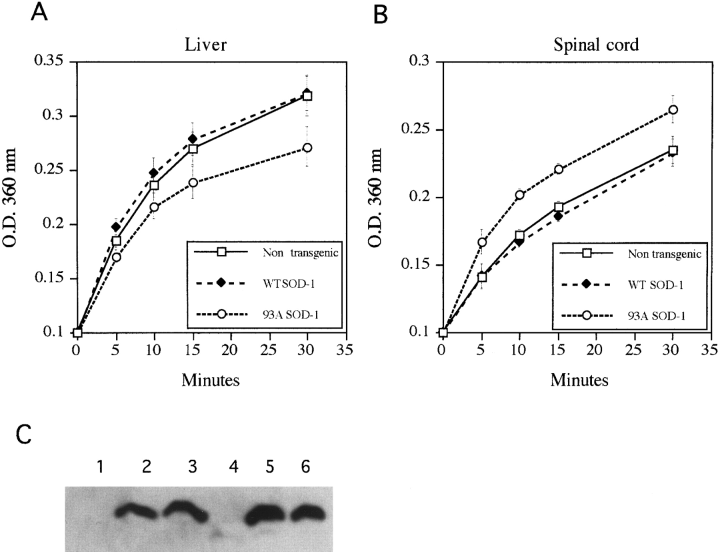
Chaperoning activity is altered in tissues from mutant SOD-1-expressing transgenic mice. Extracts were prepared from various tissues of 5-month-old transgenic mice expressing wild-type or mutant (G93A) SOD-1 and nontransgenic litter-mates and were evaluated for their ability to prevent aggregation of heat-denatured proteins. Shown is the increase in optical density (O.D.) over time representing aggregation of catalase in the presence of liver extract (A) and extract of lumbar spinal cord (B). Also shown (C) is a western blot probed with anti-SOD (Binding Site), demonstrating similar SOD-1 levels in the different tissues. Lanes 1 and 4, nontransgenic mice ; lanes 2 and 5, transgenic mice expressing wild-type SOD-1 ; lanes 3 and 6, transgenic mice expressing G93A SOD-1. Lanes 1-3 are from spinal cord and lanes 4-6 from liver.
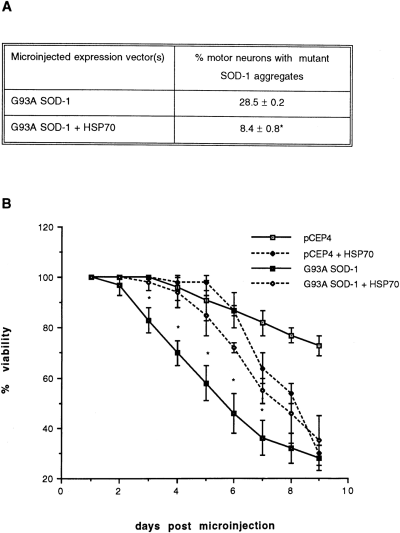
Increasing expression of HSP70 by gene transfer protects cultured motor neurons from mutant SOD-1 toxicity. HSP70 in the expression vector pcDNA3 and G93A SOD-1 in pCEP4 were co-injected into nuclei of primary motor neurons at concentrations of 5 or 10 and 200 μg/ml, respectively. A : Formation of mutant SOD-1 aggregates was significantly reduced by co-expression of HSP70 (co-injection of 10 μg/ml HSP70 expression vector). On day 3, cultures were immunolabeled with antibody specific to human SOD-1 (Sigma), and the percentage of cells in which most of the immunoreactive mutant SOD-1 was localized in punctate aggregates was counted. B : Co-expression of HSP70 (injection of 5 μg/ml expression vector) significantly prolonged viability of motor neurons expressing mutant SOD-1, despite exerting its own toxic effects at later time points. Shown are means ± SD for results obtained from three to six cultures per treatment group, 15-40 motor neurons per culture. *Significant difference between G93A SOD-1 alone and G93A SOD-1 + HSP70, p < 0.05.
In contrast to cell lines expressing mutant SOD-1, no obvious up-regulation of the stress proteins HSP70, HSP27, or αB-crystallin was detected in spinal cord of G93A SOD-1 transgenic mice compared with wild-type SOD-1 transgenic mice, either by western blotting of homogenates or by immunhistochemistry of Vibratome sections (not shown). However, this does not rule out altered expression of other proteins with chaperoning activity.
These results suggest the following hypothesis to explain how mutations of SOD-1 may lead to neurodegeneration. SOD-1 is an abundant protein that, when structurally altered as a direct consequence of mutations or subsequent posttranslational modification, becomes denatured at an elevated rate. Due to SOD-1's abundance, this is likely to divert a considerable portion of the chaperone systems toward refolding the denatured SOD-1 or targeting it for degradation by proteasomes. The depletion of the pool of freechaperones would render cells expressing mutant SOD-1 particularly vulnerable to physiological and environmental stresses, would reduce the efficiency of many cellular processes dependent on chaperone proteins, and might allow aggregates of denatured protein to form. Since most cells are able to up-regulate the levels of many chaperone proteins, they remain relatively healthy. There is evidence that motor neurons may be relatively deficient in their ability to induce certain HSPs with chaperoning activity upon suffering stresses (Manzerra and Brown, 1992 ; Morrison-Bogorad et al., 1994 ; Satoh and Kim, 1995). This may render them particularly vulnerable to damage from mutant SOD-1. They are also subjected to physiological stresses, including a high-level excitatory input, which would add to the overall burden on chaperone systems.
If depletion of free chaperones is a significant factor in the toxicity of mutant SOD-1, increasing the levels of chaperone proteins should be protective. This was tested in a primary culture model of chromosome 21-linked FALS developed in our laboratories (Durham et al., 1997). The G93A mutant was expressed in primary motor neurons of dissociated cultures of murine spinal cord by intranuclear microinjection of expression vector. This results in formation of cytoplasmic aggregates of SOD-1 and loss of viability over a 2-week period. Co-injection of expression vector encoding the murine inducible HSP70 reduced the toxicity of mutant SOD-1, dramatically decreased the percentage of motor neurons with SOD-1, aggregates (Fig. 4A), and prolonged survival (Fig. 4B). However, this protection was limited to 7 days by toxicity due to expression of HSP70.
Using three different experimental models, we have demonstrated that increased expression of proteins with chaperione activity protects cells from the toxicity of SOD-1 mutants. That these proteins have reduced stability and altered conformation and are prone to aggregation suggests that direct interactions of chaperones with mutant protein would occur. Molecular chaperones with vital for many processes, including resistance to stress and proper protein folding, intracellular transport, and degradation. Diversion of the chaperones to protect the cell from expression of mutant SOD-1 would have grave consequences, particularly if additional demands were exerted by environmental stresses. Motor neurons would be particularly vulnerable to mutant SOD-1 for a variety of reasons : They express high levels of SOD-1 ; induction of protective chaperone proteins in response to stress may be less robust in motor neurons relative to other cells ; and they are subjected to additional physiological stresses including their high level of excitatory synaptic input.
Acknowledgements
This research was supported by the Amyotrophic Lateral Sclerosis Society of Canada and the Muscular Dystrophy Association of Canada (H.D.D.), the Muscular Dystrophy Association-USA (H.D.D. and D.A.F.), NIHRO1 (D.A.F.), Le Fonds pour la Formation de Chercheurs et l'Aide à la Recherche (J.R.), and the Medical Research Council of Canada (W.E.M.). H.D.D. is a Killam Scholar.




