Differential Phosphorylation of Syntaxin and Synaptosome-Associated Protein of 25 kDa (SNAP-25) Isoforms
The present address of Dr. C. Risinger is Karolinska Institute, Department of Neuroscience, The Nobel Institute for Neurophysiology, Stockholm, Sweden.
Abbreviations used : aa, amino acids ; BSA, bovine serum albumin ; CaMKII, Ca2+ - and calmodulin-dependent protein kinase II ; CKII, casein kinase II ; GST, glutathione S-transferase ; NSF, N-ethylmaleimide-sensitive factor ; PKA, cyclic AMP-dependent protein kinase ; PKC, Ca2+ - and phospholipid-dependent protein kinase ; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis ; SNAP, soluble NSF attachment protein ; SNAP-25, synaptosome-associated protein of 25 kDa ; SNARE, SNAP receptor ; t-SNARE, target membrane SNARE ; VAMP, vesicle-associated membrane protein ; v-SNARE, vesicle membrane SNARE.
Abstract
Abstract : The synaptic plasma membrane proteins syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) are central participants in synaptic vesicle trafficking and neurotransmitter release. Together with the synaptic vesicle protein synaptobrevin/vesicle-associated membrane protein (VAMP), they serve as receptors for the general membrane trafficking factors N-ethylmaleimide-sensitive factor (NSF) and soluble NSF attachment protein (α-SNAP). Consequently, syntaxin, SNAP-25, and VAMP (and their isoforms in other membrane trafficking pathways) have been termed SNAP receptors (SNAREs). Because protein phosphorylation is a common and important mechanism for regulating a variety of cellular processes, including synaptic transmission, we have investigated the ability of syntaxin and SNAP-25 isoforms to serve as substrates for a variety of serine/threonine protein kinases. Syntaxins 1A and 4 were phosphorylated by casein kinase II, whereas syntaxin 3 and SNAP-25 were phosphorylated by Ca2+ - and calmodulin-dependent protein kinase II and cyclic AMP-dependent protein kinase, respectively. The biochemical consequences of SNARE protein phosphorylation included a reduced interaction between SNAP-25 and phosphorylated syntaxin 4 and an enhanced interaction between phosphorylated syntaxin 1A and the synaptic vesicle protein synaptotagmin I, a potential Ca2+ sensor in triggering synaptic vesicle exocytosis. No other effects on the formation of SNARE complexes (comprised of syntaxin, SNAP-25, and VAMP) or interactions involving n-Sec1 or α-SNAP were observed. These findings suggest that although phosphorylation does not directly regulate the assembly of the synaptic SNARE complex, it may serve to modulate SNARE complex function through other proteins, including synaptotagmin I.
Protein phosphorylation represents an important mechanism for regulating synaptic activity. Activity-dependent enhancement of synaptic transmission, i.e., long-term potentiation, is believed to be related to learning and memory. Cyclic AMP-dependent protein kinase (PKA), casein kinase II (CKII), Ca2+ - and phospholipid-dependent protein kinase (PKC), and Ca2+ - and calmodulin-dependent protein kinase II (CaMKII) have all been implicated in different aspects of long-term changes in synaptic efficacy (Charriaut-Marlangue et al., 1991 ; Silva et al., 1992a,b ; Abeliovich et al., 1993 ; Alberini et al., 1995 ; Wang and Kelly, 1995 ; Mayford et al., 1996). These kinases have also been shown to phosphorylate a wide variety of presynaptic proteins. The most thoroughly characterized example is the phosphorylation of synapsin I by CaMKII (Greengard et al., 1993). This reaction results in the release of synapsin I from both actin filaments and synaptic vesicles and may regulate the availability of vesicles for the docking and fusion events that result in neurotransmitter release. These vesicle docking and fusion reactions are also potential targets for phosphorylation-mediated regulation of synaptic transmission.
Transport vesicle docking and membrane fusion are fundamental steps in membrane trafficking throughout the secretory and endocytic pathways. A mechanistic model for these events, known as the soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein (SNAP) receptor (SNARE) hypothesis (Söllner et al., 1993), proposes that a vesicle membrane protein [vesicle membrane SNARE (v-SNARE)] binds to cognate proteins on the target membrane [target membrane SNARE (t-SNAREs)] to form a complex that is recognized and dissociated by the general membrane trafficking factors α-SNAP and NSF. The presynaptic plasma membrane proteins syntaxin 1 and synaptosome-associated protein of 25 kDa (SNAP-25) are t-SNAREs, whereas the synaptic vesicle protein synaptobrevin/vesicle-associated membrane protein (VAMP) is a v-SNARE. Although the precise role of the synaptic SNARE proteins in synaptic vesicle docking and fusion remains to be established, the fact that each is a target for one or more clostridial neurotoxins emphasizes their functional importance (Huttner, 1993 ; Tonello et al., 1996). Furthermore, the SNARE proteins are central components in a network of regulatory interactions that include the Ca2+ sensor synaptotagmin (Bennett et al., 1992b ; Li et al., 1995 ; Schiavo et al., 1997), synaptophysin (Calakos and Scheller, 1994 ; Edelmann et al., 1995 ; Washbourne et al., 1995), n-Sec1/Munc-18 (Hata et al., 1993 ; Pevsner et al., 1994), complexins (McMahon et al., 1995), voltagegated Ca2+ channels (Bennett et al., 1992b ; Yokoyama et al., 1997), munc-13 (Betz et al., 1997), Hrs-2 (Bean et al., 1997), and the r-sec6/sec8 complex (Hsu et al., 1996).
The synaptic t-SNAREs syntaxin 1 and SNAP-25 are each members of larger protein families. Syntaxin 1 (A and B) and SNAP-25 are highly expressed in neurons (Oyler et al., 1989 ; Bennett et al., 1992b), whereas syntaxins 2-4 (Bennett et al., 1993b) and SNAP-23 (a SNAP-25 isoform ; Ravichandran et al., 1996) have wider tissue distributions. Furthermore, the syntaxin isoforms display differential localizations in gastric parietal cells (Peng et al., 1997), pancreatic acinar cells (Gaisano et al., 1996), and polarized Madin-Darby canine kidney cells (Low et al., 1996). Additional variants of syntaxin 2 (Bennett et al., 1993b), syntaxin 3 (Ibaraki et al., 1995), SNAP-25 (Bark, 1993 ; Risinger and Larhammar, 1993), and SNAP-23 (Mollinedo and Lazo, 1997) are generated by alternative splicing or gene duplication. These observations are consistent with a role for t-SNARE isoforms in the regulation of a variety of membrane trafficking pathways.
To provide insight into the regulation of the vesicle docking and fusion machinery, as well as the molecular mechanisms underlying synaptic plasticity, we have investigated the potential phosphorylation of six different t-SNAREs by four serine/threonine kinases. Our results demonstrate differential phosphorylation patterns for the different t-SNARE isoforms and selective modulation of interactions between syntaxin 4 and SNAP-25 and between syntaxin 1A and synaptotagmin I.
MATERIALS AND METHODS
Materials
PKA, CKII, and molecular biology reagents were obtained from New England Biolabs (Beverly, MA, U.S.A.), CaMKII was a gift from Dr. M. Kennedy (California Institute of Technology, Pasadena, CA, U.S.A.), and PKC was obtained from Promega (Madison, WI, U.S.A.). Calmodulin was acquired from Calbiochem (San Diego, CA, U.S.A.). Glutathione-agarose beads, histone III-SS, bovine serum albumin (BSA), O-phospho-dl-serine, O-phospho-dl-threonine, O-phospho-dl-tyrosine, 1,2-dioleoyl-sn-glycerol, ninhydrin, and thrombin were obtained from Sigma (St. Louis, MO, U.S.A.). Prestained protein molecular weight marker and protein A-agarose were obtained from GibcoBRL (Gaithersburg, MD, U.S.A.), phosphatidylserine was from Avanti Polar Lipids (Alabaster, AL, U.S.A.), ATP was from Boehringer Mannheim (Indianapolis, IN, U.S.A.), and [γ-32P]ATP and [35S]dATP were from Du Pont-NEN (Boston, MA, U.S.A.). 125I-labeled rabbit antimouse and goat anti-rabbit antibodies were from ICN Biochemicals (Costa Mesa, CA, U.S.A.). DNA sequencing reagents were obtained from USB (Cleveland, OH, U.S.A.) and Pfu DNA polymerase was from Stratagene (La Jolla, CA, U.S.A.). Electrophoresis reagents and DC protein assay kit were from Bio-Rad Laboratories (Hercules, CA, U.S.A.). Affinity-purified rabbit polyclonal antibodies generated against SNAP-25 and syntaxins 2-4 (Berkeley Antibody Co., Richmond, CA, U.S.A.) were prepared as previously described (Gaisano et al., 1996 ; Peng et al., 1997). Anti-SNAP-25 monoclonal SMI-81 was obtained from Sternberger Monoclonals (Baltimore, MD, U.S.A.), whereas monoclonal antibodies against syntaxin 1A (HPC-1 ; Barnstable et al., 1985) and synaptotagmin I (M48 ; Matthew et al., 1981) were gifts of Dr. C. Barnstable (Yale University, New Haven, CT, U.S.A.) and Dr. L. Reichardt (University of California, San Francisco, CA, U.S.A.), respectively.
Expression of recombinant proteins
Constructs for the bacterial expression of glutathione S-transferase (GST) fusion proteins incorporating the full cytoplasmic, the amino-terminal, or the carboxyl-terminal domains of rat syntaxin 1A [amino acids (aa) 4-267, 4-193, 194-267], rat syntaxin 2C (aa 4-264 ; previously called syntaxin 2″ ; see Bennett et al., 1993b), rat syntaxin 3A (aa 4-264, 4-187, 188-264 ; identical to syntaxin 3 ; see Ibaraki et al., 1995), rat syntaxin 4 (aa 4-274, 4-196, 197-274), mouse SNAP-25 (aa 1-206), rat VAMP-2 (aa 1-94), and rat synaptotagmin I (aa 96-421, 96-265, 248-421) were generated in the pGEX-KG vector (Guan and Dixon, 1991) as previously described (Bennett et al., 1993a ; Calakos et al., 1994 ; Hao et al., 1997). A construct encoding a GST fusion protein incorporating an amino-terminal fragment of SNAP-25 (aa 1-95) was prepared by digesting the pGEX-KG/full-length SNAP-25 vector with Hin-dIII (which cuts both in the SNAP-25 insert and at the 3′ end of the pGEX-KG polylinker) followed by religation. GST fusion proteins for SNAP-23 (aa 1-211), and carboxyl-terminal SNAP-25 fragments (aa 141-206 and 125-206) were generated by PCR amplification of the indicated sequences (using SNAP-23 or SNAP-25 DNAs as templates) followed by inframe insertion of the PCR products into the pGEX-KG vector.
Expression and purification of GST fusion proteins were performed according to standard protocols (Guan and Dixon, 1991 ; Bennett et al., 1993a). For soluble protein production, thrombin cleavage was done either in 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2.5 mM CaCl2, and 0.1% β-mercaptoethanol or directly in kinase-specific phosphorylation buffers (see below). His6-α-SNAP was expressed and purified as previously described (Söllner et al., 1993). For binding assays using phosphorylated GST fusion proteins, the immobilized proteins were preincubated for 2-4 h at 4°C in binding assay buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2.5 mM CaCl2, 0.1% Triton X-100, 0.1% gelatin, and 0.1% BSA) and then washed three times with 500 μl of HKAT buffer (10 mM HEPES-KOH, pH 7.5, 140 mM potassium acetate, 1 mM MgCl2, 0.1 mM EGTA, and 0.1% Triton X-100) containing 0.1% gelatin and once with HKAT buffer alone. The samples were then suspended in kinase-specific phosphorylation buffers and phosphorylated (see below).
Immunoprecipitation
A synaptic membrane fraction (LP1) was prepared from rat brain as previously described (Bennett et al., 1992a), and protein concentration was determined by Bio-Rad DC protein assay using BSA (Pierce, Rockford, IL, U.S.A.) as a standard. Solubilization and immunoprecipitations of LP1 (2 mg/ml) were done in 2% Triton X-100 with monoclonal antibodies specific either to SNAP-25 (SMI-81) or to syntaxin 1A (HPC-1 ; Barnstable et al., 1985) as described (Bennett et al., 1993a). Antibodies were prebound to protein A-agarose beads (0.4 μl of antibody/μl of beads) before incubation with 0.3-0.5 mg of solubilized LP1 (25 μl of beads/mg of LP1 protein). After incubation for 2-4 h at 4°C, the beads were washed two times in HKAT buffer containing 0.1% gelatin and once in HKAT buffer alone. Beads with bound proteins were suspended in PKA buffer or CKII buffer and phosphorylated (see below).
Phosphorylation reactions
Phosphorylation reactions contained 0.25 U/μl PKA (catalytic subunit), 20 U/μl, CKII, 12 μg/ml CaMKII, or 0.4 U/μl PKC in a total volume of 50 μl with the following reaction buffers : PKA, 50 mM Tris-HCl, pH 7.5, and 10 mM MgCl2 ; CKII, 20 mM Tris-HCl, pH 7.5, 50 mM KCl, and 10 mM MgCl2 ; CaMKII, 50 mM Tris-HCl, pH 7.5, 20 mM MgCl2, 1 mM CaCl2, 0.4 mM EGTA, 10 μg/ml calmodulin, and 10 μM dithiothreitol ; and PKC, 20 mM Tris-HCl, pH 7.5, 20 mM MgCl2, 1 mM CaCl2, 0.5 mM EGTA, 0.4 μg of 1,2-dioleoyl-sn-glycerol, and 12 μg of phosphatidylserine. All kinases were diluted in 20 mM Tris-HCl, pH 7.5, containing 0.1 mg/ml BSA, except CaMKII, which was diluted in 10 mM Tris-HCl, pH 7.5, containing 0.2 mg/ml BSA. Samples to be phosphorylated were preincubated for 1 min at 30°C before addition of 10 μl of [γ-32P]ATP (200 μM ; 40 μCi/ml) to initiate the reactions. The reactions were continued for 30 min at 30°C. For gel analysis, the reactions were terminated by adding 10 μl of 6× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. For binding assays, the reactions were terminated by incubation on ice before subsequent use (see below). All nonphosphorylated and phosphorylated proteins were treated identically (except that 20 mM Tris-HCl, pH 7.5, was used instead of [γ-32P]ATP) before being used in binding assays.
Phosphoamino acid analysis
Identification of phosphorylated amino acid residues was done by a combination of previously described methods (Cooper et al., 1983 ; Kamps and Sefton, 1989 ; Sefton, 1995). Proteins were phosphorylated, separated by SDS-PAGE, and transferred to Immobilon filter (Millipore, Bedford, MA, U.S.A.). Phosphorylated proteins detected by autoradiography were excised and, after the filters were rewet for 1 min in methanol followed by 1 min in water, hydrolyzed in 200 μl of 6 M HCl for 2 h at 110°C. The resulting hydrolysate was dried under vacuum and dissolved in 6 μl of water. Samples of the hydrolysate (2 μl) and amino acid standards (1 μl of 1 mg/ml O-phospho-dl-serine, O-phospho-dl-threonine, or O-phospho-dl-tyrosine) were applied on a thin-layer cellulose plate (100 μm, 20 × 20 cm) and separated by chromatography in a buffer containing isobutyric acid/0.5 M ammonium hydroxide (5:3). After chromatography, the amino acid standards were visualized by staining with 0.2% ninhydrin and the 32P-labeled amino acids were detected by autoradiography.
Site-directed mutagenesis
SNAP-25 mutant T138A was generated in a two-step PCR reaction using Pfu polymerase with mutated oligonucleotides (threonine to alanine, i.e., codon change from ACA to GCA) as primers and full-length SNAP-25 cDNA as template. The first step included two PCR reactions, one for amplification of aa 135-206 with mutagenic primer A (5′-CCGCAGGGTA-GCAAACGTA-3′) and a vector specific 3′ primer and one for amplification of aa 1-140 with mutagenic primer B (5′-ATCGTTTGCTACCCTGCGG-3′) and a vector specific 5′ primer. The second step was done by combining the products from the first reactions and reamplifying with the vector specific primers only. PCR products were resolved by 1% agarose gel electrophoresis and purified with QIAEX II kit (Qiagen, Chatsworth, CA, U.S.A.). The final PCR product was cloned into the BamHI site of pGEX-KG, and the mutation was verified by DNA sequencing (Sanger et al., 1977).
Affinity chromatography assay
GST fusion proteins (SNAP-25, synaptotagmin I, or n-Sec1) bound to glutathione-agarose beads or His6-α-SNAP bound to Ni beads were incubated with different combinations of syntaxins 1A, 2C, 3A, or 4, synaptotagmin I, or VAMP-2 for 4 h at 4°C as previously described (Hao et al., 1997). Either phosphorylated or nonphosphorylated t-SNAREs were used. After the beads were washed, bound proteins were detected and quantified by SDS-PAGE and western blotting as described below.
Ligand overlay assay
SNAP-25 (100 ng ; phosphorylated or nonphosphorylated) was separated by SDS-PAGE, transferred to nitrocellulose filter as described (Hao et al., 1997), and incubated with 2 μg/ml of either syntaxin 1A, or syntaxin 1A in combination with VAMP-2. Bound syntaxin was detected with monoclonal antibody HPC-1.
SDS-PAGE and western blotting
Protein samples were separated on 12% SDS-polyacrylamide gels (Laemmli, 1970). For phosphorylation reactions, gels were stained with Coomassie Blue, dried, and exposed to x-ray film. The stoichiometry of phosphate incorporation was determined by phosphorimaging (Molecular Dynamics, Palo Alto, CA, U.S.A.) using [γ-32P]ATP as a standard. For in vitro binding assays, bound proteins were transferred electrophoretically (100 Vh) to nitrocellulose membranes (Micron Separations, Westborough, MA, U.S.A.) in bicarbonate buffer (10 mM NaHCO3 and 3 mM Na2CO3). The membranes were stained with Ponceau S (to verify equal protein loading) and then incubated with blocking buffer (5% milk in phosphate-buffered saline containing 0.05% Tween-20). All subsequent incubations were performed in blocking buffer or phosphate-buffered saline containing 0.05% Tween-20. The following primary antibodies were used : monoclonal antibodies against SNAP-25 (SMI-81 ; 1 : 10,000), synaptotagmin I (M48 ; 1 : 10,000), and syntaxin 1A (HPC-1 ; 1 : 10,000), or affinity-purified polyclonal antibodies against SNAP-25 (1 : 5,000), syntaxin 2C (1 : 1,000), syntaxin 3A (1 : 1,000), and syntaxin 4 (1 : 1,000). For quantitative analysis, the blots were probed with 125I-labeled secondary antibodies and the signals detected by autoradiography and phosphorimaging. For nonquantitative analysis, peroxidase-labeled secondary antibodies were used with a chemiluminescence detection system (Du Pont-NEN).
RESULTS
In vitro phosphorylation of t-SNAREs
To identify possible kinase targets among the t-SNAREs, we examined the ability of bacterially expressed cytoplasmic domains of syntaxins 1A, 2C, 3A, and 4, and full-length SNAP-25 and SNAP-23 to serve as substrates for PKA, CKII, PKC, and CaMKII. For each substrate/kinase combination, the phosphorylation reaction products were resolved by SDS-PAGE and analyzed by autoradiography to detect 32P incorporation (Fig. 1, upper panels) and Coomassie Blue staining to monitor protein amounts (Fig. 1, lower panels). The highest levels of phosphorylation were observed for PKA with SNAP-25 as substrate, CKII with syntaxins 1A and 4 as substrates, and CaMKII with syntaxin 3A as substrate. CKII also phosphorylated syntaxin 1B (data not shown). Quantification of phosphorylation stoichiometry revealed that each of these reactions resulted in 0.4-0.9 mol of phosphate incorporated/mol of substrate protein. PKA phosphorylation of a GST-SNAP-25 fusion protein immobilized on glutathione-agarose increased the phosphate incorporation up to 1 mol/mol of protein. The enhanced phosphorylation was not due to phosphorylation of GST, because no phosphate was associated with the GST remaining on the glutathione-agarose bead following elution of the SNAP-25 with thrombin (data not shown). Low levels of phosphorylation (stoichiometry : <0.1 mol of phosphate/mol of substrate) were observed with syntaxins 1A and 2C phosphorylated by PKC or CaMKII and with syntaxins 2C and 3A phosphorylated by CKII. SNAP-23 was not detectably phosphorylated by any of the kinases tested.
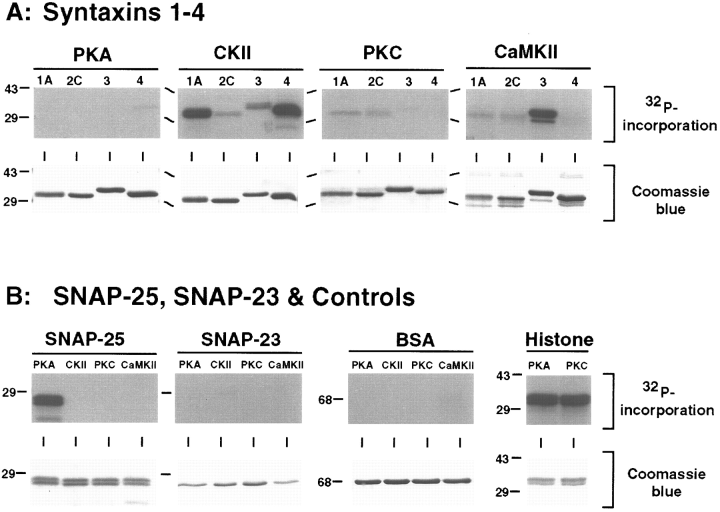
Phosphorylation of t-SNAREs with PKA, CKII, CaMKII, and PKC. Phosphorylated proteins (prepared as described in Materials and Methods) were separated by 12% SDS-PAGE, stained with Coomassie Blue (lower panels), and exposed to x-ray film (upper panels). A : Phosphorylation of 50 pmol of syntaxins 1-4. Each gel shows results for one kinase and four syntaxin isoforms (1A, 2C, 3A, and 4). B : Phosphorylation of 50 pmol of SNAP-25 and 30 pmol of SNAP-23. Each gel shows results for one substrate and four kinases (PKA, CKII, PKC, and CaMKII). Negative controls (2 μg of BSA) and positive controls for PKA and PKC (2 μg of histone III-SS) are also shown. Molecular masses are indicated by numbers (in kDa) and/or dashes to the left of each gel.
Phosphorylation site mapping
To characterize further the robust phosphorylation reactions (CKII/syntaxin 1A, CaMKII/syntaxin 3A, CKII/syntaxin 4, and PKA/SNAP-25) and identify potential phosphorylation sites, the capacity of syntaxin and SNAP-25 deletion mutants to serve as kinase substrates was examined. As shown in Fig. 2, the syntaxin isoforms examined are phosphorylated primarily within their amino-terminal domains. Stoichiometry calculations revealed that the amino-terminal fragments of syntaxins incorporate 10-fold more phosphate than the corresponding carboxyl-terminal fragments. Additional syntaxin 1A deletion mutants localized the CKII phosphorylation site(s) to between aa 8 and 76 (data not shown). Of the SNAP-25 fragments examined, only the carboxyl-terminal fragment including aa 125-206 was phosphorylated by PKA. It is interesting that a SNAP-25 carboxyl-terminal fragment encompassing aa 141-206 was not a PKA substrate, suggesting that the phosphorylation site is located between residues 125 and 141. To identify the amino acid residue(s) phosphorylated in each reaction, phosphoamino acid analysis was performed by acidic hydrolysis followed by TLC. As shown in Fig. 3A, threonine residues are primarily phosphorylated in syntaxin 3A, syntaxin 4, and SNAP-25 by CaMKII, CKII, and PKA, respectively, whereas CKII phosphorylates syntaxin 1A on both serine and threonine residues.
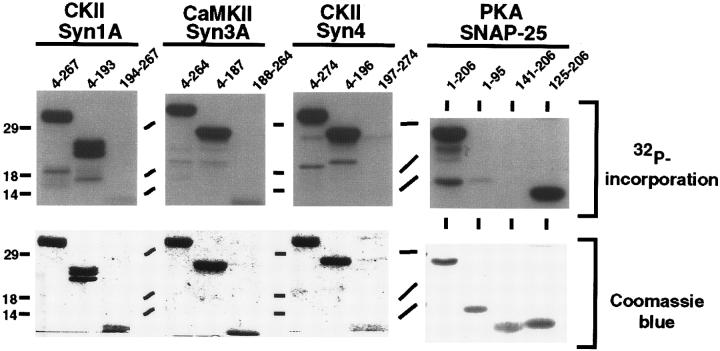
Phosphorylation of t-SNAREs deletion mutants. Full-length and deletion mutants of syntaxins (Syn1A, 3A, and 4 ; 50 pmol of full cytoplasmic and amino-terminal domains, and 10-20 pmol of carboxyl-terminal domains) and SNAP-25 (50 pmol of each domain) were phosphorylated as described in Materials and Methods. The reactions were separated by 12% SDS-PAGE, stained with Coomassie Blue (lower panels), and exposed to x-ray film (upper panels). The amino acid boundaries of the substrates are indicated by the numbers above each lane. Each gel shows one identified substrate/kinase pair. Molecular masses are indicated by numbers (in kDa) and/or dashes to the left of each gel.
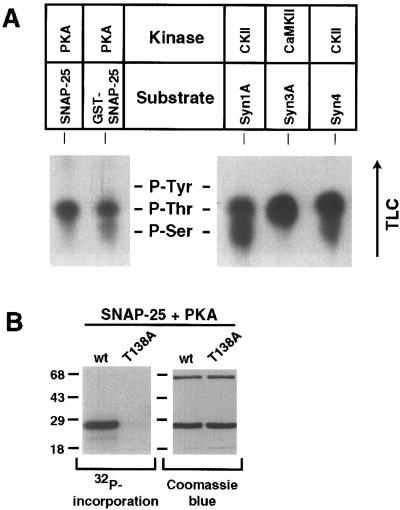
Phosphoamino acid analysis of phosphorylated t-SNAREs and identification of SNAP-25 phosphorylation site. A : Autoradiograph of TLC-separated phosphoamino acids derived from the indicated kinase/substrate combinations. The positions of unlabeled phosphoamino acid standards (P-Tyr, phosphotyrosine ; P-Thr, phosphothreonine ; P-Ser, phosphoserine) are marked. Chromatography direction is indicated by the arrow. B : PKA phosphorylation of wild type (wt ; identical to the full-length construct used in all other experiments) and T138A mutant SNAP-25. Each protein (50 pmol) was phosphorylated by PKA, separated by 12% SDS-PAGE, stained with Coomassie Blue (right panel), and exposed to x-ray film (left panel). Molecular masses are indicated by numbers (in kDa) and/or dashes to the left of each gel.
Computer predictions of potential phosphorylation sites within these proteins were performed with a motif program in the GCG package (University of Wisconsin). We have identified several potential phosphorylation sites for the syntaxin isoforms (see Discussion), but only one (Thr138) for SNAP-25. To determine whether Thr138 corresponds to the PKA phosphorylation site, site-directed mutagenesis was used to generate a SNAP-25 mutant in which the threonine at position 138 was replaced by an alanine (T138A). As shown in Fig. 3B, the SNAP-25 T138A mutation effectively eliminated phosphorylation by PKA, suggesting that Thr138 is the primary site in SNAP-25 phosphorylated by PKA.
Phosphorylation of native SNAP-25 and syntaxin 1
To determine whether native SNAP-25 and syntaxin 1 are substrates for PKA and CKII, respectively, complexes containing syntaxin 1 and SNAP-25 were immunoprecipitated from detergent extracts of rat brain membranes and analyzed by in vitro phosphorylation. As shown in Fig. 4, native SNAP-25 was efficiently phosphorylated following treatment of an anti-SNAP-25 immunoprecipitate with PKA. The level of phosphate incorporation was ~0.25 mol/mol of SNAP-25, suggesting that this protein may be an effective kinase substrate in vivo. Similarly, the syntaxin 1 recovered in an anti-syntaxin 1 immunoprecipitation was phosphorylated by CKII. It is interesting that the SNAP-25 recovered by immunoprecipitation with an anti-syntaxin 1 antibody, corresponding to SNAP-25 in binary or ternary SNARE complexes, was poorly phosphorylated. Following western blot analysis to account for the differential recovery of SNAP-25 in the two immunoprecipitates, the stoichiometry of SNAP-25 phosphorylation in the complex recovered with anti-syntaxin was estimated to be fourfold lower than that in the anti-SNAP-25 immunoprecipitate.
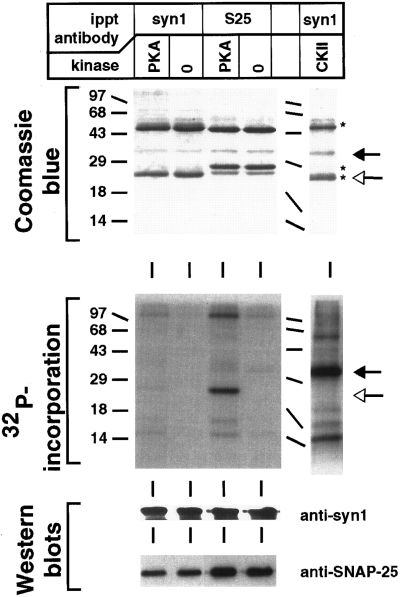
Phosphorylation of native SNAP-25 and syntaxin 1. Native syntaxin 1 and SNAP-25 were recovered from solubilized brain membranes by immunoprecipitation (ippt) with anti-SNAP-25 (S25) or anti-syntaxin 1 (syn1) antibodies. The immunoprecipitates were then subjected to phosphorylation in the presence or absence of either PKA (immunoprecipitate from 0.3 mg of membrane protein) or CKII (immunoprecipitate from 0.5 mg of membrane protein). The phosphorylation reactions were then separated by 12% SDS-PAGE and processed either by Coomassie Blue staining (upper panel) and exposure to x-ray film (middle panel), or by transfer to nitrocellulose and detection of syntaxin 1 and SNAP-25 by western blotting (lower panel). The western blots were developed with an enhanced chemiluminescence detection system. Open and filled arrows indicate the positions of SNAP-25 and syntaxin 1A, respectively. Asterisks indicate the heavy and light chains of the immunoprecipitating antibodies. Molecular masses are indicated by numbers (in kDa) and/or dashes to the left of each gel.
This result suggests that either Thr138 of SNAP-25 is inaccessible in native SNARE complexes or that it is already phosphorylated. To distinguish between these possibilities, the effect of syntaxin 1A and VAMP-2 on the phosphorylation of recombinant SNAP-25 was examined. As shown in Fig. 5, addition of syntaxin 1A or syntaxin 1A and VAMP-2 reduced the PKA-mediated phosphorylation of SNAP-25. The level of inhibition was >30% for the combination of syntaxin 1A and VAMP-2. Because it is likely that not all of the SNAP-25 in these soluble protein mixtures is associated with syntaxin and/or VAMP, we also examined the phosphorylation of recombinant SNAP-25 recovered by affinity chromatography on GST-syntaxin 1A beads. Consistent with the immunoprecipitation result, the phosphorylation of SNAP-25 that is associated with syntaxin 1A was reduced approximately fourfold relative to uncomplexed SNAP-25 analyzed in the same experiment (data not shown). Together, these results indicate that SNARE complex formation (either binary or ternary) reduces the availability of the PKA phosphorylation site in SNAP-25.
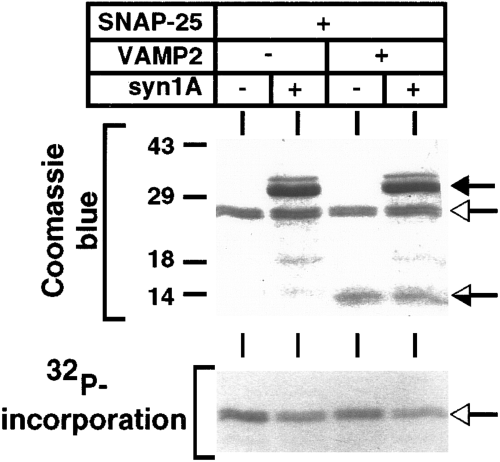
Effect of SNARE complex formation on SNAP-25 phosphorylation. SNAP-25 (50 pmol) alone or complexed with syntaxin 1A (100 pmol) and/or VAMP-2 (50 pmol) was subjected to phosphorylation with PKA. The reactions were separated by 12% SDS-PAGE, stained with Coomassie Blue (upper panel), and exposed to x-ray film (lower panel). The open arrows indicate the position of SNAP-25, and the filled and half-filled arrows indicate the positions of syntaxin 1A and VAMP-2, respectively. Molecular masses are indicated by numbers (in kDa) and dashes to the left of the gel.
Effect of phosphorylation on protein complex assembly
We next investigated the effects of syntaxin and SNAP-25 phosphorylation on the protein-protein interactions that contribute to SNARE complex assembly. First, we examined the binding properties of SNAP-25 following phosphorylation with PKA. Ternary SNARE complex assembly was monitored by the formation of heat-labile SDS-resistant complexes (Hayashi et al., 1994). As shown in Fig. 6A, phosphorylated SNAP-25 was incorporated into SDS-resistant ternary complexes when combined with syntaxin 1A and VAMP-2. The efficiency of incorporation of PKA-phosphorylated SNAP-25 into complexes was comparable to that of both nonphosphorylated SNAP-25 and T138A mutant SNAP-25. These results suggest that the phosphorylation state of SNAP-25 does not regulate ternary SNARE complex assembly and that Thr138 is not essential for complex formation or stability. Next we examined whether phosphorylation of SNAP-25 influenced its binary interactions with syntaxin 1A, VAMP-2, or synaptotagmin I. GST-SNAP-25 fusion proteins were immobilized on beads and subjected to either phosphorylation with PKA or a mock phosphorylation reaction (no ATP added). As shown in Fig. 6B, soluble syntaxin 1A, VAMP-2, and synaptotagmin bound equally well to both the phosphorylated and nonphosphorylated SNAP-25. Similar results were obtained when SNAP-25 interactions were examined with a ligand overlay assay (data not shown ; see Materials and Methods). Thus, SNAP-25 phosphorylation by PKA failed to generate significant effects on binary or ternary protein-protein interactions.
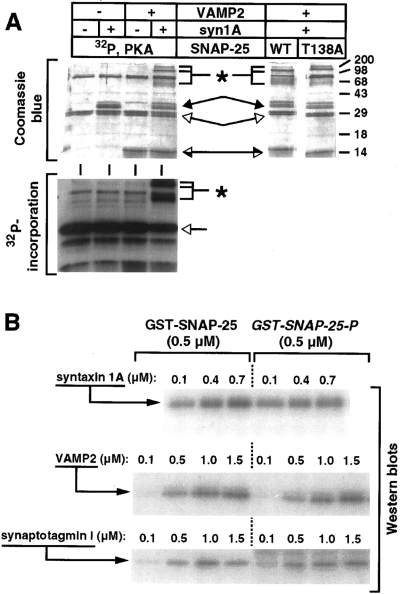
Analysis of binary and ternary complexes formed with phosphorylated SNAP-25. A : SNARE complexes were formed with the indicated combinations of syntaxin 1A (60 pmol), VAMP-2 (160 pmol), and either PKA-phosphorylated SNAP-25 (70 pmol ; left panels) or nonphosphorylated SNAP-25 (WT) and T138A mutant SNAP-25 (70 pmol each) (right panel). To detect SDS-resistant SNARE complexes, the samples were only heated to 37°C before SDS-PAGE. The samples were separated by 12% SDS-PAGE, stained with Coomassie Blue (upper panels), and exposed to x-ray film (lower panel). The open arrows indicate the position of SNAP-25, and the filled and half-filled arrows indicate the positions of syntaxin 1A and VAMP-2, respectively. SDS-resistant SNARE complexes are marked with asterisks. Molecular masses are indicated by numbers (in kDa) and/or dashes to the right of each gel. B : In vitro binding assays were performed with immobilized nonphosphorylated and phosphorylated (P) GST-SNAP-25 in combination with the indicated concentrations of soluble syntaxin 1A, VAMP-2, or synaptotagmin I as described in Materials and Methods. Bound proteins were resolved by 12% SDS-PAGE, transferred to nitrocellulose, and visualized by western blotting with the indicated antibodies using 125I-labeled secondary antibody for detection. Quantification of the western blot signal in this and similar experiments yielded the following levels of binding to phosphorylated SNAP-25 relative to nonphosphorylated SNAP-25 : syntaxin 1A, 1.0 ± 0.24 (n = 7 data points) ; VAMP-2, 1.0 ± 0.14 (n = 5 data points) ; and synaptotagmin, 0.9 ± 0.31 (n = 4 data points).
We next investigated the potential effects of phosphorylation on protein-protein interactions involving soluble recombinant syntaxin isoforms. Syntaxins 1A and 4 were phosphorylated with CKII, whereas syntaxin 3A was phosphorylated with CaMKII. In each case, the phosphorylated and nonphosphorylated syntaxins were incubated with potential binding partners immobilized on beads, and interactions were monitored by western blotting. The potential interacting partners examined included GST-SNAP-25, GST-n-Sec1, GST-synaptotagmin I, and His6-α-SNAP. In most cases, phosphorylation of syntaxins had no significant effect on binding (data not shown). However, two interactions were consistently influenced by syntaxin phosphorylation : the interaction of syntaxin 4 with SNAP-25 and the interaction of syntaxin 1A with synaptotagmin I. As shown in Fig. 7A, phosphorylated syntaxin 4 exhibited decreased binding to SNAP-25 relative to the nonphosphorylated control. No such decrease was observed with phosphorylated syntaxin 3A (Fig. 7A) or syntaxin 1A (data not shown). Quantification of this (Fig. 7B) and similar experiments indicated that phosphorylation of syntaxin 4 reduced binding to SNAP-25 by an average of 35%.
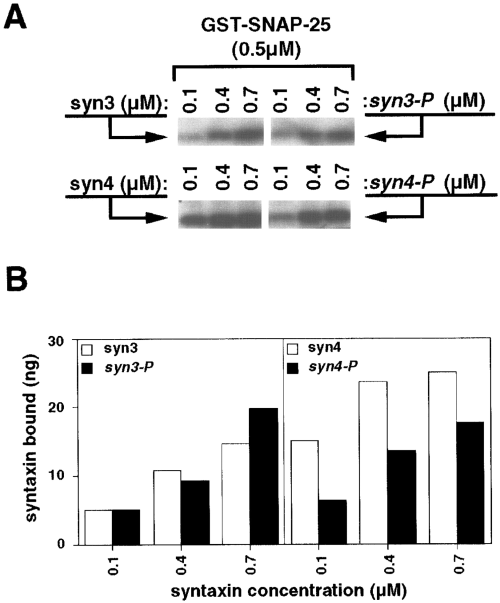
Reduced binding of phosphorylated syntaxin 4 to SNAP-25. A : In vitro binding assays were performed with immobilized GST-SNAP-25 in combination with the indicated concentrations of soluble syntaxin 3 (syn3) or syntaxin 4 (syn4). The syntaxin 3 and 4 proteins used were either nonphosphorylated or phosphorylated (P) with CaMKII or CKII, respectively. Bound proteins were resolved by 12% SDS-PAGE, transferred to nitrocellulose, and visualized by western blotting with the indicated antibodies using 125I-labeled secondary antibody for detection. B : Phosphorimager-derived quantification of the binding interactions shown in A. The western blot signals were converted to nanogram values by comparison with a standard curve generated with known amounts of syntaxins 3 and 4. The relative levels of phosphorylated versus nonphosphorylated syntaxin binding to SNAP-25 were calculated from this and similar experiments. For phosphorylated syntaxin 4, binding averaged 0.65 ± 0.15 (n = 11 data points from three experiments) relative to the nonphosphorylated form, whereas the relative binding of phosphorylated syntaxin 3 averaged 1.1 ± 0.26 (n = 6 data points from two experiments).
In contrast to the inhibition of binding observed between phosphorylated syntaxin 4 and SNAP-25, the CKII phosphorylation of syntaxin 1A potentiated its interaction with the cytoplasmic domain of synaptotagmin I (Fig. 8A). To determine the domain of synaptotagmin responsible for this effect, fragments of synaptotagmin corresponding to the entire cytoplasmic domain (encompassing both C2-homologous domains ; C2A&B), the first C2-homologous domain (C2A), and the second C2-homologous domain (C2B) were tested for their ability to bind syntaxin 1A. As shown in Fig. 8B, the interaction of syntaxin 1A with the C2B domain of synaptotagmin is selectively potentiated by phosphorylation. Quantification of these results demonstrated that the potentiation was greater than twofold for both the full cytoplasmic domain and the C2B domain (Fig. 8C). Little or no potentiation was observed with the C2A domain in either the presence (Fig. 8) or absence (data not shown) of Ca2+.
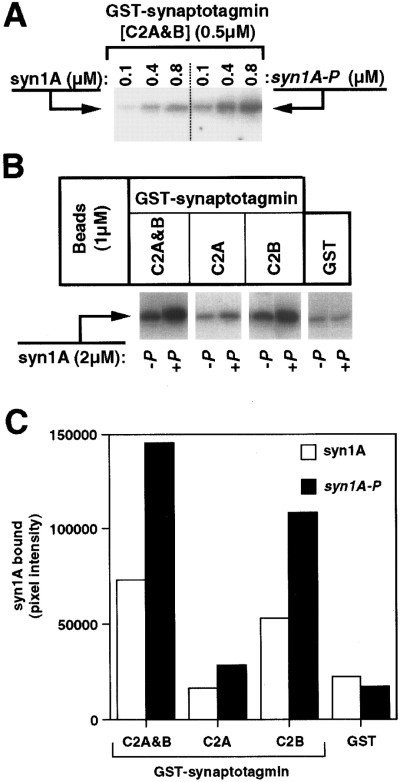
Enhanced binding of phosphorylated syntaxin 1A to synaptotagmin I. A : In vitro binding assays were performed with immobilized GST-synaptotagmin I containing both C2A and C2B domains (C2A&B) in combination with the indicated concentrations of either nonphosphorylated or CKII-phosphorylated (P) syntaxin 1A (syn1A). Bound syntaxin 1A was resolved by 12% SDS-PAGE, transferred to nitrocellulose, and visualized by western blotting using 125I-labeled secondary antibody for detection. The result presented is representative of three separate experiments in which phosphorylation potentiated syntaxin 1A binding to synaptotagmin I an average of 3.1 ± 1.0-fold. B : In vitro binding assays were performed with immobilized GST or GST-synaptotagmin I containing the indicated C2 domain(s) in combination with either nonphosphorylated (-P) or CKII-phosphorylated (+P) syntaxin 1A. Bound syntaxin 1A was resolved by 12% SDS-PAGE, transferred to nitrocellulose, and visualized by western blotting using 125I-labeled secondary antibody for detection. C : Phosphorimager-derived quantification of the binding interactions shown in B.
DISCUSSION
We have characterized the in vitro phosphorylation of six recombinant t-SNAREs (syntaxins 1A, 2C, 3A, and 4, as well as SNAP-25 and SNAP-23) by four serine/threonine kinases (PKA, PKC, CaMKII, and CKII). In addition to identifying active kinase/substrate combinations, we have performed phosphorylation domain and site mapping and investigated the consequences of phosphorylation on protein-protein interactions involving the relevant t-SNAREs.
Phosphorylation of SNAP-25 and SNAP-23
Of the kinases examined, only PKA phosphorylated SNAP-25. The phosphorylation site was mapped to a Thr residue at position 138. This residue is part of a PKA consensus site that is highly conserved among species variants of SNAP-25 and SNAP-23. Isoforms of both SNAP-25 and SNAP-23 are also generated by alternative splicing (Bark, 1993), gene duplication (Risinger and Larhammar, 1993), or exon deletion (Mollinedo and Lazo, 1997). With one exception, all isoforms contain the conserved Thr residue corresponding to position 138 of SNAP-25. The exception is SNAP-23B, which has lost this site as a result of the apparent deletion of the equivalent of exon 6 in SNAP-25 (Bark, 1993 ; Mollinedo and Lazo, 1997 ; Risinger et al., 1997). Despite this high degree of conservation, recombinant SNAP-23 was not phosphorylated by PKA (or any other kinase examined). Although the bacterially expressed SNAP-23 is unlikely to be grossly misfolded, it is possible that it is folded in a way that makes the PKA site inaccessible. This possibility is supported by the observation that bacterially expressed SNAP-23, unlike in vitro translated SNAP-23 (Ravichandran et al., 1996), interacts poorly with syntaxins and VAMPs (J. Hao and M. K. Bennett, unpublished observation). The extent to which these observations reflect authentic differences between SNAP-25 and SNAP-23 remains to be determined.
Previously published results have shown that treatment of PC12 cells with phorbol ester, which stimulates the PKC pathway, results in phosphorylation of SNAP-25 on a serine residue (Shimazaki et al., 1996). We were unable to detect significant PKC-mediated phosphorylation of SNAP-25. One possible explanation for this observation is that bacterially expressed SNAP-25 is not an effective PKC substrate due to misfolding or lack of palmitoylation. The well characterized binding properties of the recombinant SNAP-25 (Chapman et al., 1994 ; Hayashi et al., 1994 ; Hao et al., 1997 ; Schiavo et al., 1997 ; Poirier et al., 1998) argue against the misfolding possibility. The role of palmitoylation will require further investigation. Alternatively, the phorbol ester-stimulated phosphorylation of SNAP-25 could be mediated by activation of another kinase downstream of PKC. It is unlikely that such a kinase is PKA, because it phosphorylates SNAP-25 on a threonine residue. Furthermore, phosphorylation of SNAP-25 in PC12 cells reduces its binding to syntaxin (Shimazaki et al., 1996), which is not observed with PKA-phosphorylated SNAP-25.
Phosphorylation of syntaxins
The four syntaxin isoforms were differentially phosphorylated by the kinases examined. Syntaxin 1A was phosphorylated by CKII (Bennett et al., 1993a ; Hirling and Scheller, 1996 ; this study) on both serine and threonine residues, syntaxin 3A was phosphorylated by CaMKII on threonine residue(s), and syntaxin 4 was phosphorylated by CKII mainly on threonine residue(s). Only syntaxin 2C was not significantly phosphorylated. In each case, phosphorylation of syntaxins occurred within their amino-terminal domain, whereas the H3 domain (aa 199-243), a predicted coiled-coil domain required for numerous protein-protein interactions (Chapman et al., 1994 ; Hayashi et al., 1994), was not phosphorylated (see below). We have not determined how many phosphorylation sites there are in each substrate, but we know from phosphoamino acid analysis that there are at least two sites in syntaxin 1A and at least one site in both syntaxin 3A and syntaxin 4. The predicted phosphorylation sites for syntaxin 1A are Ser14 and Thr71, both of which are CKII consensus sites. For syntaxin 3A, the predicted phosphorylation sites are Thr14 and Thr164. Although these sites are not optimal consensus sites for CaMKII (RxxS/T) because they contain a lysine instead of an arginine at position -3, it is worth noting that a recently described CaMKII phosphorylation site in VAMP-2 (Ser61) also has a lysine instead of an arginine at position -3 (Hirling and Scheller, 1996). It is surprising that computer motif prediction failed to identify any potential CKII site(s) for threonine in syntaxin 4. Thus, the phosphorylation site for syntaxin 4 remains to be determined. Syntaxin 1A has recently been reported to be a substrate for CaMKII in vitro (Hirling and Scheller, 1996 ; Yokoyama et al., 1997). However, we have consistently observed only weak phosphorylation of syntaxin 1A with CaMKII (<0.06 mol of phosphate/mol of syntaxin 1A). Because of this low stoichiometry, the physiological significance of this phosphorylation reaction in uncertain.
Functional consequences of t-SNARE phosphorylation
Recent protease-protection experiments have demonstrated that the synaptic SNARE complex core incorporates two separate domains of SNAP-25, aa 1-94 and aa 136-206 (Poirier et al., 1998). The PKA phosphorylation site within SNAP-25 (Thr138) lies at the beginning of the second protected domain. This suggests that the reduced phosphorylation of SNAP-25 we observed following SNARE complex assembly may be at least partly due to inaccessibility of the phosphorylation site. It also indicates that complex formation may be an effective negative regulator of SNAP-25 phosphorylation. However, we were unable to demonstrate any changes in SNAP-25 interactions with syntaxin 1A, VAMP-2, or synaptotagmin I following phosphorylation with PKA. This is consistent with the observation that aa 136-141 are not essential for complex assembly (M. A. Poirier and M. K. Bennett, unpublished observation). Thus, although Thr138 is present in the core of SNARE complex, its phosphorylation state does not regulate in vitro complex assembly.
The differential phosphorylation of the syntaxin isoforms indicates that any functional consequences associated with syntaxin phosphorylation will not be general regulatory events, but rather will be specific to the membrane trafficking pathway(s) involving an individual syntaxin isoform. The fact that all of the syntaxins are phosphorylated outside of the critical H3 domain is consistent with our observation that phosphorylation has minimal impact on SNARE complex assembly. The one exception is the apparent reduction in binding of SNAP-25 to CKII-phosphorylated syntaxin 4. It has been shown that the amino-terminal domain of syntaxin 1A can negatively regulate the interaction of the H3 domain of syntaxin 1A with VAMP-2 (Calakos et al., 1994). The phosphorylation of syntaxin 4 may promote a similar negative regulatory interaction.
The most intriguing consequence of phosphorylation that we observed is the enhanced interaction of CKII-phosphorylated syntaxin 1A with synaptotagmin I. This effect is of particular interest because synaptotagmin I binds Ca2+ and has been proposed to be the Ca2+ sensor for regulated neurotransmitter release (Südhof and Rizo, 1996 ; Bennett, 1997). Furthermore, synaptotagmin may mediate its regulatory effects through an interaction with syntaxin, raising the possibility that phosphorylation of syntaxin 1A could directly modulate neurotransmitter release. Synaptotagmin I and syntaxin 1A interact through Ca2+-dependent mechanisms, mediated by the C2A domain of synaptotagmin I, and Ca2+-independent mechanisms, mediated by the C2B domain of synaptotagmin I (Chapman et al., 1995 ; Li et al., 1995 ; Kee and Scheller, 1996). These interactions have been shown to require the H3 domain of syntaxin 1A (Chapman et al., 1995 ; Kee and Scheller, 1996), although an involvement of the amino-terminal region of syntaxin has also been demonstrated (Shao et al., 1997). We have found that syntaxin 1A phosphorylation primarily promotes the Ca2+-independent interaction with the C2B domain of synaptotagmin I. As previously noted, CKII phosphorylation of syntaxin 1A occurs between aa 8 and 76. Whether phosphorylation of the amino-terminal domain of syntaxin 1A promotes interaction with synaptotagmin through a conformational change or through a direct effect on the binding site remains to be determined. One possibility based on the recent proposal that Ca2+ binding to synaptotagmin I generates an electrostatic switch (Shao et al., 1997) is that the negative charge added to syntaxin by phosphorylation is directly involved in the increased binding.
Our results demonstrate that phosphorylation of SNAP-25 and syntaxin 1A have minimal effects on the in vitro assembly of the synaptic SNARE complex. One explanation for these observations might be that syntaxin or SNAP-25 phosphorylation is insufficient to influence significantly the formation of the remarkably stable ternary synaptic SNARE complex (Hayashi et al., 1994 ; Poirier et al., 1998). Indeed, SNARE complex assembly can tolerate mutations (amino acid substitutions, deletions) in each of its components (Chapman et al., 1994 ; Hayashi et al., 1994 ; Kee et al., 1995 ; Hao et al., 1997 ; Poirier et al., 1998). Although we cannot rule out the possibility that other protein kinases might function to regulate the assembly of the SNARE complex core, it is important to note that phosphorylation could regulate SNARE complexes in vivo by influencing the regulatory proteins that control SNARE complex assembly and function. Syntaxin and SNAP-25 are central components in a network of regulatory interactions that include α-SNAP (Söllner et al., 1993), synaptotagmin (Bennett et al., 1992b ; Li et al., 1995 ; Schiavo et al., 1997), n-Sec1/Munc-18 (Hata et al., 1993 ; Pevsner et al., 1994), complexins (McMahon et al., 1995), voltage-gated Ca2+ channels (Bennett et al., 1992b ; Yokoyama et al., 1997), munc-13 (Betz et al., 1997), Hrs-2 (Bean et al., 1997), and the r-sec6/sec8 complex (Hsu et al., 1996). Several of these regulatory interactions are modulated by phosphorylation. For example, phosphorylation of α-SNAP by PKA reduces its interaction with the synaptic SNARE complex (Hirling and Scheller, 1996), whereas PKC phosphorylation of n-Sec1 reduces its interaction with syntaxin (Fujita et al., 1996). Our observation of an enhanced interaction between phosphorylated syntaxin 1A and synaptotagmin I extends this trend. Clearly, additional experiments will be required to determine if other protein interactions are targets of regulation by t-SNARE phosphorylation both in vitro and in vivo. It is also possible that the functional consequences of syntaxin or SNAP-25 phosphorylation may be manifested in properties more subtle than the in vitro protein-protein interactions we have examined, such as the regulation of membrane fusion (Weber et al., 1998). The results we have described provide the foundation for further studies aimed at clarifying the role of t-SNARE phosphorylation in the regulation of the vesicle docking and fusion machinery, as well as the molecular mechanisms underlying synaptic plasticity.
Acknowledgements
This work was supported by fellowship B-PD 10905-302 from the Swedish Natural Research Council (C.R.), National Institutes of Health grant GM-51313 (M.K.B.), and the McKnight Fund for Neuroscience Scholar Award (M.K.B.).




