Role of Protein Kinase C (PKC) in Agonist-Induced μ-Opioid Receptor Down-Regulation
I. PKC Translocation to the Membrace of SH-SY5Y NeuroblastomaCells Is Induced by μ-Opioid Agonists
Abbreviations used : DAMGO, [d-Ala2, N-Me-Phe4, Gly-ol] enkephalin ; DIPR, diprenorphine ; DPDPE, [Tyr-d-penicillamine-Gly-Phe-d-penicillamine]enkephalin ; DSLET, Tyr-d-Ser-Gly-Phe-Leu-Thr ; PBS, phosphate-buffered saline ; PDBu, phorbol 12,13-dibutyrate ; PKC, protein kinase C ; PMA, phorbol 12-myristate 13-acetate ; PTX, pertussis toxin.
Abstract
Abstract : Agonist-induced down-regulation of opioid receptors appears to require the phosphorylation of the receptor protein. However, the identities of the specific protein kinases that perform this task remain uncertain. Protein kinase C (PKC) has been shown to catalyze the phosphorylation of several G protein-coupled receptors and potentiate their desensitization toward agonists. However, it is unknown whether opioid receptor agonists induce PKC activation under physiological conditions. Using cultured SH-SY5Y neuroblastoma cells, which naturally express μ- and δ-opioid receptors, we investigated whether μ-opioid receptor agonists can activate PKC by measuring enzyme translocation to the membrane fraction. PKC translocation and opioid receptor densities were simultaneously measured by 3H-phorbol ester and [3H]diprenorphine binding, respectively, to correlate alterations in PKC localization with changes in receptor binding sites. We observed that μ-opioid agonists have a dual effect on membrane PKC density depending on the period of drug exposure. Exposure for 2-6 h to [d-Ala2, N-Me-Phe4, Gly-ol]enkephalin or morphine promotes the translocation of PKC from the cytosol to the plasma membrane. Longer periods of opioid exposure (>12 h) produce a decrease in membrane-bound PKC density to a level well below basal. A significant decrease in [3H]diprenorphine binding sites is first observed at 2 h and continues to decline through the last time point measured (48 h). The opioid receptor antagonist naloxone attenuated both opioid-mediated PKC translocation and receptor down-regulation. These results demonstrate that opioids are capable of activating PKC, as evidenced by enhanced translocation of the enzyme to the cell membrane, and this finding suggests that PKC may have a physiological role in opioid receptor plasticity.
Opioid receptors are members of the superfamily of seven-transmembrane, G protein-coupled receptors (Lohse, 1993). Many opioid-mediated responses, including the inhibition of forskolin-stimulated cyclic AMP accumulation, the activation of an inwardly rectifying K+ channel, and antinociception, are diminished by repeated exposure to selective agonists (Mestek et al., 1995 ; Narita et al., 1996a,b ; Wang et al., 1996b ; Cai et al., 1997). This loss of receptor sensitivity and function results predominantly from two separate but related physiological processes : desensitization and down-regulation (Law et al., 1983).
Desensitization and down-regulation each occur with their own characteristic time course. Desensitization occurs within minutes of agonist exposure, and down-regulation follows the former in periods ranging from hours to days (Chakrabarti et al., 1995 ; Kovoor et al., 1995 ; Zhang et al., 1996 ; Breivogel et al., 1997 ; Kaneko et al., 1997). Desensitization is a rapid, reversible loss of agonist affinity and receptor function, produced by an uncoupling of the receptor from its G protein (Harada et al., 1990 ; Hausdorff et al., 1990 ; Harrington et al., 1994). Concurrent with this functional decrease, the G protein-coupled receptor complex becomes reversibly internalized into intracellular endosomes via the clathrin-coated vesicular pathway (Keith et al., 1996). These acute phases of receptor regulation, however, do not produce a net loss of receptor sites from the cell surface (no change in receptor Bmax). Conversely, after longer periods of agonist exposure, the receptor-G protein complex will undergo down-regulation, i.e., a net loss of these protein units from the plasma membrane due to their degradation via a separate mechanism (Law et al., 1991 ; Nestler, 1992 ; Carter and Medzihradsky, 1993 ; Raynor et al., 1994 ; Arden et al., 1995 ; Pak et al., 1996 ; Sternini et al., 1996 ; Breivogel et al., 1997). Both of these processes are presently under intensive study because they have been hypothesized to contribute to the development of opiate tolerance.
A common mechanism that links receptor desensitization and down-regulation appears to be the phosphorylation of the opioid receptor protein. μ-Selective agonists such as morphine and [d-Ala2, N-Me-Phe4, Gly-ol]enkephalin (DAMGO) have been shown to promote the phosphorylation of the μ-opioid receptor (Arden et al., 1995 ; Sadée and Wang, 1995 ; Zhang et al., 1996 ; Yu et al., 1997). Phosphorylation of specific amino acid residues on the opioid receptor uncouples it from the G protein and reduces its affinity for agonists, leading to the need for higher concentrations of opioids to achieve a given response (Wang et al., 1996b). Receptor phosphorylation also appears to be required for down-regulation, because the mutation of specific threonine residues within a protein kinase C (PKC) consensus site significantly attenuates this response in the δ-opioid receptor (Trapaidze et al., 1996). Consequently, opioid receptor phosphorylation is important during both the short- and long-term phases of receptor regulation.
Several protein kinases, including β-adrenergic receptor kinase, protein kinase A, and protein tyrosine kinases, have been hypothesized to contribute to alterations in opioid receptor sensitivity (Harada et al., 1989 ; Wimpey and Chavkin, 1992 ; Lefkowitz, 1993 ; Nakano et al., 1994 ; Pei et al., 1995). Other lines of evidence support the involvement of Ca2+-regulated kinases such as calcium/calmodulin kinase and PKC in opiate tolerance (Mao et al., 1995 ; Mestek et al., 1995 ; Fundytus and Coderre, 1996 ; Narita et al., 1996a ; Zhang et al., 1996). PKC is of considerable interest due to its role in the regulation of other G protein-coupled receptors (Raymond, 1991 ; Harrington et al., 1994). However, comprehensive studies focusing on the relationship between acute or prolonged opioid receptor stimulation and PKC activity have not appeared in the literature.
Several reports have demonstrated that prior activation of PKC by phorbol esters potentiates the agonist-mediated loss of μ- and δ-opioid receptor function (Gucker and Bidlack, 1992 ; Chen and Yu, 1994 ; Hayashi et al., 1995 ; Wang et al., 1996a ; Cai et al., 1997 ; Narita et al., 1997). Furthermore, intrathecal pretreatment of mice with a specific PKC inhibitor, calphostin C, prevents the development and expression of acute antinociceptive tolerance to repeated spinal applications of deltorphin-II (Narita et al., 1996a). Although many of the studies have focused on desensitization rather than down-regulation, a consensus opinion on PKC's contribution to opioid receptor plasticity has not been established for either process. In addition, the physiologic activation of PKC by μ-opioids has yet to be reported, and many of the earlier reports have used artificial means to activate cellular PKC.
As part of our investigation of the relationship between PKC and opioid receptor regulation, we investigated whether μ-selective opioids are capable of inducing a physiologic translocation/activation of PKC in SH-SY5Y neuroblastoma cells. We report here that opioid-mediated PKC translocation is observed and that the kinetics suggest a role in longer-term regulation, such as down-regulation, rather than the faster process of desensitization.
MATERIALS AND METHODS
Drugs and cell culture
Morphine sulfate, DAMGO, [Tyr-d-penicillamine-Gly-Phe-d-penicillamine]enkephalin (DPDPE), and Tyr-d-Ser-Gly-Phe-Leu-Thr (DSLET) were received from the National Institute on Drug Abuse (Bethesda, MD, U.S.A.). Nimodipine and chelerythrine chloride were purchased from Research Biochemicals International (Natick, MA, U.S.A.), and pertussis toxin (PTX) was from GibcoBRL (Grand Island, NY, U.S.A.). Phorbol 12-myristate 13-acetate (PMA) and 4α-PMA were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Undifferentiated SH-SY5Y neuroblastoma cells were a generous gift from Dr. Wolfgang Sadée (University of California at San Francisco, San Franciso, CA, U.S.A.), and were plated at an initial density of 5.0 × 105 cells in 75-cm2 flasks. Cells were maintained in medium consisting of a 1 : 1 mixture of Dulbecco's modified Eagle's medium/F-12 (GibcoBRL) supplemented with 2 mMl-glutamine, 10% fetal bovine serum (Gemini Bio-Products, Calabasas, CA), 50 μg/ml gentamicin sulfate (Gemini Bio-Products), and 1 mM nonessential amino acids (GibcoBRL) at 37°C in 7% CO2/humidified air. After 24 h, SH-SY5Y cells were differentiated toward the neuronal phenotype by addition of 10 μM retinoic acid (Sigma), and the retinoic acid-containing medium was changed every 3 days until the cell monolayer reached confluency (5-7 days). On reaching confluency, the cells were incubated in the above medium without fetal bovine serum and exposed to drugs and/or phorbol esters at the indicated concentrations for various intervals.
Cell harvesting and membrane preparation
After drug incubation, the culture media were aspirated, and the cells were washed twice with sterile phosphate-buffered saline (PBS ; pH 7.4). Cells were harvested in a solution of PBS containing 1 mM EDTA and centrifuged at 3,000 g at 4°C for 10 min in a Sorvall centrifuge using an SS-34 rotor. The supernatant was discarded, and the pellet was resuspended in a receptor preparation buffer containing (mM) Tris-HCl (38.5), Tris-base (11.5), EDTA (2.0), EGTA (0.5), dithiothreitol (0.8), phenylmethylsulfonyl fluoride (100 μM), leupeptin (2 μg/ml), and aprotinin (2 μg/ml) at pH 7.4. The lysed cells were homogenized for 5 s using a Brinkmann tissue homogenizer at a setting of 2 and centrifuged at 45,000 g at 4°C for 20 min. The pellet (P2 fraction), containing the washed membrane preparation, was resuspended at a protein concentration of 0.2-0.4 mg/ml (as measured by the BCA method ; Pierce) in a receptor binding buffer containing (mM) Tris-HCl (38.5), Tris-base (11.5), NaCl (100), and CaCl2 (1.3), pH 7.4. This membrane preparation was stored at —70°C until used.
[3H]Diprenorphine ([3H]DIPR), [3H]DSLET, [3H]DAMGO, and [3H]horbol 12,13-dibutyrate ([3H]PDBu) binding
The nonselective opiate antagonist [3H]DIPR was used to quantify the basal level of opioid receptors in washed membrane homogenates. An antagonist was used instead of a radiolabeled agonist because its binding kinetics are unaffected by the presence of Na+ in the assay buffer, which is necessary for simultaneous 3H-phorbol ester binding experiments (Simon et al., 1975). For 3H-opioid binding (opioid binding sites), 590 μl of membranes were allowed to equilibrate in the absence or presence of 10 μM (—)-naloxone (National Institute on Drug Abuse) to determine specific binding. Scatchard analyses of saturation binding data were performed to determine [3H]DIPR (Amersham ; specific activity, 39.0 Ci/mmol), [3H]DAMGO (47 Ci/mmol ; Multiple Peptide Systems, San Diego, CA, U.S.A.), or [3H]DSLET (21.6 Ci/mmol ; Multiple Peptide Systems) binding parameters (KD and Bmax) in a total well volume of 1 ml for 120 min at room temperature. In some experiments, single-point determinations were used to assay the number of opioid binding sites using a saturating concentration of [3H]DIPR (2.0 nM). The tissue was harvested onto Titertek filtermats [coated with 0.1% polyethylenimine to reduce nonspecific binding] using a Brandel cell harvester. The filters were placed in scintillation vials containing 3.0 ml of Liquiscint (National Diagnostics). Samples were counted for radioactivity for 5.0 min in a Beckman liquid scintillation counter (efficiency, 50%). The cpm data were converted to femtomoles of [3H]DIPR bound per milligram of protein. For [3H]PDBu binding (PKC binding sites), 300 μl of membranes were allowed to equilibrate with receptor binding buffer in the absence or presence of 10 μM PMA (Sigma) to determine specific binding. Scatchard analyses of saturation binding data were performed to determine [3H]PDBu binding parameters (KD and Bmax) using [3H]PDBu (NEN ; specific activity, 18.0 Ci/mmol) in a total well volume of 1 ml for 120 min at room temperature. In some experiments, single-point determinations were used to assay the number of PKC binding sites using a single concentration of [3H]PDBu (1.0 nM). A single [3H]PDBu concentration near its KD was used to minimize the nonspecific binding of this highly lipophlic molecule. The tissue was harvested, and radioactivity was quantified as described above. The cpm data were converted to femtomoles of [3H]PDBu bound per milligram of protein.
Data analysis and statistical methods
[3H]PDBu and [3H]DIPR binding curves were generated, and all regression analyses were performed using the LIGAND curve-fitting program (Munson and Rodbard, 1980). All graphs were produced using Sigmaplot for Windows (ver. 4.0). IC/EC50 values were determined using the equation of Cheng and Prusoff (1973). One- and two-way ANOVAs and the post hoc Tukey's test were used for multiple comparisons at a minimal significance level of p≤ 0.05. Student's t test was used for simple two-sample tests at the same significance level. Statistical data were expressed as mean ± SE values of the indicated number of observations. In some figures, a representative graph is used to illustrate the results of a particular experiment that was repeated at least four times.
RESULTS
Basal parameters of [3H]DIPR and [3H]PDBu binding in SH-SY5Y neuroblastoma membranes
Scatchard analysis of saturation curves of [3H]DIPR binding demonstrated a KD of 0.6 ± 0.04 nM and a Bmax of 265 ± 21.4 fmol/mg of protein in control cell membranes, which represents the binding of both μ- and δ-receptor types (Table 1). Individual populations of μ- and δ-opioid receptors were identified by [3H]DAMGO (KD = 0.5 ± 0.1 nM, Bmax = 204 ± 32.3 fmol/mg of protein) and [3H]DSLET (KD = 0.3 ± 0.02 nM, Bmax = 50.7 ± 11.2 fmol/mg of protein) binding, respectively, and each showed binding characteristics consistent with previous studies. SH-SY5Y cells consistently maintained a ratio of μ- to δ-opioid receptors of ≈4:1 throughout the study (Table 1). Saturation binding analysis revealed that [3H]PDBu binds with high affinity to a single population of sites in cell membrane homogenates with a KD of 1.3 ± 0.10 nM and a Bmax of 2,001 ± 60.1 fmol/mg of protein (Table 1).
| [3H]DIPR | [3H]DAMGO | [3H]DSTLE | [3H]PDBu | |
|---|---|---|---|---|
| K D (nM) | 0.6 ± 0.04 | 0.5 ± 0.1 | 0.3 ± 0.02 | 1.3 ± 0.10 |
| B max (fmol/mg of protein) | 265.1 ± 21.4 | 204.3 ± 32.3 | 50.7 ± 11.2 | 2,001.1 ± 60.6 |
Down-regulation of opioid receptors by morphine, DAMGO, and DPDPE
Opioid receptor down-regulation was assessed after a 24-h exposure of SH-SY5Y cells to morphine, DAMGO, or the δ-specific agonist DPDPE (0.01 nM-10 μM). All ligands produced concentration-dependent decreases in number of membrane opioid receptor binding sites. However, differences exist in the potency of each drug. The IC50 values for DAMGO, DPDPE, and morphine were 42.2 ± 7.0, 37.3 ± 2.5, and 104 ± 9.1 nM, respectively (Fig. 1A). At the highest concentration tested (10 μM), DAMGO produced the most robust loss of membrane [3H]DIPR binding sites (58.3%), followed by morphine (37.7%) and DPDPE, which produced only a minor decrease (8.8%) in specific DIPR binding. Moreover, as expected from its selectivity, DAMGO produced no significant change in the density of [3H]DSLET-labeled, δ-opioid receptor sites in identically prepared membrane homogenates (data not shown). The ability of DAMGO to decrease homologously μ-receptor number, coupled with the predominant expression of this receptor type in SH-SY5Y cells, guided us to use DAMGO for the balance of our experiments. DAMGO (1 μM) treatment for 24 h results in the loss of [3H]DIPR binding sites without a change in radioligand affinity (control, KD = 0.30 ± 0.1 nM, Bmax = 220 ± 30.1 fmol/mg of protein ; DAMGO-treated, KD = 0.35 ± 0.08 nM, Bmax = 90.7 ± 15.7 fmol/mg of protein ; p≤ 0.01 ; Fig. 1B).
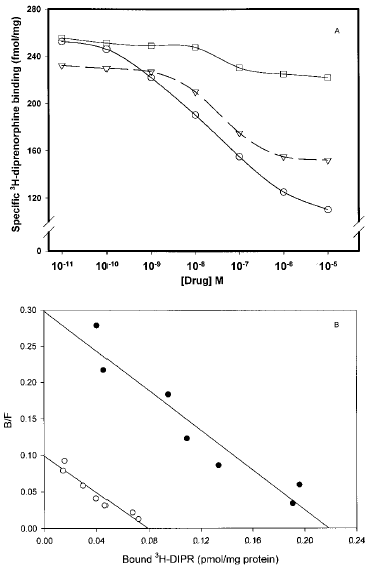
A : Down-regulation of membrane opioid receptors by prolonged exposure to DAMGO, morphine, or DPDPE is SHSY5Y neuroblastoma cells. Eighty-percent confluent cells were exposed for 24 h to the opioid agonists DAMGO (○), morphine (▿), or DPDPE (□) at concentrations ranging from 0.01 nM to 10 μM. Cells were washed extensively, and membranes were prepared as described in Materials and Methods before [3H]DIPR binding to quantify opioid receptor number. The individual binding curves were generated using the four-parameter logistic equation [f1 = if(b<O, yO + a), f2 = if(b>O, yO + a/(1 + abs(x/xO)^b)), yO + a*abs ((x/xO)) ^ (abs(b))/(1 + (abs(x/xO)) ^ (abs(b))), f=if (x< =0, f1, f2), fit f to y], which was resident within the Sigmaplot 4.0 program. Graphed results are representative of an experiment that was repeated four times. B : Scatchard analysis of [3H]DIPR binding to membranes prepared from SH-SY5Y neuroblastoma cells exposed for 24 h to medium or 1 μM DAMGO. Membranes prepared from control (•) and DAMGO-treated (○) cells were incubated with [3H]DIPR (0.1-10 nM) as described in Materials and Methods. DAMGO significantly reduced the density of μ-opioid receptors in SH-SY5Y neuroblastoma cells without altering ligand affinity (untreated cells, KD = 0.30 ± 0.1 nM, Bmax = 220 ± 30.1 fmol/mg of protein ; DAMGO-treated cells, KD = 0.35 ± 0.08 nM, Bmax = 90.7 ± 15.7 fmol/mg of protein). Graphed results are representative of an experiment that was repeated four times. KD and Bmax values are mean ± SE values of conditions performed in quadruplicate. B/F, bound/free.
Time course of PKC translocation induced by DAMGO
The next question was whether concentrations of DAMGO, able to produce down-regulation, elicit PKC translocation. DAMGO (1 μM) produced time-dependent changes in the density of membrane-bound [3H]PDBu binding sites. However, membrane PKC density was altered in a bimodal fashion depending on the length of the agonist exposure (1 μM DAMGO). Membrane PKC density was increased after incubation times ranging from 2 to 6 h, but no differences were observed at any of the earlier time points examined (0, 5, 15, 30, or 60 min). PKC translocation rose to 217% of control at 4 h (F0.01, 7, 32 = 5.57, p≤ 0.01 by ANOVA ; Fig. 2A). Membrane PKC density returned to control levels by 12 h. When DAMGO incubation was extended for periods of ≥18 h, the number of membrane [3H]PDBu binding sites was reduced to levels well below those in control cultures. This “reverse PKC translocation” was observed through the final time points (48 h) and represented a reduction of 73.3%. Both PKC translocation and reverse translocation represented changes in the number of membrane [3H]PDBu binding sites, as indicated by changes in the Bmax of [3H]PDBu binding measured at 4 and 24 h, respectively, with no changes in ligand affinity observed (Table 2). During opioid-mediated PKC translocation and reverse translocation, there was a parallel decrease or increase in PKC protein content and number of [3H]PDBu binding sites in the cytosolic fraction (data not shown).
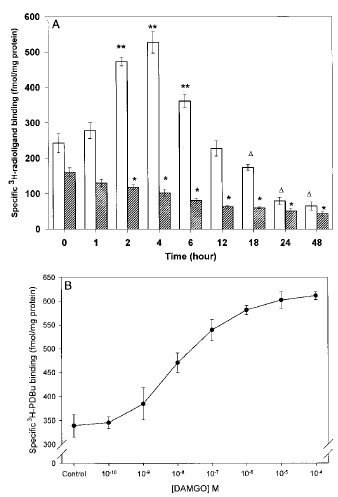
A : Effect of DAMGO (1 μM) on membrane [3H]PDBu and [3H]DIPR binding in SH-SY5Y neuroblastoma cells with time. Confluent cells were incubated with 1 μM DAMGO for the times indicated on the x-axis. Cells were washed extensively, and membranes were prepared as described in Materials and Methods before [3H]DIPR binding ([UNK]) to quantify opioid receptor number or [3H]PDBu (□) to determine the extent of PKC translocation. Nonspecific binding, determined in the presence of 10 μM naloxone and 10 μM PMA for [3H]DIPR and [3H]PDBu binding, respectively, represented ≤10% of the total binding for each ligand. Data are mean ± SE (bars) values from four independent experiments. *p≤ 0.05 by ANOVA and the post hoc Tukey's test (F0.05, 7, 32 = 5.57) compared with control (t = O h) ; **p≤ 0.01 by ANOVA and the post hoc Tukey's test compared with control (t = O h) ; ▵p≤ 0.05 by ANOVA and the post hoc Tukey's test compared with control (t = O h). B : Concentration dependency of DAMGO-stimulated PKC translocation in SH-SY5Y neuroblastoma cells. Confluent cells were incubated with DAMGO (0.1 nM-100 μM) for 4 h. Cells were washed extensively, and membranes were prepared as described in Materials and Methods before [3H]PDBu binding to determine the extent of PKC translocation. Nonspecific binding, determined in the presence of 10 μM PMA, represented ≤10% of the total binding for each ligand. Data are mean ± SE values (bars) from four independent experiments.
| [3H]PDBu | [3H]DIPR | ||||
|---|---|---|---|---|---|
| Condition | Incubation time (h) | K D (nM) | B max [fmol/mg] (% control) | K D (nM) | B max [fmol/mg] (% control) |
| Control | 4 | 1.7 ± 0.2 | 2,002.2 ± 200.7 (100) | 0.53 ± 0.2 | 241.6 ± 17.2 (100) |
| 1 μM DAMGO | 4 | 1.5 ± 0.1 | 4,111.5 ± 761.1 (205)a | 0.61 ± 0.1 | 153.9 ± 28.6 (63)a |
| 1 μM DAMGO | 24 | 1.8 ± 0.2 | 870.2 ± 53.3 (43)b | 0.73 ± 0.1 | 76.0 ± 9.9 (31)b |
- a p≤ 0.05 by the post hoc Tukey's test from control (q0.05,12,3 = 3.77) ;
- b p≤ 0.01 by the post hoc Tukey's test from control (q0.01,12,3 = 5.04).
Simultaneous quantification of opioid binding sites showed that the onset of significant receptor down-regulation roughly parallels PKC translocation to the membrane and progresses in a time-dependent manner (Fig. 2A). It should be noted that μ-receptor down-regulation was still observed during the periods when membrane PKC density was significantly below control. Subsequent studies were carried out at 4 h to take advantage of the period of peak PKC translocation by DAMGO. A doseresponse curve revealed that DAMGO-induced PKC translocation was observed at a concentration of 10 nM and continued to increase until it reached a plateau at 1 μM (EC50 = 89.3 nM ; Fig. 2B). Morphine was similarly effective at promoting PKC translocation at concentrations consistent with its affinity for the μ-opioid receptor and for inducing down-regulation (data not shown).
Effect of naloxone on PKC translocation in the presence or absence of opioid agonists
4-h DAMGO treatment. The characteristics of 4-h DAMGO-mediated PKC translocation and opioid receptor down-regulation are presented in Table 3. A total inhibition of DAMGO-mediated PKC translocation was observed after pretreatment with the opioid antagonist naloxone. Naloxone pretreatment also prevents the loss of membrane μ-opioid receptors induced by DAMGO, although naloxone alone had no significant effect on membrane PKC or opioid receptor density at this time point. The specific δ-opioid receptor antagonist naltrindol had no effect on either DAMGO-mediated PKC translocation or the reduction in [3H]DIPR binding, confirming μ-opioid receptor mediation of the PKC response.
| [3H]PDBu | [3H]DIPR | ||||
|---|---|---|---|---|---|
| Condition | Inhibitor/± DAMGO incubation time (h) | K D (nM) | B max [fmol/mg] (%control) | K D (nM) | B max [fmol/mg] (%control) |
| Control | 0/0 | 1.2 ± 0.4 | 2,005.0 ± 210.6 (100) | 0.58 ± 0.02 | 273.3 ± 33.3 (100) |
| 1 μM DAMGO | 0/4 | 1.1 ± 0.1 | 3,943.5 ± 512.8 (195)a | 0.71 ± 0.04 | 192.3 ± 15.6 (70)a |
| 1 μM naloxone/medium | 2/4 | 1.3 ± 0.1 | 1,864.8 ± 99.7 (90) | 0.52 ± 0.02 | 291.2 ± 25.1 (106) |
| 1 μM naltrindol/medium | 2/4 | 1.0 ± 0.3 | 1,798.2 ± 109.7 (85) | 0.66 ± 0.04 | 264.6 ± 11.2 (90) |
| Naloxone/DAMGO | 2/4 | 0.9 ± 0.2 | 1,844.0 ± 59.9 (90)b | 0.75 ± 0.03 | 307.4 ± 50.2 (112)b |
| Naltrindol/DAMGO | 2/4 | 0.85 ± 0.1 | 4,611.4 ± 812.4 (230)a | 0.73 ± 0.22 | 176.3 ± 36.9 (64)a |
- a p < 0.01 by the post hoc Tukey's test from control (q0.01,50,10 = 5.45) ;
- b p < 0.01 by the post hoc Tukey's test from 4-h DAMGO alone (q0.01,50,10 = 5.45).
24-h DAMGO treatment. When the coapplication of DAMGO and naloxone was increased to 24 h, this treatment produced very interesting results (Fig. 3). At naloxone concentrations of ≥10 nM, there was a significant up-regulation of [3H]DIPR binding sites. In addition, naloxone-mediated opioid receptor up-regulation was matched by a significant increase in number of membrane-bound [3H]PDBu binding sites (Fig. 3). These latter effects are also observed when the antagonist is used at similar concentrations in the absence of DAMGO.
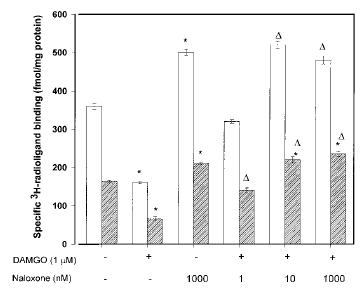
Naloxone's effects on opioid receptor down-regulation and PKC translocation in SH-SY5Y neuroblastoma cells treated for 24 h with DAMGO. Confluent cells were incubated with 1 μM DAMGO for 24 h without or with naloxone (1, 10, or 1,000 nM). Naloxone was added 15 min before DAMGO. At the end of treatment, cells were washed extensively, and membranes were prepared as described in Materials and Methods before [3H]DIPR binding ([UNK]) to quantify opioid receptor number or [3H]PDBu (□) to determine the extent of PKC translocation. Nonspecific binding, determined in the presence of 10 μM naloxone and 10 μM PMA for [3H]DIPR and [3H]PDBu binding, respectively, represented ≤10% of the total binding for each ligand. Data are mean ± SE (bars) values from four independent experiments. *p≤ 0.05 by ANOVA and the post hoc Tukey's test compared with control ; ▵p≤ 0.05 by ANOVA and the post hoc Tukey's test compared with DAMGO (1 μM) alone.
DISCUSSION
Phosphorylation of the μ-opioid receptor can be stimulated by morphine or DAMGO or directly by the PKC activator PMA, and each drug produces this effect with a similar time course (5-20 min) (Zhang et al., 1996 ; Yu et al., 1997). Receptor phosphorylation induced by all three compounds has also been shown to diminish receptor function (Zhang et al., 1996 ; Narita et al., 1997). As stated in the introductory section, the literature is divided on whether opioid receptor phosphorylation by PKC occurs during agonist-mediated receptor plasticity. We set out to try to resolve this controversy by examining whether or not opioid agonists can physiologically induce PKC activation in cultured cells that naturally express opioid receptors. We assessed PKC activation by measuring its translocation from the cytosol to the plasma membrane, which is widely accepted as evidence of enzyme activation (Nishizuka, 1992). Our results clearly demonstrate that DAMGO and morphine induce a naloxone-reversible, bimodal change in membrane PKC translocation in μ-opioid receptor-expressing SH-SY5Y neuroblastoma cells. Exposure to DAMGO for 2-6 h elicited a pronounced increase in number of membrane-bound 3H-phorbol ester binding sites. Longer incubations (>12 h) produced a significant decrease in number of membrane [3H]PDBu binding sites well below that in cells not exposed to DAMGO. Our results are consistent with those of Mayer et al. (1995), who showed that chronic exposure to morphine increased membrane PKC density in the spinal cord of rats rendered tolerant to its antinociceptive effects. To our knowledge, our results are the first evidence that μ-opioids, at concentrations that produce receptor down-regulation, induce PKC translocation/activation in cells that express opioid receptors.
Nonlinear regression analysis showed that the t1/2 for the down-regulation of [3H]DIPR binding sites by DAMGO was 5.2 h, which is consistent with previous studies using this cell line (Zadina et al., 1993 ; Sternini et al., 1996 ; Breivogel et al., 1997). Our own analysis of [3H]DIPR binding confirms that this decrease was due to a loss of cell surface binding sites, without a change in ligand affinity. Both morphine and DAMGO decreased opioid receptor density at concentrations consistent with their binding affinities for μ-receptors (Prather et al., 1994). The loss of [3H]DIPR binding sites after morphine treatment represents the loss of μ- as well as some δ-opioid receptors from the cell surface, whereas DAMGO only reduces the density of μ-receptors at the concentrations used (Zadina et al., 1994). Our experiments also show that the nonselective opioid antagonist naloxone prevents DAMGO-mediated PKC translocation to the plasma membrane by preventing access to the receptor's binding site. This evidence, coupled with the ineffectiveness of the δ-receptor antagonist naltrindol, confirms that DAMGO is mediating PKC translocation by stimulating μ-opioid receptors.
In contrast, reports of the t1/2 for opioid receptor desensitization range from 8 to 90 min (Kovoor et al., 1995 ; Cai et al., 1996). We showed that the t1/2 for DAMGO-induced PKC translocation was ~2.5 h. PKC translocation to the membrane fraction peaked at 4 h before it began to decline toward control levels and then well below basal levels by ≥24 h. Thus, the half-maximal translocation of [3H]PDBu binding sites precedes the half-maximal loss of μ-opioid receptors from SH-SY5Y cells. Therefore, the kinetics of opioid-mediated PKC translocation are consistent with a participatory role for this kinase in opioid receptor down-regulation rather than the faster processes of desensitization and internalization. Different protein kinases, such as β-adrenergic receptor kinase or other G protein receptor kinases, i.e., GRK-3, appear to be likely candidates for controlling the more rapid processes of desensitization and receptor internalization (Keith et al., 1996 ; Zhang et al., 1996).
As has been shown previously (Yoburn et al., 1990 ; Zadina et al., 1993), the continued presence of naloxone (24 h) produces a pronounced up-regulation of membrane opioid binding sites (Fig. 3). It is interesting that this treatment also induces a pronounced translocation of PKC to the cell membrane (Fig. 3). Both effects occurred whether naloxone was used alone or added in the presence of DAMGO. Several laboratories have reported that μ-opioid receptors exist under a minimal level of phosphorylation (Wang et al., 1996a,b ; Zhang et al., 1996), and it is possible that PKC is involved in this regulatory process. Further studies are necessary to determine whether PKC-mediated phosphorylation is required for up-regulation of μ-opioid receptors.
An interesting finding was the loss of membrane [3H]PDBu sites that was observed when the DAMGO incubation period was extended for >12 h. In the presence of DAMGO, membrane PKC density gradually diminished from its maximum (217% of control) at 4 h to its lowest measured density (26.6% of control) after 48 h. This decrease in [3H]PDBu binding reflects the loss of PKC-α and -ε from the membrane fraction. Favaron et al. (1990) have observed that continuous PKC activation by phorbol esters results in the time-dependent disappearance of PKC from the cytosol and membrane fractions. In vivo, the loss of particulate [3H]PDBu binding sites occurs in the hippocampus of rats after periods of significant and prolonged PKC translocation produced by focal cerebral ischemia (Onodera et al., 1989). Moreover, in A431 human epidermoid carcinoma cell cultures, stimulation of the bradykinin-2 receptor produces a similar pattern of enhanced PKC translocation (to 140% of control from 2-10 min)—as measured by [3H]PDBu binding—followed by reverse translocation after longer periods of drug exposure (to 50% of control at t≥ 15 min) (Liebmann et al., 1996). The loss of membranebound [3H]PDBu sites over time may occur to limit the duration of kinase activation.
The PKC (ATP : protein-phosphotransferase, EC 2.7.1.37) family of serine/threonine kinases is composed of at least 10 isoforms (α, β1, βII, γ, δε, ξ, η, θ, and λ) (Nishizuka, 1992). These 10 members have been subdivided into groups based on their common structural characteristics, regional localization, and dependencies on Ca2+, phospholipids, and diacylglycerol (DAG) for activity. Individual PKCs are classified into the following groups : conventional (α, β1, βII, and γ ; Ca2+ - and DAG-regulated), novel (δ, ε, η, and θ ; DAG-regulated), and atypical (ξ and λ ; regulated by neither DAG nor Ca2+). All PKC isoforms exist in an inactive state within the cytosol but become translocated to the plasma membrane by various stimuli, including activation of G protein-coupled receptors (Nishizuka, 1992 ; Tippmer et al., 1994). The conventional and novel PKC isoenzymes can also be translocated by tumor-promoting phorbol esters, which are analogues of DAG (Castanga et al., 1982 ; Wolf et al., 1985 ; Cardell et al., 1990). The translocation of PKC is believed to be its primary mode of activation in mammalian cells (Nishizuka, 1986 ; Favaron et al., 1988).
The loss of membrane-bound PKC-α and -ε over time (as measured by [3H]PDBu binding) did not halt the progression of opioid receptor down-regulation (Fig. 2A). This finding could be interpreted to mean that PKC does not have a role in the down-regulation process. This has, in fact, been suggested during the characterization of agonist-mediated phosphorylation, desensitization, and down-regulation of the δ-opioid receptor (Pei et al., 1995 ; Trapaidze et al., 1996). Only a relatively brief period of PKC activation may be necessary for the processes that control receptor down-regulation to commence. Alternatively, we believe that the activation of other, non-DAG-regulated PKC isoforms may also contribute to opioid receptor down-regulation. It is interesting that changes in the in vivo expression of individual PKC isoforms have been reported in the postmortem brains of opiate addicts who died of heroin or methadone overdose, where there was a significant loss of PKC-α immunoreactivity, whereas levels of PKC-ξ immunoreactivity were unchanged (Garcia-Sevilla et al., 1997). The opioid sensitivity of a non-DAG-regulated PKC isoform (PKC-ξ), which is also expressed within the SH-SY5Y neuroblastoma cell line, and its involvement in μ-receptor down-regulation are addressed in our accompanying article (Kramer and Simon, 1999).
In summary, we demonstrate that μ-selective opioid agonists are capable of producing the prolonged translocation of DAG-sensitive PKC isoforms to the plasma membrane of SH-SY5Y neuroblastoma cells, an effect that roughly parallels the early phase of receptor downregulation. After longer periods of opioid agonist exposure, the membrane density of PKC is decreased relative to that of untreated cells, whereas opioid receptor down-regulation continues unabated. The finding that μ-opioid agonists induce PKC translocation to the plasma membrane strengthens the hypothesis that PKC contributes to opioid receptor regulation.
Acknowledgements
The authors acknowledge the help of Drs. Theresa Gioannini, Jacob Hiller, and Matthew Andria in the completion of the manuscript. This work was supported by training grant T32-DA07254 and research grant DA00017 to E.J.S. from the National Institute on Drug Abuse.




