Cytokine Induction of Inducible Nitric Oxide Synthase in an Oligodendrocyte Cell Line
Role of p38 Mitogen-Activated Protein Kinase Activation
Abbreviations used : ERK, extracellular signal-regulated kinase ; GAPDH, glyceraldehyde-3-phosphate dehydrogenase ; IFN-γ, interferon-γ ; IL-1, interleukin-1 ; iNOS, inducible nitric oxide synthase ; JNK, c-Jun N-terminal kinase ; LPS, lipopolysaccharide ; MAPK, mitogen-activated protein kinase ; MS, multiple sclerosis ; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide ; L-NAME, nitro-L-arginine methyl ester ; NFkB, nuclear factor kB ; NO, nitric oxide ; TNFα, tumor necrosis factor-α.
Abstract
Abstract : The induction of inducible nitric oxide synthase (iNOS) by proinflammatory cytokines was studied in an oligodendrocyte progenitor cell line in relation to mitogen-activated protein kinase (MAPK) activation and cytokine-mediated cytotoxicity. When introduced individually to cultures of CG4 cells, the cytokines, i.e., tumor necrosis factor-α (TNFα), interleukin-1 (IL-1), and interferon-γ (IFNγ), had either minimal (TNFα) or no (IL-1 and IFNγ) detectable stimulatory effect on the production of nitric oxide. However, combinations of these factors, in particular, TNFα plus IFNγ, elicited a strong enhancement of nitric oxide synthesis and, as revealed by western blot and RT-PCR analysis, the expression of iNOS. TNFα and IL-1 were able to activate p38 MAPK in a time- and dose-dependent manner and together showed a combinatorial effect. In contrast, IFNγ neither activated on its own nor enhanced the activation of p38 MAPK in response to TNFα and IL-1. However, a specific inhibitor of p38 MAPK, i.e., SB203580, inhibited the induction of iNOS in cytokine combination-treated cells in a dose-dependent manner, thereby suggesting a role for the MAPK cascade in regulating the induction of iNOS gene expression in cytokine-treated cells. Blocking of nitric oxide production by an inhibitor of iNOS, i.e., nitro-L-arginine methyl ester, had a minimal protective effect against cytokine-mediated cytotoxicity that occurred before the elevation of nitric oxide levels, thereby indicating temporal and functional dissociation of nitric oxide production from cell killing.
Nitric oxide (NO), a short-lived, highly reactive free radical, has been implicated in several inflammatory and degenerative diseases of the CNS (Dawson and Dawson, 1996 ; Bolaños et al., 1997 ; Parkinson et al., 1997 ; Brosnan et al., 1998). Whereas NO derived from neuronal NO synthase may play a role in neuronal degeneration and death (Dawson and Dawson, 1996) besides playing a role in neurotransmission, that produced by inducible NO synthase (iNOS ; the high output isoform of NO synthase) is thought to contribute significantly to the pathogenesis of the inflammatory demyelinating disease, multiple sclerosis (MS) (Parkinson et al., 1997 ; Brosnan et al., 1998). There is evidence for the transcriptional induction of iNOS in the CNS associated with MS and experimental allergic encephalitis, an animal model of MS (Koprowski et al., 1993 ; Bagasra et al., 1995). In these and other inflammatory conditions, iNOS is thought to be expressed mainly by activated astrocytes and microglia, the two glial cell types involved in intracerebral immune regulation (Mucke and Eddleston, 1993 ; Perry et al., 1993 ; Merrill and Jonakait, 1995 ; Kreutzberg, 1996). Among the possible inducers of iNOS are proinflammatory cytokines, i.e., interleukin-1 (IL-1), tumor necrosis factor-α (TNFα), and interferon-γ (IFNγ), which are known to accumulate in the diseased brain (for reviews, see Coyle, 1996 ; Merrill and Benveniste, 1996 ; Navikas and Link, 1996 ; Navikas and Link, 1996 ; Ledeen and Chakraborty, 1998). Several in vitro studies have documented the induction of iNOS in glial cells treated with these proinflammatory cytokines and with microbial products, such as bacterial lipopolysaccharide (LPS), or a combination of the two (Murphy et al., 1993 ; Brosnan et al., 1998). In vitro studies have also demonstrated a direct toxicity of NO on oligodendrocytes, suggesting a role for NO in cell injury (Mitrovic et al., 1995). Although astrocytes and microglia may account for most of the NO produced in CNS inflammation, oligodendrocytes themselves could be induced to express iNOS with the possibility of self-injury (Merrill et al., 1997). Thus, an understanding of the regulation of iNOS expression in oligodendrocytes and its possible implications for cell survival/death becomes important with respect to pathogenetic mechanisms of MS and other demyelinating diseases.
We (Bhat et al., 1998) and others (Da Silva et al., 1997) have shown recently that mitogen-activated protein kinase (MAPK) cascades are involved in cytokine and LPS-mediated iNOS induction in astrocytes and microglial cells. In particular, the p38 subgroup of MAPK was found to play an important role as evidenced by a strong inhibition of iNOS gene expression by a specific inhibitor of the kinase, i.e., SB203580. In the present study, we have investigated the expression of iNOS in an oligodendrocyte cell line treated with proinflammatory cytokines in relation to MAPK activation. The results show that, as in the case of astrocytes and microglia, the stress-activated protein kinase pathway, i.e., p38 MAPK, plays a role in the cytokine induction of iNOS gene expression in oligodendrocyte lineage cells. The results also suggest the noninvolvement of endogenously produced NO in the mechanism of cytokine combination (i.e., TNFα plus IFNγ)-mediated cytotoxicity.
MATERIALS AND METHODS
Materials
[γ-32P]ATP and [γ-32P]CTP were purchased from Du Pont-NEN (Boston, MA, U.S.A.). Calf serum (supplemented), fetal calf serum, Dulbecco's modified Eagle medium, and MLV reverse transcriptase were from GibcoBRL (Grand Island, NY, U.S.A.). Nitro-l-arginine methyl ester (l-NAME), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and antibiotic-antimycotic mixture were from Sigma (St. Louis, MO, U.S.A.). IFNγ was from Pepro Technology (Rocky Hill, NJ, U.S.A.). Recombinant TNFα and IL-1 were from R&D Systems (Minneapolis, MN, U.S.A.). Anti-phospho-specific p38 MAPK (Tyr182) antibody was purchased from New England Biolabs (Beverly, MA, U.S.A.). Anti-total p38 MAPK was from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Monoclonal anti-iNOS antibodies were obtained from Transduction Laboratories (Lexington, KY, U.S.A.). Taq DNA polymerase was from Boehringer-Mannheim Corp. (Indianapolis, IN, U.S.A.). SB203580 was either a gift from Dr. J. C. Lee (SmithKline Beecham, Philadelphia, PA, U.S.A.) or purchased from Calbiochem Corp. (San Diego, CA, U.S.A.). Six-well, 12-well, and 100-mm culture dishes were purchased from Corning-CoStar (Cambridge, MA, U.S.A.). Pregnant rats (Sprague-Dawley strain) were procured from Harlan Sprague-Dawley (Indianapolis, IN, U.S.A.).
Cell culture
The cultures of CG4 cells, maintained as described previously (Bhat and Zhang, 1996), were subcultured in 30% B104 neuroblastoma-conditioned medium and after 2 days changed to conditioned medium-free chemically defined medium. After an additional 24 h, the cultures were treated with the cytokines and assayed for nitrite production and iNOS expression.
Western blot analysis
Western blot was performed for the analysis of p38 MAPK activation (using antibodies specific for the phosphorylated form of the kinase) and for the detection of iNOS. In brief, protein samples (cell extracts, 20 μg of protein equivalents) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto a polyvinylidene difluoride membrane. The membrane was blocked with 5% bovine serum albumin, 1% milk powder in 10 mM Tris-HCl containing 150 mM NaCl, and 0.5% Tween 20 (TBST) for 1 h and incubated overnight with suitably diluted primary antibodies. Following extensive washing with TBST, the membrane was incubated with anti-IgG-alkaline phosphatase conjugate. The blot was finally developed with the alkaline phosphatase substrate 5-bromo-4-chloro-3-indolyl phosphate (50 μg/ml) along with nitroblue tetrazolium (100 μg/ml) in sodium glycinate buffer (pH 9.6) in the presence of 4 mM MgCl2. In some cases, the enhanced chemiluminescence method was used to detect the bands.
Measurement of nitrite production
NO production was determined by measurement of nitrite in the medium as described previously (Bhat et al., 1998), based on the Griess reaction (Stuehr and Nathan, 1989). An aliquot of the spent medium was mixed with an equal volume of a 1:1 mixture of 1% sulfanilamide in water and 0.1% N-1-naphthyl-ethylenediamine dihydrochloride in 5% phosphoric acid. The absorbance was then read at 570 nm. Sodium nitrite dissolved in the culture medium was used as the standard.
PCR analysis of iNOS gene expression
A semiquantitative RT-PCR assay was used to determine the mRNA levels of iNOS in relation to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) message as described previously (Bhat et al., 1998). In brief, 2 μg of total RNA, extracted by using the RNA-STAT kit (Tel-Test, Friendwood, TX, U.S.A.), was used for cDNA synthesis by reverse transcription with 20 units of reverse transcriptase in RT buffer in the presence of a 0.5 mM concentration of each dNTP, 20 units of RNase inhibitor, and random hexamers as primers. The thermal cycler (Cetus 480) was programmed for 50 min at 42°C, 1 min at 25°C, and 5 min at 95°C. A 1% concentration of each cDNA synthesized in the RT reaction was used for PCR amplification in the presence of 1 unit of Taq DNA polymerase in Taq buffer, a 0.2 mM concentration of each dNTP, and a 1 μM concentration of each primer. Each sample was amplified for 35 cycles using a three-step program (15 s at 95°C, 30 s at 60°C, and 30 s at 72°C). After amplification, the products were separated on an agarose (1%) gel (cast in the presence of ethidium bromide) and visualized under UV light. In some cases, the separated products were transferred to nylon and the blots probed with end-labeled oligonucleotides internal to the PCR primer sequences.
MTT assay
The MTT assay was performed as described by Mosmann (1983). MTT was dissolved in phosphate-buffered saline at 5 mg/ml. The stock solution was filtered through a Millipore filter (0.22 μm) and added to the culture medium at a dilution of 1:10. The plates were incubated at 37°C for 2 h. The dark brown formazan crystals formed after the reduction of the tetrazolium ring by the mitochondria of living cells were dissolved in acidified (40 mM HCl) isopropanol, and after centrifugation, the optical density of the supernatant was read at 570 nm.
RESULTS
NO production in cytokine-treated CG4 cultures was determined as nitrite (a stable oxidation product of NO) released into the culture medium by using a colorimetric method (see Materials and Methods). As shown in Fig. 1A, the individual cytokines, IL-1 and IFNγ, had no apparent effects, and TNFα had only a slight stimulatory effect on the production of NO. However, both IL-1 and IFNγ synergized with TNFα to induce NO synthesis. IFNγ, in particular, enhanced NO synthesis by six- to sevenfold. To determine the expression of iNOS that catalyzes the production of NO in cytokine-treated cultures, we carried out immunoblot analysis using antibodies specific for iNOS. The data illustrated in Fig. 1B confirm the induction of iNOS synthesis in response to cytokine combination. The cultures treated with TNFα and IFNγ individually did not contain detectable immunoreactive iNOS protein (data not shown).
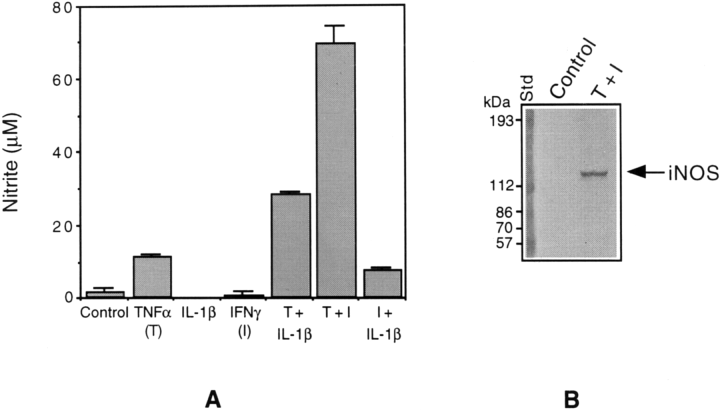
Cytokine stimulation of nitrite production and iNOS synthesis in CG4 cells. A : The cultures were treated with TNFα (50 ng/ml), IL-1 (50 ng/ml), and IFNγ (15 ng/ml) individually and in different combinations for 3 days, and aliquots of the culture medium were mixed with Griess reagent for the estimation of nitrite produced (Materials and Methods). The optical density of the colored product was measured at 570 nm. The values shown are means ± SD of data from triplicate determinations. T, TNFα ; I, IFNγ. B : Aliquots of the cell extracts (20 μg of protein equivalents) prepared from control and cytokine (TNFα plus IFNγ)-treated cultures were subjected to immunoblot analysis using antibodies specific for iNOS. Note : Each experiment described in the present study has been repeated at least three times with identical or similar results.
The steady-state levels of mRNAs for iNOS and GAPDH (a housekeeping gene product used as internal control) in glial cultures treated with the cytokines, individually and in combination, were determined by RT-PCR. Southern hybridization using labeled probes internal to the primers was performed to confirm the identities of the two gene products (data not shown). Figure 2A shows iNOS mRNA levels in cultures exposed to TNFα, IFNγ, and a combination of the two for 40 h. Consistent with the above findings on NO production and iNOS protein expression, a combination of TNFα and IFNγ resulted in a strong induction of iNOS mRNA. The time course of iNOS expression shown in Fig. 2B indicates a linear increase in mRNA levels starting as early as 4 h and reaching a maximal steady state by 48 h following treatment with cytokine combination.
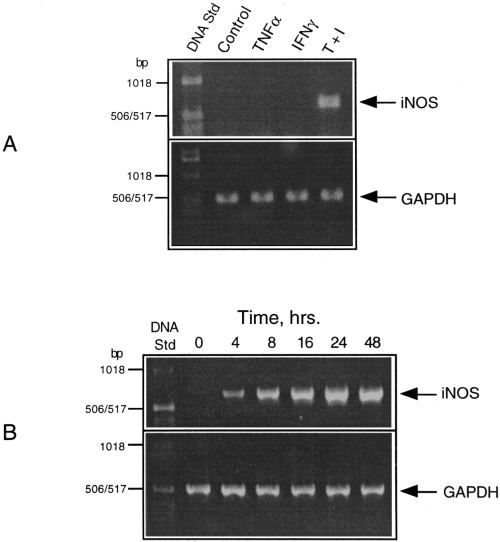
RT-PCR analysis of iNOS gene expression in cytokine-treated CG4 cultures. A : Samples of RNA isolated from cultures treated with TNFα, IFNγ, and a combination of the two (T + I) for 6 h were subjected to RT-PCR using the primers specific for iNOS and GAPDH as described in Materials and Methods. B : Time course of iNOS expression in response to cytokine combination.
Our previous studies have indicated a key role of MAPK cascades, in particular, p38 MAPK, in LPS and cytokine induction of iNOS in astrocytes and microglia (Bhat et al., 1998). To test the activation of p38 MAPK in response to cytokine treatment, immunoblot analysis was performed using antibodies specific for the phosphorylated form of p38 kinase. The validity of using this technique to determine the activation of the kinase has been confirmed previously (Bhat et al., 1998). As shown in Fig. 3A and B, there was a concentration- and time-dependent activation of p38 MAPK in response to TNFα treatment. Parallel blots run as controls using antibodies directed against the total antigen did not show any changes (Fig. 3B). The immunoblot profile and its quantitative analysis shown in Fig. 3DI indicate that IFNγ neither stimulated the phosphorylation of p38 MAPK on its own nor enhanced the kinase response to TNFα. Figure 3C shows the time-dependent activation of p38 MAPK in IL-1-treated cultures in the presence and absence of TNFα. There was rather a strong activation of p38 MAPK by IL-1, which was enhanced further by TNFα as confirmed by densitometric analysis (Fig. 3DII).
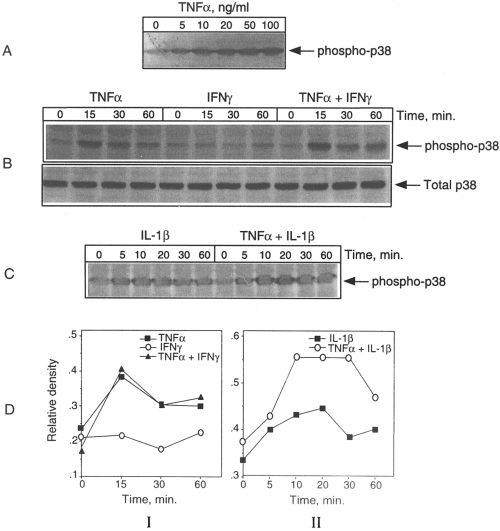
Cytokine activationofp38 MAPK in CG4 cells. The cultures were fed fresh medium and, after 2 h, treated with TNFα either atdifferent concentrations (A) or at 50 ng/ml with and without IFNγ (15 ng/ml) for various time periods (B) The cultures were also treated with IL-1 ineither the absence or presence of TNFα for various time periods (C). Aliquots of the cell lysates were then subjected to immunoblot analysis using antibodies specific for the active (phosphorylated) form of p38 MAPK. Parallel blots run with anti-total p38 MAPK antibodies served as controls (e.g., B) The blots shown in B and C were analyzed densitometrically and the results included as DI and DII, respectively. It is clear that IFNγ neither had a significant effect by itself nor enhanced the effect of TNFα on p38 MAPK activation. However, there was a combinatorial effect of TNFα and IL-1on p38 phosphorylation.
Specific inhibitors of MAPKs have been useful in testing the roles of the kinase pathways in cellular responses to agents that activate MAPKs. We have described previously the use of SB203580, a highly specific inhibitor of p38 MAPK, to delineate the role of p38 kinase activation in LPS induction of iNOS expression in astrocytes and microglia (Bhat et al., 1998). As shown in Fig. 4A, the inclusion of SB203580 in the presence of the two cytokines drastically suppressed the induction of NO production. The block of NO synthesis correlated with an almost complete inhibition of the expression of iNOS protein as determined by immunoblot analysis (Fig. 4B).
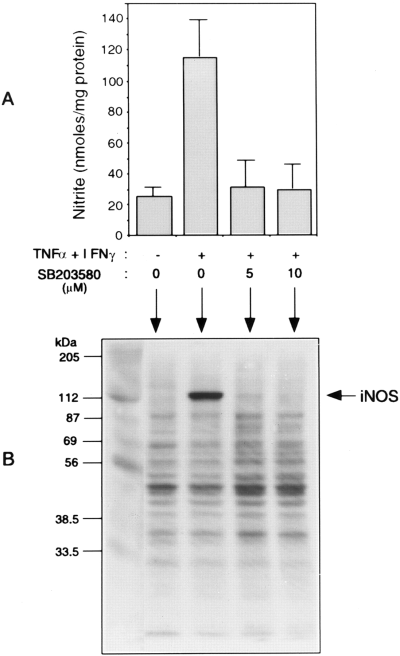
Inhibition of cytokine-induced nitrite production and iNOS expression by the p38 MAPK inhibitor, SB203580. A : CG4 cultures were treated with IFNγ plus TNFα in the presence and absence of the MAPK inhibitor (5 and 10 μM) for 72 h. Aliquots of the culture medium were analyzed for nitrite production. B : The cell extracts from cultures treated with the cytokine combination in the presence and absence of SB203580 (10 μM) and untreated control were analyzed for iNOS expression by immunoblot using anti-iNOS antibodies. The values for nitrite synthesis are means ± SD of data from triplicate determinations.
We next examined the effect of the kinase inhibitor on iNOS mRNA levels in cytokine-treated cultures. CG4 cultures were treated with the two cytokines in either the presence or absence of different concentrations of SB203580 for 40 h before RNA preparation followed by RT-PCR. As shown in Fig. 5A, the kinase inhibitor suppressed the expression of iNOS mRNA in a dosedependent manner. The sensitivity of iNOS expression to the kinase inhibitor was evident throughout the induction period as depicted in Fig. 5B.
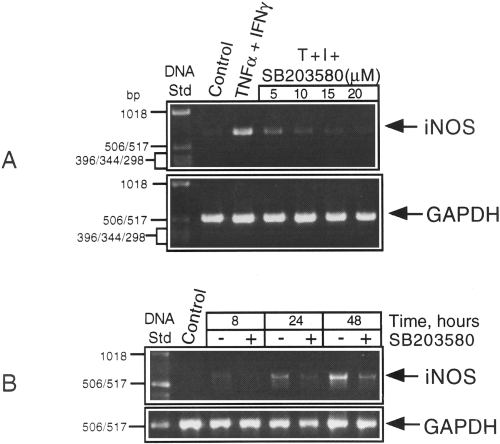
Effects of the MAPK inhibitor on iNOS mRNA levels in cytokine-treated CG4 cultures. A : RNA samples were isolated from cultures treated with TNFα (T) plus IFNγ (I) either alone (control) or in the presence of different concentrations of SB203580 for 40 h and subjected to RT-PCR using the primer pairs specific for iNOS and GAPDH. B : Cultures treated with the cytokine combination for different times in the presence and absence of a constant amount (10 μM) of SB203580 were extracted for RNA followed by RT-PCR. The products were run on a 1% agarose gel impregnated with ethidium bromide and the bands visualized under UV and photographed.
In a related study, we have found that a combination of TNFα and IFNγ exerts a potent cytotoxic effect on CG4 cells and in primary oligodendrocyte progenitors (Andrews et al., 1998). Because a possible mechanism of cytotoxicity could involve NO production, it was of interest to examine the viability of the cells treated with the cytokine combination in the presence of an inhibitor of iNOS activity, i.e., L-NAME. As shown in Fig. 6A, although the compound was quite effective in blocking the production of NO in cytokine-treated cultures (upper panel), it could only modestly reverse the cytotoxic effect (quantified using the MTT reduction assay as described in Materials and Methods) of the cytokine combination (Fig. 6, lower panel). The kinase inhibitor SB203580 was also ineffective in suppressing the cytokine toxicity as depicted in Fig. 6B.
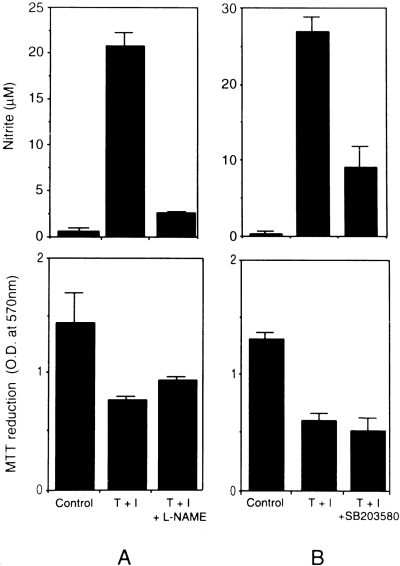
Effect of blocking nitrite productionof CG4 cell survival/death in response to cytokine treatment. The cultures were treated with the cytokine combination (T + I) in the presence or absence of (A) the iNOS inhibitor, l-NAME (1 mM), and (B) the kinase inhibitor, SB203580 (10 μM), for 72 h and analyzedfor nitrite production and cell survival by MTT reduction as described in Materials and Methods.
DISCUSSION
The results presented in this report demonstrate cytokine-mediated induction of iNOS gene expression in an oligodendrocyte cell line and a key role of p38 MAPK signaling in this process. Specifically, treatment of CG4 cells with cytokine combinations, in particular, TNFα plus IFNγ, resulted in an induced production of NO and the expression of iNOS as determined by western blot and RT-PCR analysis of the protein and the message levels, respectively. A specific inhibitor of p38 kinase (i.e., SB203580) inhibited cytokine-induced production of NO and the expression of iNOS, thereby suggesting a role for this kinase cascade in cytokine-induced iNOS gene expression. The findings parallel previously reported results with astrocytes and microglia on MAPK regulation of the expression of iNOS (Bhat et al., 1998).
RT-PCR data on iNOS mRNA levels have indicated the regulation of iNOS gene expression at the level of transcription and/or mRNA stability. It is known that besides transcriptional activation, iNOS gene expression is regulated at the level of mRNA stability and translational efficiency (Lowenstein et al., 1993 ; Xie et al., 1993). Transcriptional regulation of iNOS is complex, involving a number of factors including nuclear factor κB (NFκB), AP-1, and various members of the C/EBP, ATF/CREB, and STAT family (Lowenstein et al., 1993 ; Xie et al., 1993). Several of these (e.g., AP-1, ATF, and CREB) are either direct or indirect targets of p38 MAPK (Cohen, 1997) and, thus, may account for the observed inhibition of iNOS gene expression by a specific inhibitor of the kinase. It is interesting that p38 MAPK [and the extracellular signal-regulated kinase (ERK)] pathway, in addition to signaling to nuclear factors, can exert translational control involving downstream kinases and their target proteins that specifically bind mRNA. One of these targets is the translation initiation factor, eIF-4E, which plays a key role in the regulation of translation in mammalian cells (Flynn and Proud, 1996). It is phosphorylated at Ser209 by a Ser/Thr kinase termed MAPK-interacting kinase 1 (MNK1), which lies downstream of p38 MAPK and ERK (Fukunaga and Hunter, 1997 ; Waskiewicz et al., 1997). The phosphorylation enhances the affinity of eIF-4E for the 5′ cap structure of mRNAs, resulting in translational activation. Although the details on the posttranscriptional regulation of iNOS gene expression are not well understood, the presence of many cis and trans determinants controlling mRNA stabilization and translational efficiency point toward regulatory protein-mRNA interactions. For example, iNOS mRNA from rat astrocytes and mouse macrophages contain several AUUUA motifs in the 3′-untranslated region (Galea et al., 1994) that confer instability on mRNA and are the targets of specific AU-binding factors (Chen and Shyu, 1995). It has been suggested that in glial cells (astrocytes), iNOS mRNA stability may be both translation- and transcription-dependent (Park and Murphy, 1996). Future experiments would determine the rate of iNOS mRNA turnover in the presence and absence of the kinase inhibitors and the factors that might regulate mRNA stability.
Our results show that iNOS expression requires combinatorial actions of the cytokines. This effect is consistent with several findings showing a synergistic action of IFNγ with other cytokines, such as TNFα and IL-1β, to induce iNOS mRNA expression and NO production in many cell types, including astrocytes (Murphy et al., 1993 ; Kopnisky et al., 1997). The synergism seems to involve activation of multiple intracellular signaling pathways, many of which may be essential but not sufficient for the response to occur. Thus, although the data presented indicate an essential role for p38 MAPK in cytokine combination-induced iNOS expression, the activation of the kinase alone does not correlate with the observed response. For example, the level of activation of p38 in response to TNFα is the same in the presence and absence of IFNγ, although the combination of the two cytokines proves to be a potent inducer of iNOS expression. Similarly, despite a strong activation of p38 MAPK, IL-1 cannot increase NO production. That p38 MAPK activation alone is insufficient to induce iNOS has also been brought out by a recent study with rat pancreatic cells responding to hyperosmolarity, which, despite a substantial activation of the kinase, cannot induce iNOS expression (Larsen et al., 1998). Our recent findings show that activation of a related MAPK pathway, i.e., ERK, is also required for iNOS expression in astrocytes and microglia (Bhat et al., 1998). However, in the present cell system (i.e., oligodendrocytes), the cytokines were unable to activate this kinase. Other mechanisms that may contribute to the combinatorial effects of the cytokines include the activation of c-Jun N-terminal kinase (JNK), NFκB, JAK-STAT, and related interferon-regulatory factor-1. Both NFκB and interferon-regulatory factor-1 are known to be required for optimal expression of iNOS gene (Lowenstein et al., 1993 ; Xie et al., 1993 ; Kamijo et al., 1994). Although the role of JNK in iNOS expression is not yet clear, we have observed a strong activation of JNK in CG4 cells treated with TNFα (Zhang et al., 1996 ; Andrews et al., 1998). The cytokine also synergizes with IFNγ to activate NFκB in these cells (Andrews et al., 1998).
Our observation of the ability of oligodendrocyte lineage cells to express iNOS is in agreement with a previous study with primary oligodendrocyte cultures (Merrill et al., 1997). In that study, however, the cells were stimulated with a combination of IFNγ and LPS. We could not detect an effect of this combination on NO production by CG4 cells (data not shown). iNOS induction by cytokine combination in oligodendrocyte lineage cells as demonstrated in our study may be relevant to the perpetuation of the inflammatory processes occurring in MS brain, wherein both TNFα and IFNγ are known to accumulate. As suggested by Merrill et al. (1997), a physiological consequence of NO production in oligodendrocytes may be self-injury. In this context, we have found that the two cytokines in combination elicit severe cytotoxicity towards CG4 cells and primary oligodendrocyte progenitors (Andrews et al., 1998), prompting a consideration of the possibility that the NO produced may mediate the toxic effect of the cytokines. Two observations in the present study are at odds with this idea. First, there is a temporal difference between the development of toxicity (<24 h) (Andrews et al., 1998) and the production of significant levels of NO (2-3 days). Second, an inhibition of NO production by an inhibitor of iNOS had only modest protection against cytokine toxicity. It will be of interest to test if endogenously produced NO has distinct cellular targets and effects, and if it renders the cell population producing it either resistant or hypersensitive to NO-mediated toxicity, a scenario described in a recent study with a macrophage cell line (Mohr et al., 1998).
Acknowledgements
This study was supported by grants (RG2481 and RG2849) from the National Multiple Sclerosis Society. We thank Ms. Elaine Terry and Ms. Sallie Bendt for excellent technical and secretarial assistance.




