Molecular Cloning of a Putative Cyclic Nucleotide-Gated Ion Channel cDNA from Limulus polyphemus
The nucleotide sequence for Lcng1 has been deposited in the GenBank database under GenBank accession no. AF091302.
Abbreviations used : cGMP, cyclic GMP ; CNG, cyclic nucleotide-gated ; eag, ether-a-go-go ; LCNG1, a putative Limulus cyclic nucleotide-gated channel subunit ; ORF, open reading frame ; pfu, plaque-forming units ; RPA, RNase protection assay ; SH3, Src homology domain 3 ; UTR, untranslated region.
Abstract
Abstract : Cyclic nucleotide-gated channels have been proposed to mediate the electrical response to light in the ventral photoreceptor cells of the horseshoe crab, Limulus polyphemus. However, a cyclic nucleotide-gated channel has not been identified from Limulus. We have cloned a putative full-length cyclic nucleotide-gated channel cDNA by screening cDNA libraries constructed from Limulus brain using a probe developed from Limulus ventral eye nerves. The putative full-length cDNA was derived from two overlapping partial cDNA clones. The open reading frame encodes 905 amino acids ; the sequence shows 44% identity to that of the α subunit of the bovine rod cyclic GMP-gated channel over the region containing the transmembrane domains and the cyclic nucleotide binding domain. This Limulus channel has a novel C-terminal region of ~200 amino acids, containing three putative Src homology domain 3 binding motifs and a putative coiled-coil domain. The possibility that this cloned channel is the same as that detected previously in excised patches from the photoreceptive membrane of Limulus ventral photoreceptors is discussed in terms of its sequence and its expression in the ventral eye nerves.
Cyclic GMP (cGMP) is the intracellular second messenger for phototransduction in vertebrate photoreceptors, where it directly activates cyclic nucleotide-gated (CNG) channels in the plasma membrane of the outer segment (Fesenko et al., 1985 ; Kaupp et al., 1989). It has also been proposed as an intracellular second messenger that activates ion channels in horseshoe crab (Limulus polyphemus) photoreceptors. Injection of cGMP into Limulus ventral photoreceptor cells partially depolarizes the photoreceptors, and channels in excised patches from the photoreceptive membranes can be activated directly by cGMP (Johnson et al., 1986 ; Bacigalupo et al., 1991).
As a first step toward resolving the role of CNG channels in Limulus phototransduction, we have cloned a putative CNG channel cDNA, Lcng1, from Limulus. This putative Limulus CNG channel subunit (LCNG1) shares significant homology with the α subunit of the bovine rod cGMP-gated channel. The sequence also reveals a novel long C terminus. RT-PCR experiments indicate that the Lcng1 gene is expressed in both Limulus brain and ventral eye nerve.
MATERIALS AND METHODS
Materials and tissue preparation
Horseshoe crabs were either purchased from the Martin Fish Co. (Ocean City, MD, U.S.A.) or the Marine Biological Laboratory (Woods Hole, MA, U.S.A.) or caught in the Indian River of Florida. Dissected, live tissues were either used immediately or frozen in liquid nitrogen and stored at—80°C.
Ventral eye nerves were cut at least several millimeters away from the brain to avoid brain tissue contamination. In desheathing nerves, both the sheath around the nerve and the modified patch of cuticle at the eye terminal were removed. Brains were dissected free of surrounding nerves. The coax extensor muscle of the leg was chosen for muscle total RNA isolation. Hemolymph was withdrawn from the pericardial cavity by inserting a needle (18 gauge) through the membrane of the hinge and immediately mixed with a 0.4 volume of an ice-cold solution containing 5% dimethyl sulfoxide in 3% NaCl to avoid coagulation. Limulus amebocytes were pelleted by centrifugation at 1,300 g for 15 min at 4°C.
RNA isolation
For large-scale total RNA preparation, CsCl centrifugation was used (MacDonald et al., 1987). A typical isolation used 12-18 Limulus brains. Samples prepared by this method were used for cDNA library construction, primer extension experiments, and RT-PCR.
For small-scale preparation, a TriZOL RNA isolation method (GibcoBRL, Gaithersburg, MD, U.S.A.) was used. One typical isolation used one brain, one to six ventral eye nerves, one to eight desheathed ventral eye nerves, 1 g of muscle, or the amebocytes collected from 20-50 ml of hemolymph. RNA samples isolated by this protocol were used for RT-PCR and RNase protection assay (RPA).
The mRNA was isolated using FastTrack or Micro FastTrack mRNA isolation kits (Invitrogen, San Diego, CA, U.S.A.). Each large-scale isolation used 12-18 Limulus brains. Each small-scale isolation used one brain or four to 10 ventral eye nerves. The mRNA was used for cDNA library constructions and RT-PCR.
RT-PCR
First-strand cDNA was synthesized from 1-10 μg of RNA or from the entire RNA preparation of a small amount of tissues. RNA was denatured at 68°C for 5 min in the presence of primer immediately before reactions. A typical reaction mixture (20 μl) contained RNA, each deoxynucleotide triphosphate at 0.25 mM, 50 pmol of primer, and 200 units of reverse transcriptase (SuperScript II ; Gibco BRL). Reactions were carried out at 42-45°C for 1-2 h.
A typical PCR assay (50 μl) contained 10-25% of one RT reaction as the template, 1 mM MgCl2, each deoxynucleotide triphosphate at 0.1-0.2 mM, 10-40 pmol of each primer, and 2.5 units of Taq DNA polymerase. The PCR was typically programmed as follows : a first denaturing step at 94°C for 2 min followed by one cycle of amplification with an annealing step at 60-65°C for 2 min and an extension step at 72°C for 2 min and then 30-35 cycles with a denaturing step at 94°C for 30 s, an annealing step at 56°C for 1 min, and an extension step at 72°C for 1-2 min.
cDNA probe development
Three partially degenerate primers were synthesized according to three motifs within the cyclic nucleotide binding domain in the CNG channel family. These three motifs were found to have almost identical amino acids and highly similar nucleotide sequences in all of the α subunits of the six CNG channel sequences published by 1993 (Kaupp et al., 1989 ; Dhallen et al., 1990, 1992 ; Ludwig et al., 1990 ; Goulding et al., 1992 ; Pittler et al., 1992). Their nucleotide and amino acid (in parentheses) sequences are as follows : upstream primer 1, CC(T/C)GG(A/C)GA(T/C)TA(T/C)AT(T/C)TGC (PGDYIC, amino acids 533-538 ; bp 2,069-2,086) ; upstream primer 2, GG(A/C)(A/C)(A/G)NGAGATGTACAT (GKEMYI, amino acids 544-549 ; bp 2,102-2,119), where N stands for any of the four nucleotides ; and downstream primer, AG(A/T/G)AT(A/G)CT(A/G)AT(T/C)TC(A/G)CC(A/G)AA (FGEISI, amino acids 575-580 ; bp 2,195-2,214).
The primer pair composed of upstream primer 1 and the downstream primer was used to amplify cDNA primed by oligo(dT) from brain total RNA. The RT-PCR procedures, which generated bands of ~150 bp, were further screened by the nested primers (upstream 2 and downstream). One ~150-bp band, from which a 130-bp band was amplified using the nested primers, was subcloned and sequenced. One 146-bp fragment was identified as having 63% identity with the cGMP binding domain of the bovine rod CNG channel α subunit. The PCR protocol used for these initial RT-PCR procedures was slightly different from those that were used later. The annealing temperature for the first 10 cycles was slowly decreased from 65 to 56°C. The reaction continued for another 20 cycles with an annealing temperature of 56°C.
The 146-bp fragment was used as a probe to screen a genomic library described below. One partial genomic clone contained three open reading frames (ORFs), which were later shown to be exons (labeled C1, C2, and C3 in Fig. 2A). The adjoining regions of the first two exons (C1 and C2) are identical to the 146-bp probe. Two primers near the ends of the first two exons (bp 1,800-1,821 and 2,319-2,338) were used to amplify a 539-bp cDNA fragment by RT-PCR from ventral eye nerve mRNA. The sequence of this 539-bp fragment was identical to a continuous sequence combined from exons C1 and C2. This 539-bp fragment was then used as a hybridization probe in cDNA library screenings. A detailed description and all the experimental results regarding the development of the cDNA probe have been described in a Ph.D. dissertation (Chen, 1997).
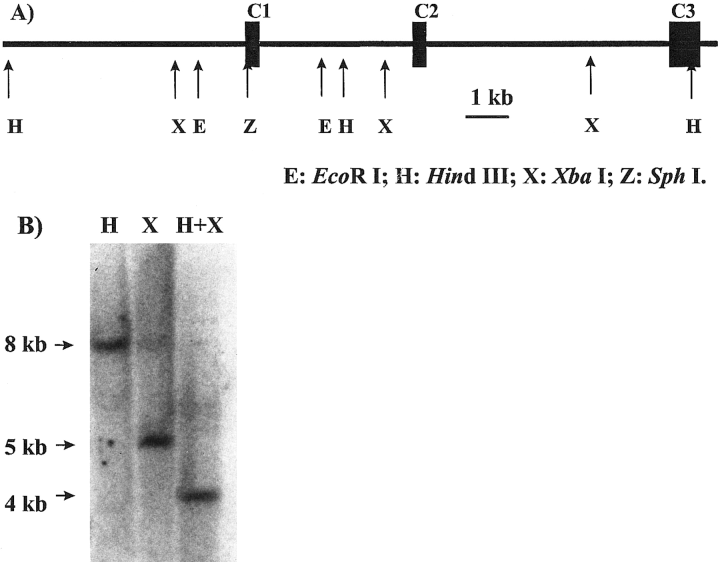
Genomic Southern blot demonstrates the presence of Lcng1 in Limulus genome. A : The restriction map for a partial genomic clone corresponding to the 3' end of Lcng1. E, EcoRl ; H, Hindll ; X, Xbal ; Z, Sphl. B : Genomic Southern blot corresponding to the digest by Hindlll (H), Xbal (X), or Hindlll + Xbal (H + X). The probe was a sense riboprobe of 294 bases corresponding to exon C1 as depicted in A and was labeled by 32P.
cDNA library construction and screening
Brains were chosen for cDNA library construction because brain tissue could be more readily obtained in great quantity than ventral eye nerves. It has also been shown that ventral photoreceptors are present not only in the ventral eyes and along the length of the ventral eye nerves, but also at the base of the nerves and in the brain (Clark et al., 1969 ; Chamberlain and Wyse, 1986).
Two cDNA libraries were used to obtain the full-length cDNA sequence. The first library was constructed from Limulus brain mRNA and primed by an oligo(dT) primer. The cDNA was cloned into the λZap-Express vector (Stratagene, La Jolla, CA, U.S.A.). The primary library contained 3.6 × 106 primary plaque-forming units (pfu) and was amplified once. The majority of the synthesized cDNA was between 1.5 and 2 kb. About 4 × 106 plaques of the amplified library were screened by the 539-bp cDNA probe. One clone of 4.3 kb corresponding to the 3′ cDNA sequence was obtained.
The second library was constructed from cDNA primed by a sequence-specific primer. First-strand cDNA was synthesized from brain total RNA using an antisense primer (5′-GAGTC-TACCGCCACTTCT-3′) derived from the 3′ cDNA clone (bp 2,678-2,695). The cDNA was cloned into the λZap-Express vector. The primary library contained 3.8 × 105 pfu. The entire library was amplified once. About 350,000 plaques were screened by the same 539-bp cDNA probe. Three overlapping clones of 265 putative positive plaques were selected for further screening. The longest one (2.7 kb) contained the overlapping region, and the 5′ upstream region extended from the 3′ cDNA clone.
Genomic DNA isolation from Limulus amebocytes
The genomic DNA intended for library construction was purified from amebocytes using a CsCl centrifugation method (Bingham et al., 1981). A suspension of amebocytes was incubated for 1 h on ice with 2% Sarkosyl. The lysate was digested by protease K (100 μg/ml) at 50°C for 3 h. After the protease K digest, CsCl was added at 1.0 g/ml. The sample was centrifuged at 278,000 g (Beckman 70.1Ti rotor) for 72 h at 15°C. After the centrifugation, the tube was punctured near the bottom of the tube, and the viscous fractions containing DNA were collected. The DNA sample was combined and dialyzed against three changes of 1 L of TE buffer [10 mM Tris-HCl (pH 7.4) and 1 mM EDTA] every 8 h at 4°C to remove CsCl. The size of the isolated DNA appeared to be >100 kb on a 0.5% agarose gel.
To isolate the genomic DNA for Southern blot analysis, a commercial kit (Easy-DNA ; Invitrogen) was used. Amebocytes collected from 60 ml of Limulus hemolymph were used for one isolation. The purified DNA had a ratio of A260 to A280 between 1.6 and 1.8. The size appeared to be > 50 kb on a 0.5% agarose gel.
Genomic DNA library construction
A genomic DNA library was constructed into a Lambda Fix II vector (Stratagene). Genomic DNA from amebocytes was partially digested by a series of different concentrations of Sau3AI for 1 h at 37°C to determine the optimal enzyme/DNA ratio for a partial digest. The same enzyme/DNA ratio was then applied to a large-scale partial digest with 100 μg of DNA. The partially digested DNA was separated by an equilibrium centrifugation in a continuous sucrose gradient (10-40%, 154,000 g for 48 h). DNA with sizes between 15 and 25 kb was used as the insert. The primary titer of the library was 1.2 × 107 pfu. About 1.5 × 106 primary phages were screened with a probe derived from a 146-bp PCR product within the cGMP binding domain of LCNG1 (see above) to obtain four overlapping genomic clones that contained three exons coding for the C terminus of LCNG1.
Genomic Southern blot analysis
One hundred micrograms of genomic DNA was digested by 500-2,500 units of restriction enzymes (GibcoBRL) in a final volume of 1 ml. The digested DNA was precipitated by ethanol and loaded onto a 0.8% agarose gel. After electrophoresis, the DNA was fragmented in 0.2 M HCl for 30 min and then transferred onto a nylon membrane overnight under alkaline transfer conditions.
A riboprobe of 294 bases corresponding to exon C1 (see Fig. 2A) was used for hybridization. It was generated by an in vitro transcription method and labeled with [α-32P]CTP. The membrane was washed with 0.05× standard saline citrate (SSC) at 60°C for 1 h.
Primer extension
Two nested primers derived from the 5′ untranslated region (UTR) of Lcng1 were used. Ex-1 (5′-CATTCTATT-TCATCCTTCCT-3′, bp 31-50) is immediately downstream from Ex-3 (5′-TCACAGTACTAATAAAATATA-ATCATCC-3′, bp 3-30). Primers were labeled by 32P at their 5′ ends by phosphorylation using T4 DNA kinase. Total RNA (equivalent to one brain) isolated from Limulus brains by CsCl centrifugation was hybridized with 40 pmol of antisense primer at 30°C overnight. RNA was denatured at 80°C for 10 min in the presence of primer immediately before hybridization. After hybridization, RNA primer was precipitated by ethanol and resuspended in diethyl pyrocarbonate-treated water.
First-strand cDNA was extended from the annealed primers by SuperScript II at 42°C for 1.5 h. The resultant cDNA was analyzed on a sequencing gel. The experiment was repeated once.
The molecular size markers were generated from a sequencing reaction using a template of the 5′ cDNA clone in pBKCMV. α-35S-dATP was used in the sequencing reaction.
RPA
An antisense riboprobe of 609 bases was generated by in vitro transcription and labeled with [α-32P]CTP, corresponding to the 539-bp cDNA fragment from the cyclic nucleotide binding domain of LCNG1 and 70 bases from the vector sequence. The full-length probe was purified on a 5% acrylamide gel.
A DirectProtect RPA kit (Ambion, Austin, TX, U.S.A.) was used with modification. Total RNA from four to 20 ventral eye nerves or one brain was isolated using the TriZOL method. The RNA pellet was dissolved in the hybridization solution (40 μl). The RNA and probe (0.005-0.02 pmol, 4 × 106-1.2 × 108 dpm/pmol) were incubated at 37°C overnight. After hybridization, an RNase A/T1 mixture was used to digest RNA. The RNases were removed by protease K digest. The products were precipitated by isopropanol and analyzed from a 5% acrylamide gel.
RESULTS
A putative novel CNG ion channel cDNA was isolated from L. polyphemus
A full-length cDNA of 6,237 bp was constructed from two overlapping cDNA clones by screening two Limulus brain cDNA libraries. A 4.3-kb clone was first obtained by screening an oligo(dT)-primed cDNA library using, as a probe, a 539-bp RT-PCR fragment amplified from Limulus ventral eye nerve mRNA (see Materials and Methods). The 4.3-kb clone contained an ORF of 1,318 bp and a 3,050-bp noncoding region, including a poly(A) tract of 19 adenosine residues at the 3′ end. The typical eukaryotic polyadenylation signal [AAUAAA (Proudfoot, 1984)] is present 14 bp upstream of the poly(A) tract. A portion (469 bp) of the 539-bp probe overlapped with the 5′ end of this clone. A second cDNA clone of 2.7 kb was cloned from a cDNA library primed by a gene-specific primer, located 826 bp downstream of the 5′ end of the first cDNA clone. This clone contains a 472-bp noncoding sequence at the 5′ end and an ORF of 2,215 bp and has an overlap of 818 bp with the previous clone (8 bp was presumably removed in creating blunt ends of cDNA during library construction). The putative full-length cDNA contains an ORF of 2,715 bp and UTRs of 472 bp at the 5′ end and 3,050 bp at the 3′ end. The translation initiation codon was assigned to be the first AUG (bp 473-475) in the ORF (the second in-frame AUG is 231 bp downstream). The in-frame termination codon was UGA (bp 3,188-3,190). This cDNA is named Lcng1.
Figure 1 shows the deduced amino acid sequence of LCNG1, which is aligned with the cGMP-gated channel α subunit from bovine rod (CNG1) and Drosophila melanogaster (DmCNG), and its hydropathicity profile. The deduced protein is 905 amino acids long, and its calculated molecular size is 102,956 daltons. This protein shares 44% identical amino acids with the α subunit of the bovine rod cGMP-gated channel and 46% identical amino acids with the Drosophila channel over the region of 452 amino acids containing the transmembrane domains, the voltage sensor S4, the pore, and the cGMP binding domain (Fig. 1A). The hydropathicity plot of the entire protein shows five strongly hydrophobic regions (Fig. 1B). The hydropathicity profile is very similar to those found in all other cloned CNG channels, suggesting that LCNG1 shares a similar topology with CNG channels.

Sequence alignment between LCNG1 and the α subunit of the bovine rod cGMP-gated channel (CNG1) and Drosophila CNG channel (DmCNG) and the hydropathicity profile of LCNG1. A : Sequence alignment. Identical residues are indicated by dark shading, and similar residues are indicated by light shading. Gaps are indicated with hyphens. Transmembrane domains including the S4 voltage sensor, the pore loop, and the cGMP binding domain are underlined with broken lines. Three putative SH3 binding motifs in the C terminus of LCNG1 are boldfaced. CNG1 data are from Kaupp et al. (1989), and DmCNG data are from Baumann et al. (1994). B : The hydropathicity profile indicates five hydrophobic regions (I-V). The hydropathicity plot was calculated according to the methods of Kyte and Doolittle (1982) with a window size of 20 amino acids. The profile was generated with the Pepplot program in the Wisconsin Package (ver. 8, September 1994, Genetics Computer Group, Madison, WI, U.S.A.).
To confirm that the putative cGMP-gated channel cDNA is a Limulus gene and not from a contaminating source, we performed a genomic Southern blot using genomic DNA isolated from Limulus amebocytes and compared the results of the blot with the predictions of a restriction map of a partial genomic clone of Lcng1. We first isolated overlapping partial genomic clones from a Limulus genomic DNA library using a 146-bp probe derived from the cGMP binding region of LCNG1 (see Materials and Methods). Partial sequencing of one of those clones revealed three putative exons, C1-C3 (Fig. 2A). The combined sequences of these exons were identical to a continuous sequence comprising the 3′ end of Lcng1 (C1, bp 1,800-2,093 ; C2, bp 2,094-2,399 ; C3, bp 2,400-3,187). None of the identified partial genomic clones contained any more ORFs upstream of these three exons. The genomic sequence corresponding to the 5′ cDNA is therefore unavailable. A restriction map of the partial genomic clone is shown in Fig. 2A.
A Southern blot was then performed on genomic DNA using a 294-base riboprobe corresponding to the first exon C1 in Fig. 2A as a probe. The genomic DNA was digested by HindIII, XbaI, or HindIII and XbaI (Fig. 2B). According to the restriction map of the genomic clone, the HindIII and XbaI digest should generate 8- and 5-kb fragments, respectively, that can be hybridized by the probe. Double digestion with these two enzymes should produce a 4-kb fragment hybridized by the probe. All three fragments were detected. The restriction digest products by several other enzymes and/or combinations of enzymes shown in Fig. 2A were also detected by the same probe under similar conditions in a total of six Southern blots (data not shown).
To determine how close the 5′ end of Lcngl cDNA was to the beginning of the transcript, a primer extension experiment was performed using two nested antisense primers (EX-1, bp 31-50 ; Ex-3, bp 3-30). Both primers were in the 5′ UTR of Lcng1. They were 20 bases apart at their 5′ ends. Two dominant bands of 116 and 96 bases were synthesized from Limulus brain total RNA by RT from these two primers, respectively (Fig. 3, lanes 6 and 7). These two distinct bands likely represent specific reverse-transcribed products from the channel mRNA because these extended products differed in length by the expected 20 nucleotides. Negative controls (lanes 8 and 9) of RNA samples treated with RNase confirmed that these two bands were synthesized from RNA. This experiment suggests that transcription of the Lcng1 gene likely starts 66 bases upstream of the 5′ end of Lcng1, a conclusion supported by the existence of 14 in-frame stop codons in the 5′ UTR. In addition, the primer extension experiment also demonstrates that the Lcng1 transcript is present in the Limulus brain.
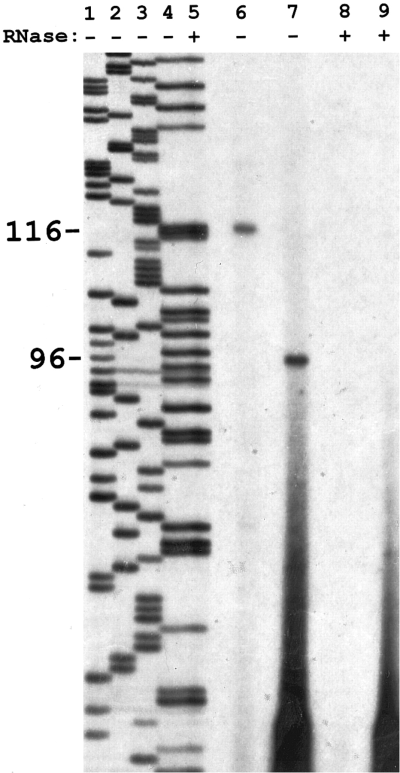
Primer extension determines that the 5' end of Lcng1 cDNA is 66 bases downstream from the transcription initiation site. Two nested antisense primers in the 5' UTR were used in the RT of Limulus brain total RNA. Lanes 6 and 8 were primed by Ex-1 and lanes 7 and 9 by Ex-3. A distinct band is seen at 116 bases in lane 6 and 96 bases in lane 7. Lanes 8 and 9 are negative controls treated with RNase. Lanes 1-5 are molecular size markers generated by a sequencing reaction from the 5' partial clone of Lcng1 in pBK-CMV using Ex-1 as the primer. Lane 5 is the same reaction as in lane 4 but with added RNase to confirm that the RNase used in negative controls was DNase-free. The reverse-transcribed product was labeled by 32P, and the molecular size marker was labeled by 35S. Details of the experiments are given in Materials and Methods. This experiment was repeated once.
Lcng1 gene is expressed in Limulus brain and ventral eye nerves
To test further whether this putative cGMP-gated channel is expressed in the brain and ventral eye nerve, we use an RPA, a method that does not require amplification of the transcript. A 609-base antisense riboprobe corresponding to the cyclic nucleotide binding domain was hybridized to aliquots of total RNA isolated from the brain and the ventral eye nerve. The riboprobe contained the 539-base cDNA sequence and 70 bases of vector sequence. The specific protection of the riboprobe by Lcng1 mRNA should result in a 539-base labeled product. This product was present in an RNA sample from a single brain (Fig. 4A, lane 3). By contrast, no 539-base band was detected in an RNA sample from four ventral eye nerves after the film was exposed overnight (Fig. 4A, lane 2). Increasing the number of ventral eye nerves to 20 allowed the detection of a faint 539-base band in one experiment after exposure for 1 week (Fig. 4B, lane 2). We were concerned that our isolation of RNA from ventral eye nerves might not be sufficiently efficient to obtain enough RNA to detect Lcng1 in small numbers of nerves. However, we could readily detect in the extracts from four nerves the presence of a transcript coding for opsin, the major photoreceptor gene product, using a similar RPA protocol (data not shown ; but see Battelle et al., 1997). The RPA experiments demonstrate the presence of Lcng1 in single brains but suggest that the Lcng1 messenger level in the ventral eye nerve is too low to be detected in small numbers of dissected ventral nerves.
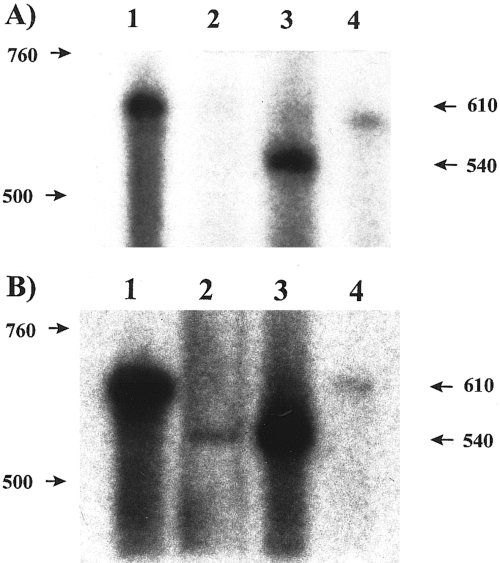
RPAs demonstrate high abundance of Lcng1 transcript in Limulus brain but not in ventral eye nerves. A and B : Lane 1 is the probe control without RNA sample. Lane 2 is the total RNA from either four (A) or 20 (B) undesheathed ventral eye nerves. Lane 3 is the total RNA from one brain. Lane 4 is the negative control without RNA. All four reactions contained the same amount of probe. RNase A/T1 mixture was added to samples in lanes 2-4. The entire reactions were assayed on a 5% acrylamide gel, with the exception of the probe control (lane 1). Only a fraction (5%) of the probe control was assayed to avoid overloading. The antisense riboprobe corresponding to the putative cyclic nucleotide binding domain of LCNG1 was labeled with [α-32P]CTP.
Owing to our failure to detect the transcript in small numbers of ventral eye nerves using RPA, we investigated the presence of the Lcng1 transcript in Limulus ventral eye nerve by RT-PCR. Although the initial cDNA probe was obtained from the ventral eye nerve mRNA, we decided to use RT-PCR once again to test for the presence of the Lcng1 transcript in Limulus ventral eye nerve, this time taking particular care to avoid possible tissue contamination from the brain and genomic DNA contamination in RNA isolations. Ventral nerves were cut several millimeters away from the brain. Primers were chosen from different exons. The sense primer was from exon C1 (bp 1,800-1,821), and the antisense primer was from exon C3 (bp, 2,940-2,957), ~1.2 kb downstream. These primers were also located outside the region that overlaps both partial cDNA clones to avoid contamination from the purified clones present in the laboratory. Owing to the extremely long 3′ UTR, a sequence-specific primer within the coding region (bp, 3,122-3,140) was used for first-strand cDNA synthesis.
A band ~1.2 kb was amplified from RNA material equivalent to one ventral eye nerve (Fig. 5A, lane 1) but not from the same amount of RNA sample treated with RNase (lane 3) or the water control (lane 4). No bands were detected in another negative control of an RNA sample without added reverse transcriptase (data not shown). The sequence of the 1.2-kb band was verified by several restriction enzyme digests. Lane 2 shows one digest by SacII, which generated two bands of the expected sizes. Because the primers used span three exons and are located in two different partial cDNA clones and as the negative control samples produced no amplified products, it is unlikely that the RT-PCR product was amplified from contaminated genomic DNA or any of the two partial cDNA clones in the laboratory.
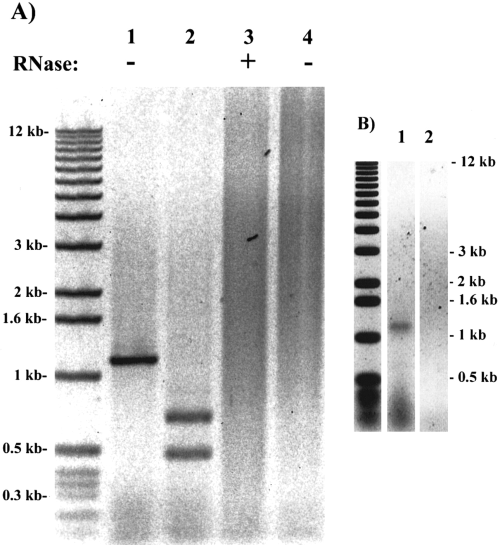
Lcng1 gene is expressed in Limulus brain and ventral eye nerves. The sequence for the antisense primer used in RT was 5'-TTATCGGGCTAGTCTCACC-3' (bp 3,122-3,140). The primer sequences for PCR were 5'-AGGCTTGATGTCGTAAAGAC-3' (bp 1,812-1,821, sense) and 5'-TGTCCGAGTGAGGTGTG-3' (bp 2,941-2,957, antisense). The TriZOL RNA isolation kit was used for the RNA isolations. A : RT-PCR on undesheathed nerves. Lane 1 was the RT-PCR product amplified from total RNA equivalent to one ventral eye nerve after one round of amplification, and lane 2 was the Sacll-digested product of lane 1. Lane 3 was an RNase (DNase-free)-treated control, and lane 4 was a water control. B : RT-PCR on desheathed nerves. Lane 1 was amplified from total RNA isolation from two desheathed nerves after one round of amplification. Lane 2 was the artificial seawater control.
We further investigated the expression of the Lcng1 gene in desheathed ventral eye nerves. Ventral eye nerves are covered with a tough sheath of connective tissue (Millecchia et al., 1966). In electrophysiological experiments, desheathing is required to expose photoreceptors. Both the sheath and the modified patch of cuticle near the ventral eye terminal were removed in this experiment. The same 1.2-kb fragment was amplified from RNA sample equivalent to two desheathed ventral eye nerves (Fig. 5B, lane 1) but not from an artificial seawater control (lane 2). A weaker band was detected when RNA from one desheathed nerve was used (data not shown). The intensity of the amplified band from one desheathed nerve was substantially lower than the band amplified from one undesheathed ventral eye nerve. This could suggest that the transcript is present in sheath cells as well as the neurons in the nerve, but we note that the dissection removes some photoreceptors from the nerve as well as the photoreceptors in the terminal plate beneath the ventral eye spot.
We also tested the tissue distribution of the Lcng1 transcript by the RT-PCR approach using total RNA samples from Limulus brain, blood, and muscle. The 1.2-kb band was detected only in the brain (Fig. 6, lane 5) and not in the blood, muscle, or control samples (lanes 1-4, 6, and 7).
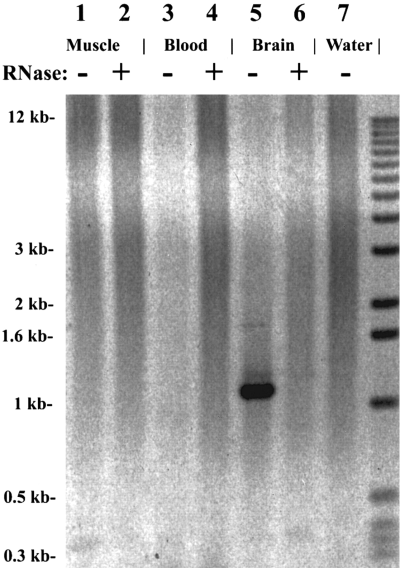
Tissue-specific distribution of Lcng1 transcript by RT-PCR : Limulus muscle (lanes 1 and 2), blood (lanes 3 and 4), or brain (lanes 5 and 6). Lanes 2, 4, and 6 were RNase-treated samples. Ten micrograms of total RNA was used in the RT reactions. Lane 7 was a water control. The same primers as in Fig. 5 were used.
We could readily detect the Lcng1 transcript in brain tissues by both RT-PCR and RPA. We detected the Lcng1 transcript in RNA extracted from small numbers of ventral eye nerves by RT-PCR but not by RPA. This suggests that the Lcng1 transcript is more abundant in the brain than it is in the ventral eye nerve. However, the RT-PCR experiments on both desheathed and undesheathed ventral eye nerves constitute evidence that the Lcng1 gene is expressed in a tissue where photoreceptors are present.
DISCUSSION
We have cloned a putative CNG channel cDNA from Limulus. Our evidence suggests that this putative channel gene is expressed in Limulus brain and ventral eye nerve.
LCNG1 shows strong overall homology with CNG channel subunits
LCNG1 is 40% identical at the amino acid level to other cloned CNG channel α subunits in common regions. About 60% of the amino acids in the cyclic nucleotide binding domain are identical. The high sequence identity between LCNG1 and other CNG channels suggests that the LCNG1 gene belongs to the CNG channel family (Finn et al., 1996).
Among the cyclic nucleotide-modulated channels, a new family of voltage-gated potassium channels, originally isolated from the ether-a-go-go (eag) Drosophila mutant, has been identified at the molecular level (Warmke et al., 1991). The eag channels are gated by voltage, but cyclic AMP shifts the voltage dependence toward more negative values (Brüggemann et al., 1993). Their primary sequences show ~20% identity to the Shaker family of potassium channels and the CNG channels in their transmembrane domains (Warmke and Ganetzky, 1994). The sequence identity between eag and CNG channel families in their putative cyclic nucleotide binding domains is also quite low [~17% (Warmke and Ganetzky, 1994)]. The overall amino acid sequence identity between LCNG1 and Drosophila eag protein in the cyclic nucleotide binding and transmembrane domains is only 14%. LCNG1 is therefore most likely a CNG channel subunit and not a cyclic nucleotide-modulated channel subunit.
A region homologous to the S4 voltage sensor is present
CNG channels possess a segment homologous to the S4 voltage sensor region of voltage-gated channels in their fourth transmembrane domain. The S4 region features four to eight amino acid triplets with positively charged groups in the third (or fourth) positions (Noda et al., 1984). Hydrophobic amino acids lie in between the positively charged amino acids (Fig. 7A). The amino acid sequence between I302 and R323 in LCNG1 is different from its counterpart in the bovine rod CNG channel ; however, the typical S4 voltage sensor features are present (Fig. 7A).

: Sequence alignment for the S4 voltage sensor from LCNG1, the bovine rod CNG channel α subunit (CNG1), the voltage-gated Shaker K channel (Shaker A), and the first domain of the eel Na channel (Na channel). The first four charged residues (R or K) are each separated by two hydrophobic residues. There are two more charged residues that can be aligned with either the Shaker K channel or bovine rod CNG1 channel. The positively charged groups are boldfaced. B : Sequence alignment for the cyclic nucleotide binding domains from LCNG1, the CNG channel α subunit of bovine rod (CNG1) or Drosophila (DmCNG), and a bacterial cyclic AMP-activated protein (CAP). The cyclic nucleotide binding pocket in CAP consists of an N-terminal α helix (A helix) preceding an eight-stranded β-strand, followed by two α helices (B and C) (Weber and Steitz, 1987). It has been proposed that the cyclic nucleotide binding domain in a CNG channel has a similar three-dimensional structure. Double-underlined sequences form α helices in the ligand binding pocket. Underlined sequences form β-sheets. The asterisks indicate the amino acids that interact with the cyclic nucleotide ribose phosphate moiety as determined in the CAP crystal structure or are invariant features of cyclic nucleotide binding domains (Shabb and Corbin, 1992). Boldfaced amino acids are believed to control ligand specificity in CNG channels. The secondary structure features have only been demonstrated for CAP (Weber and Steitz, 1987). C : Pore region sequence alignment. The pore regions are from LCNG1, the CNG channel α subunit of the bovine rod photoreceptor (CNG1), human rod (hCNG1), and Drosophila (DmCNG), and the voltage-gated Shaker K channel (Shaker A), the first domain of the rabbit brain Ca channel (Ca channel), and the eel Na channel (Na channel) of the voltage-gated channel family. The amino acid involved in the blockage of monovalent cation passage by Ca2+ in CNG1 is boldfaced. Amino acids involved in regulating potassium specificity in the Shaker K channel are underlined. Amino acids identical between LCNG1 and the bovine rod CNG channel are indicated by vertical bars. Gaps are indicated by hyphens. CNG1 data are from Kaupp et al. (1989), DmCNG from Baumann et al. (1994), Na channel from Noda et al. (1984), hCNG1 from Dhallan et al. (1992), Shaker A from Pongs et al. (1988), Ca channel from Mori et al. (1991), and CAP from Weber and Steitz (1987).
The cyclic nucleotide binding domain is similar to that of the bovine rod cGMP-gated channel
Cyclic nucleotide gating of a CNG channel is controlled by a cyclic nucleotide binding domain of ~130 amino acids located at the C terminus, just downstream from the transmembrane domains (Kaupp et al., 1989 ; Altenhofen et al., 1991 ; Goulding et al., 1994 ; Varnum et al., 1995). This domain shows homology to the cyclic AMP binding domain in the catabolite gene activator protein in Escherichia coli (Fig. 7B). The putative cyclic nucleotide binding domain (F518-A644) of LCNG1 is similar to the counterpart in both catabolite gene activator protein and CNG channels (Fig. 7B), including amino acids G541, G553, G576, E577, R590, and A592, which are invariant across species (Shabb and Corbin, 1992). The aspartic acid residue at position (D638) in helix C, necessary for cGMP selectivity in other cGMP-gated channels (Varnum et al., 1995), is also conserved. Therefore, it is likely that LCNG1 is cGMP-selective.
Homomeric channels encoded by LCNG1 are unlikely to be potassium-selective
The region between transmembrane domains S5 and S6 in CNG channels has been proposed to be the pore region. This hypothesis has been confirmed by chimeric mutagenesis experiments (Goulding et al., 1993 ; Sun et al., 1996), and the recent determination of the structure of the pore region of a potassium channel establishes the physical principles underlying selective potassium conductance (Doyle et al., 1998). In LCNG1, the region between the two putative transmembrane domains S5 and S6 (Y380-P398) is similar to the counterpart in both voltage-gated ion channels and CNG channels (Fig. 7C). It is likely that this same region forms the ion pathway and selectivity filter in the functional channel. Two residues (Tyr and Gly) typically present in the second half of this region in voltage-gated potassium channels (Heginbotham et al., 1992) are not present in the LCNG1 putative pore region, and it is therefore unlikely that LCNG1 can form monomeric channels that are potassium-selective.
Novel oncogene Src homology region 3 (SH3) binding motifs
An intriguing new sequence feature in LCNG1 is the presence of a long C terminus. There are three short proline-rich motifs of ~10 amino acids in this long C terminus (Fig. 1A).
These short proline-rich motifs are of particular interest because they are similar to regions in other proteins that bind to SH3 domains (Cicchetti et al., 1992 ; Ren et al., 1993 ; Cohen et al., 1995). SH3 binding motifs are composed of an ~10-amino acid sequence rich in proline, with a minimal consensus sequence of X-P-p-X-P (PPII helix), where X can be any amino acid and p tends to be another proline (Ren et al., 1993 ; Yu et al., 1994 ; Pawson, 1995). The minimal consensus SH3 binding consensus sequence X-P-p-X-P is present in all three proline-rich motifs in LCNG1.
Examples of proteins that contain SH3 domains or SH3 binding motifs include oncogene products, catalytic enzymes such as protein kinases (Mayer and Baltimore, 1993), cytoskeleton proteins such as microtubulins (Herskovits et al., 1993), and channel proteins. However, actual binding between these putative SH3 domains and PPII helices has been shown only in a few cases (Cicchetti et al., 1992 ; Ren et al., 1993 ; Yu et al., 1994 ; Lee et al., 1996). Even less is known about the functions of the SH3-mediated interaction. An example with possible relevance to LCNG1 is the possible role of SH3 binding motifs in targeting an epithelial sodium channel to the apical membrane of epithelial cells (Rotin et al., 1994). The epithelial sodium channel is localized adjacent to the actin filaments via SH3 domain interaction between the channel and actin (Cantiello et al., 1991). Actin is the cytoskeleton component of the microvilli in Limulus photoreceptors (Calman and Chamberlain, 1992).
A human voltage-gated potassium channel (Kv 1.5) is reportedly regulated by direct interaction with the SH3 domain of Src tyrosine kinase (Holmes et al., 1996). The coexpression of both Kv1.5 and Src tyrosine in HEK 293 cells suppressed the current of Kv1.5. This result could be relevant because the CNG channels and the voltage-gated channels belong to the same superfamily. Recently, a putative CNG channel from mouse brain, BCNG-1, has been identified as also interacting with the neural form of Src (Santoro et al., 1997). LCNG1 shows only weak homology (15-20% identity) with BCNG-1 in their proline rich C-terminal regions. However, one of the motifs in LCNG1 (TPLPPSP, amino acids 764-770) has a pattern similar to the PPII sequence that is responsible for the interaction in Kv1.5 (RPLPPLP).
A putative coiled-coil domain is present in the C terminus
A stretch of ~50 amino acids (E834-C880) near the end of the LCNG1 C terminus resembles that of several cytoskeletal proteins, including myosin and actin. A homologous region is also found in amino acids 666-712 of the C terminus of a chick cone photoreceptor CNG channel (Bönigk et al., 1993). Most of these amino acids are capable of forming α helices. Homologous regions in cytoskeleton proteins form a very regular structure composed of coiled-coils of α helices (Wiche et al., 1991). The coiled-coil structure is the structural basis for the formation of muscle filaments and for the binding of proteins to the cytoskeleton. Together with the SH3 binding motifs, this extra-long C terminus may mediate channel localization via the guidance of the cytoskeleton. This C terminus may also anchor other regulatory proteins that may be important for this putative Limulus CNG channel to function.
Can LCNG1 be a candidate channel subunit for photoreception ?
The phototransduction mechanism in Limulus has not been fully resolved. A major problem remaining is the identification of the channel responsible for the electrical response to light. Experiments performed on excised patches indicate that channels that are active in the light can be activated by cGMP (Bacigalupo et al., 1991) and are not cation-selective. Our analysis of the structure of LCNG1 indicates that its predicted basic properties are consistent with those of the native channel recorded in situ. However, we have not yet localized Lcng1 transcript at the cellular level or LCNG1 protein at the subcellular level, and although we have shown that the gene transcript is present in Limulus ventral eye nerves, the transcript is clearly not abundant at the tissue level. The nerve is a complex tissue comprising photoreceptor cells, glia, and efferent axons (Fahrenbach, 1981). The cDNA will enable us to attempt an accurate localization of the channel using in situ hybridization and immunostaining and to determine the biophysical properties of the putative channel.
Acknowledgements
Acknowledgment : We thank Laura N. Antar, Grigory Charny, Alain Dabdoub, Hsiao-hseng Hsiao, and Kyrill Ukhanov for dissecting the animals and Robert M. Greenberg at the Whitney Laboratory of University of Florida for his help in the cDNA probe development. This work is primarily funded by grants EY07743 to R.P. from the National Eye Institute, partially funded by a Research-in-Aid grant to F.H.C. from the Sigma Xi Society, a grant to S.T. from the National Institutes of Health, and grant IBN-9211327 to B.-A.B. from the National Science Foundation.




