Low plasma levels of dehydroepiandrosterone (DHEA) and incidence of lipodystrophy
Dhea and hiv
Dehydroepiandrosterone (DHEA) is an abundant adrenal steroid[1] that is present in plasma in the form of its sulphate derivative, DHEA-S. Plasma levels of DHEA-S are the highest of all steroids. The concentration of DHEA-S peaks during the third decade of life and then declines progressively with age[2,3]. Plasma levels of DHEA-S have been found to be decreased in HIV-infected patients, with a trend toward greater reductions in patients with advanced HIV disease[4]. Two large cohorts have indicated that low serum DHEA levels are associated with an increased risk of progression to AIDS, independently of CD4 cell counts[5,6]. Beneficial effects of replacement therapy with oral DHEA (50 mg/day) have been observed in the elderly, associated with restoration of DHEA and DHEA-S serum levels to concentrations similar to those observed in younger individuals[7,8]. Preliminary data in HIV-seropositive patients have indicated that DHEA doses of up to 2250 mg daily over 16 weeks were well tolerated without dose-limiting side effects[9]. Recently, the efficacy and safety of the oral administration of DHEA as replacement therapy in patients with advanced HIV disease have been demonstrated in a trial that was primarily aimed at assessing quality of life[10].
Association of low DHEA levels and lipodystrophy
Antiretroviral therapy may result in the occurrence of lipodystrophy, a syndrome of fat redistribution, hyperlipidaemia and insulin resistance[11,12,13,14,15,16]. The pathogenesis of lipodystrophy remains unclear[17]. DHEA to cortisol ratios in plasma have been found to be lower in patients with lipodystrophy than in patients without, whereas levels of plasma DHEA were in the normal range[18], and it has been suggested that lipodystrophy results from an imbalance between peripheral lipolysis and lipogenesis related to a defect in the regulation of steroid hormones.
Previously reports have shown that 24 h urinary cortisols are elevated, as are cortisol stimulatory hormones, whereas total serum cortisol may be normal, in HIV-infected individuals exhibiting lipodystrophy, suggesting an underlying alteration in production and destruction of serum cortisols which may also contribute to the pathogenesis of lipodystrophy[19].
Clinical study
Here we report an increased risk of lipodystrophy according to plasma levels of DHEA-S in a group of 17 patients with advanced HIV infection. Plasma levels of DHEA-S were measured in these patients prior to the introduction of protease inhibitors (PIs) in 1995 and again, 4 years later, in 1999. In the interval, all patients received a triple combination antiretroviral therapy, including indinavir in 13 patients and ritonavir in four patients. During the follow-up, seven patients were changed to another PI including nelfinavir in four patients and saquinavir combined with low-dose ritonavir (100 mg twice a day) in three patients.
Levels of DHEA-S were measured by means of an immunoenzymatic assay, using the Serono SR1 analyser, as described in a previous study[20], in serum samples immediately frozen and stored at −80 °C. Mean normal values for DHEA-S vary with age: 8.22 μm/L and 7.24 μm/L between 30–34 years of age and 35–39 years of age, respectively. At the time of the second determination of DHEA-S, nine of the 17 patients (53%) exhibited lipodystrophy, defined as moderate to severe fat loss in at least two areas (including face, legs and arms) plus abdominal lipid accumulation. No significant differences in the PI used were observed between patients with and without lipodystrophy.
The results of CD4 cell counts, plasma HIV-RNA and plasma DHEA-S before and 3 years after highly active antiretroviral therapy are shown in Table 1. Prior to treatment with PIs and during follow-up, the mean CD4 cell count in the two groups was not significantly different. Before treatment with PIs, the mean plasma levels of DHEA-S were higher in the group with lipodystrophy than in those without (9.6 vs. 4.3 μm/L; P = 0.04) (Fig. 1). Despite a mean increase of 180 CD4 cells during the 39 months of PI therapy, a significant decrease in plasma DHEA-S levels was observed in the group of patients with lipodystrophy versus those without (− 3.8 μm/L vs. + 0.7 μm/L; P = 0.01; Mann–Whitney U-test). However, no significant difference was established between the two groups regarding the DHEA-S plasma level at the time of diagnosis of lipodystrophy.
| CD4 cell count (× 106/L) | HIV-RNA plasma level (log10/mL) * | DHEA-S plasma level μm/L | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | Male | Mean ± SEM | |||||||
| Age (y) Mean ± SEM | Male | Mean duration of HAART | 1995 | 1999 | 1995 | 1999 | 1995 | 1999 | |
| Study population | 41.4 ± 1.4 | 15/17 | 39.1 ± 0.5 | 47.8 ± 21.2 | 336.2 ± 42.8 | 4.68 ± 0.2 | 2.27 ± 0.9 | 7.1 ± 1.4 | 5.4 ± 0.9 |
| n = 17 | P = 0.0003** | P = 0.0004 | NS | ||||||
| Lipodystrophy | 41.3 ± 2.5 | 7/9 | 39.7 ± 0.5 | 63.7 ± 38.1 | 359 ± 61.2 | 4.61 ± 0.3 | 2.13 ± 0.6 | 9.6 ± 1.9 | 5.8 ± 1.6 |
| n = 9 | P = 0.007 | P = 0.01 | P = 0.02 | ||||||
| No lipodystrophy | 41.6 ± 1.1 | 8/8 | 38.3 ± 0.7 | 29.8 ± 15.8 | 310 ± 62.7 | 4.77 ± 0.2 | 2.25 ± 0.3 | 4.3 ± 1.7 | 4.9 ± 0.9 |
| n = 8 | P = 0.01 | P = 0.01 | NS | ||||||
- *The lower limit of detection of the branched-chain DNA assay (Chiron) was 1.7 log 10 copies/mL; **comparison between 1995 and 1999 values, using the Wilcoxon test.
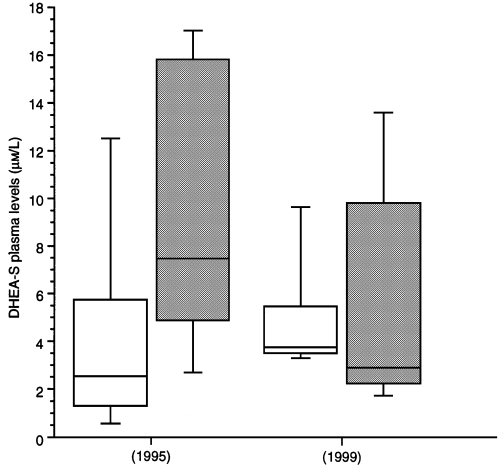
(D)HEA-S plasma levels before (1995) and during highly active antiretroviral therapy. White bars represent patients without lipodystrophy, grey bars the patients with lipodystrophy. The boxes delimit the 25th and 75th percentiles; the line within the box is the median. The end of the vertical lines show the 10th and 90th percentiles.
Recommendation
The results of a recently conducted, double-blind placebo-controlled trial indicate that a replacement dose of DHEA at 50 mg/day by the sublingual route for 4 months is safe and may improve the subjective perception of the quality of life in individuals with advanced HIV disease[10]. A statistically significant increase in the levels of DHEA-S was observed in the treated group throughout the study despite a poor oral bioavailability of DHEA. Taken together, the available data emphasize the need for further evaluation of DHEA therapy to treat or to prevent lipodystrophy.




