The vertical distribution of pronophiline butterflies (Nymphalidae, Satyrinae) along an elevational transect in Monte Zerpa (Cordillera de Mérida, Venezuela) with remarks on their diversity and parapatric distribution
Abstract
Aim The distribution of neotropical butterflies of the tribe Pronophilini (Lepidoptera, Satyridae) was analysed with the aim of determining (i) the elevational ranges of distribution of each species (ii) the elevational gradient in diversity, and (iii) the existence of parapatric distributions of some closely related species pairs.
Location The field study was carried out in the middle and upper section of the valley of the Río Albarregas, a tributary of the Río Chama on the southern slopes of the Serranía de la Culata, in the central part of the Cordillera de Mérida, Venezuela.
Methods The material was collected along an elevational transect set on a trail leading from Merida to the Páramo de Los Conejos in the area known as Monte Zerpa. It consisted of a series of 32 collection sites set at every 25 m of altitude and covered an elevation from 2250 m to 3025 m.
Results The ranges of Lymanopoda obsoleta, L. albocincta, L. zapatoza, Corades chelonis, C. pannonia, C. medeba, Mygona irmina, Pedaliodes epidipnis, P. japhleta, P. montagna and P. panyasis were all restricted to the lower half of the cloud forest zone and L. diezti, C. pax, P. polla, P. ornata and P. ferratilis were all restricted to the upper part. Two species, Erethris porphyria and Steroma bega, crossed the entire elevational range of the cloud forest zone.The biodiversity, as measured by Shannon’s index, attained a maximum at 2700 m, beyond which it gradually decreased. A parapatric distribution was confirmed for three pairs of closely related species: L. obsoleta and L. diezti, C. chelonis and C. pax and P. montagna and P. ferratilis.
Main conclusions The peak in diversity at middle elevations seems to result from the overlapping ranges of species distributed over lower and upper parts of the cloud forest. Correlation of species composition at particular transect sites with elevation showed that increase in elevation was associated with an increase in species composition difference. Post-mating isolation was suggested as a primary factor responsible for maintaining the parapatric distributions of species occupying the upper and lower zones and preventing their respective distributions from expanding.
Introduction
Questions about how insects are distributed in mountains and the patterns of their diversity have repeatedly stimulated researchers interested in mountain biogeography. During the past century, the elevational distribution of various groups of insects and their diversity have been investigated in different mountainous regions of the world (Janzen, 1973; Janzen et al., 1976; Claridge & Singhrao, 1978; Gagne, 1979; Lawton et al., 1987; Hanski & Niemelä, 1990; McCoy, 1990; Olmstead et al., 1990; Gutiérrez & Menendéz, 1995; Menendéz & Gutiérrez, 1996; Davis et al., 1999). The elevational distribution and diversity of butterflies and moths belonging to the order Lepidoptera have also been studied in various mountains at different latitudes. However, most research on distribution has been carried out on Lepidoptera in North American (Boggs & Murphy, 1997; Fleishman et al., 1998), European (Randall, 1982; Lawton et al., 1987; Sánchez-Rodríguez & Baz, 1995; Gutiérrez, 1997), Asian (Hebert, 1980; Holloway et al., 1990) and African mountains (Harmsen, 1989). By contrast, surprisingly little is known about the elevational distribution and diversity of neotropical Lepidoptera in the Andes, especially in humid areas covered by cloud forest. With the exception of the work of Adams (1985) and our previous studies of Colombian Pronophilini butterflies (Pyrcz & Wojtusiak, 1999), some results have been presented only by Wolda (1987) on the elevational distribution of hawk moths (Sphingidae) in Panama. To some extent it is to true that other information on elevational distribution among neotropical butterflies can be derived partially from various publications on their systematics and faunistics and to some extent also from labels attached to specimens in museum collections from around the world. However, labels on specimens collected in the nineteenth century often lack precise altitude data.
Working since 1990 on the systematics of neotropical Satyridae butterflies of the group Pronophilini, we have found that this group can serve as a useful model for studying the distribution patterns and species diversity of cloud forest insects along elevational gradients in the Andes. Pronophilini (Nymphalidae, Satyrinae) constitute the most diverse group of butterflies that inhabit the environment of the cloud forest belt from Venezuela to Bolivia, extending from 1400 m up to the boundary with open field páramo vegetation at 3200–3400 m above sea level. Most known species are restricted to the cloud forest and only some are associated with Poaceae plants, typical for the alpine páramo vegetation (Brown, 1941; Pelz, 1997). Each part of the Andean mountains that is isolated from its neighbours by deep valleys contains its own species composition of Pronophilinae, and seems to harbour the largest number of species at an elevation of approximately 2500 m.
At these locations, there are over 400 described species (Adams, 1985) but the estimated number is probably much higher and may exceed 600. The number of species varies considerably depending on site location and is highest in the Central Andes, decreasing gradually toward the North. Most species exhibit very low vagility and adults usually fly close to their larval host plants, different species of neotropical bamboo of the genus Chusquea (Poaceae) (Schultze, 1929; Adams & Bernard, 1981; DeVries, 1987), a characteristic component of the cloud forest vegetation in the Andes.
On average, about 30% of taxa are endemic at species and subspecies levels in the main Andean Cordilleras, but in more isolated mountainous ranges, such as Sierra Nevada de Santa Marta or Cordillera de Mérida, this percentage is even higher (Adams & Bernard, 1977). The largest number of endemic species is always found in the upper part of the cloud forest zone (Adams, 1985). Prior to Adams’ research (above), little was known about the vertical ranges of Pronophilini. Only some data obtained by two naturalists in the early twentieth century, Fassl (1911, 1915 and 1918) and Krüger (1924, 1925), can be taken today as a source of reliable information. In the late 1920s, Pronophilini became a ‘forgotten’ group of Neotropical butterflies and the difficulty in identification of similar-looking, dark coloured species, especially those of the genus Pedaliodes, discouraged entomologists from undertaking any zoogeographical studies for a long time.
The purpose of the present study was to determine the elevational distribution of Pronophilini butterflies in the cloud forest of the Cordillera de Mérida, using the method of sampling along an elevational transect. We used this high accuracy method to study the elevational distribution of Pronophilini in the Tambito cloud forest of Western Cordillera in Colombia (Pyrcz & Wojtusiak 1999). No other field studies, either on the elevational distribution of Pronophilini, or any other group of Andean butterflies, have been performed previously by means of the same method. The present study aimed to identify the upper and lower ranges of each species and to discover the pattern of their diversity and abundance along the entire cloud forest up to its boundary with the zone of paramo. Special attention was paid to species that according to Adams (1985) may have parapatric distributions.
Materials and methods
The field study was carried out in the middle and upper section of the valley of the Río Albarregas, a tributary of the Río Chama (Lat. 08°36′N, Long. 71°10′W), on the southern slopes of the Serranía de la Culata, in the central part of the Cordillera de Mérida, between February and April 1996. This area is known as Monte Zerpa. The mean annual temperature in the vicinity of Monte Zerpa, La Mucuy, at different altitudes are as follows: 2000 m, 14.8 °C; 2500 m, 11.8 °C; 3000 m, 8.7 °C. The mean precipitation in Mérida amounts to 1743 mm at an elevation of 1600 m, and in La Mucuy it reaches 2025 mm at an elevation of 2000 m (Veillon, 1989). During the dry season from January to the end of March, slopes of mountains are only free of clouds early in the morning. Later in the day, cloud mounts up quickly so that the sun is completely obscured between 11 and 12 a.m. The cloud gradually thickens and usually at about midday rain starts and continues until the late afternoon.
The transect was set along a trail leading from Merida to the Páramo de Los Conejos. It consisted of a series of 32 collection sites, which were set at every 25 m of altitude. The lowest collection site was set at the bottom of a steep ridge at an elevation of 2250 m and the upper most at an elevation of 3025 m, in the forest-paramo ecotone. The cloud forest vegetation along the ridge was relatively undisturbed, largely exhibiting its primary character, except in the vicinity of the trail predominantly covered with Chusquea bamboo.
Butterflies were collected at each site of the transect by means of a butterfly net. All specimens sitting on the ground or flying above it were caught during a period of 5 minutes. As almost all pronophiline butterflies show a high feeding preference for decaying organic matter, they were attracted to collecting sites of the transect by bait. The same quantity of dung was used as a bait at each site and was daily smeared on the ground. Butterflies, which were missed or observed in flight only, were excluded from the analyses.
Collecting along transects always started at 9 a.m. and continued to 1 p.m. On cloudy days, it was impossible to complete samplings along the entire transect. Such incomplete data were omitted from the calculations. The entire material constituting the basis for further analysis consisted of 1007 individuals belonging to 20 species accumulated during 13 full transect samplings.
Statistical methods
To quantify Pronophilini biodiversity at different elevations in the area of our research we used Shannon’s index (Magurran, 1988):
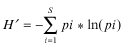
where S is the total number of species in a whole sample, pi is the proportion of the i-th species in the sample (pi = Ni/N), Ni is the number of individuals of the i-th species, and N is the number of all species in a sample.
Since an estimator of this index is biased when samples are not numerous (Magurran, 1988), original data collected every 25 m were grouped to make one data set for every 100 m. Confidence intervals of 95% for Shannon’s index were established by means of a bootstrap technique (Manly, 1991), so that each group for which the confidence interval was calculated, was resampled 2000 times.
To compare similarity of species composition at different sites along a transect we calculated Jaccard’s coefficients (Magurran, 1988). The value of the coefficient is 1 when samples from two sites have the same species composition, and equals 0 when both sites share no species in common. As a measure of a difference in species composition between the two different sites we used 1-Jaccard coefficient. To find out whether an increase in species compositional difference is associated with increasing elevation we calculated Mantel’s correlation coefficient. It is used to calculate correlations between the two square matrices containing information about the distance between pairs of objects (Manly, 1991). To test for parapatric distribution patterns among three pairs of species we used Mann–Whitney U-tests for each pair (Sokal & Rohlf, 1995).
Results
Species distributions
The following species of Pronophilini were recorded in Monte Zerpa: Corades chelonis rubeta Thieme, C. pax Watkins, C. medeba columbina Thieme, C. pannonia ploas Thieme, Eretris porphyria (C. & R. Felder), Lasiophila zapatoza meridae Adams & Bernard, Lymanopoda obsoleta Westwood, L. dietzi Adams & Bernard, L. albocincta albocincta Hewitson, Mygona irmina (Doubleday), Panyapedaliodes jephtha Thieme, P. panyasis (Hewitson), Pedaliodes proerna fumaria Thieme, P. japhleta Butler, P. montagna Adams & Bernard, P. ferratilis Butler, P. polla Thieme, P. ornata Grose-Smith & Kirby, Pronophila epidipnis Thieme and Steroma bega Westwood.
Two species, Erethris porphyria and S. bega, appeared to have the widest zones of distribution and their individuals were found almost along the entire transect. Medians of their distribution corresponded to 2600 m and 2650 m a.s.l., respectively (Fig. 1). Four species, Pronophila epidipnis, Panyapedaliodes panyasis, C. pannonia ploas and Mygona irmina appeared to have relatively narrow spans of elevational distribution, ranging from 200 to 250 m. Zones of L. zapatoza meridae, C. chelonis rubeta and P. japhleta were approaching 400 m in span, and a zone of P. montagna almost 500 m. As shown in Fig. 1, medians of the distributions of all species mentioned above, except Erethris porphyria and S. bega, were comprised in a belt extending from 2350 m to 2525 m of altitude.
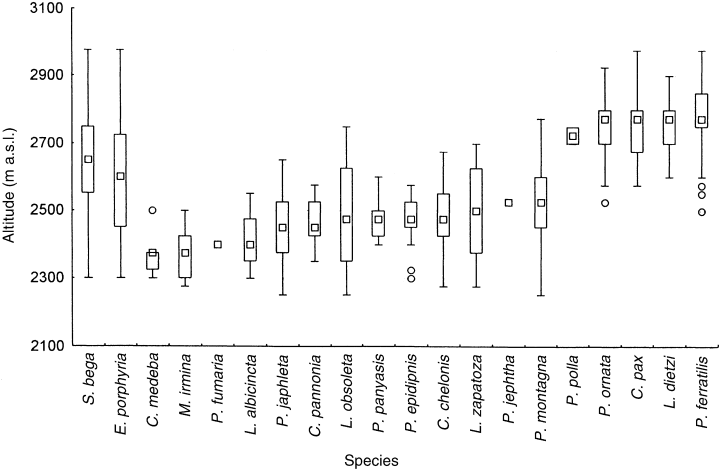
Ranges of vertical distribution of species of Pronophilini collected in Monte Zerpa. Box and whisker plot with median, lower and upper quartile, minimum and maximum and outside values of altitude range.
Unfortunately, it was not possible to estimate precisely the ranges of C. medeba columbina, P. proerna fumaria and Pan. jephtha, because of small sample size. However, medians of their distributions also fell within the 2350–2525-m altitude belt.
Results obtained in our fieldwork indicate that most of the species are distributed in the lower half of the elevational span of the cloud forest. Only five species, L. diezti, C. pax, P. polla, P. ornata and P. ferratilis, are confined to the upper half, with their ranges extending from 2500 m to the upper forest limit. Such a pattern of vertical distribution of ranges of all species is reflected in species richness (Fig. 2). The highest number of species appears at the middle elevations 2400–2600 m, where ranges of species of the lower and upper parts of the cloud forest overlap.

Distributional ranges of Pronophilini butterflies along the altitudinal transect in Monte Zerpa. (a) Histogram of species richness. (b) Histogram of number of specimens collected.
The number of individuals of all collected species varied in relation to elevation, peaking at middle elevations (Fig. 3). However, the distribution pattern shows pronounced decrease in number of specimens at elevations between 2600 and 2700 m. This is the zone in which populations of species occurring in the lower part of the forest are reaching the upper limits of their ranges and are therefore in low densities, whilst populations of species characteristic of the upper part of the cloud forest reach the lower limits of their distribution and are also at low densities.

Biodiversity in Monte Zerpa by means of Shannon’s index (±0.95% confidence intervals). For details see Materials and methods.
The biodiversity distribution pattern of Pronophilini along the cloud forest in Monte Zerpa was relatively similar, with an increase at 2700 m and decreasing at higher elevations. Finally, species composition was positively correlated with elevation (Mantel correlation coefficient, r = 0.86, P < 0.001).
Parapatric species
One of the most interesting results of our research on Monte Zerpa butterflies was the confirmation of parapatric distributions of three closely related pairs of species L. obsoleta and L. diezti, C. chelonis and C. pax, P. montagna and P. ferratilis. In their research (1981, 1986), Adams and Bernard also classified the same species as parapatric, but they did not measure precisely the altitude for each of them. They used the term ‘parapatric’ for those species of Pronophilini that replace each other along an elevational gradient. Moreover, Adams (1985), in his work on Columbian Pronophilini, even identified several putative duos, trios or even quartets of parapatric species in this group of butterflies.
In Monte Zerpa, the population of L. obsoleta occurs from an elevation of 2250 m up to 2750 m and the population of L. dietzi from an elevation of 2600–2900 m. The median abundance of L. obsoleta corresponds to an elevation of 2480 m and that for L. dietzi to an elevation of 2780 m (U43,34 = 63 P < 0.0001). As shown in Fig. 5, the zone of distribution of L. obsoleta is wider. Ranges of both species overlap in a zone of 150 m in span and the point where their populations are at similar densities occurs at the elevation of about 2700 m.
The second parapatric pair of species is composed by C. chelonis and C. pax (Fig. 5). The population of C. chelonis occupies the zone that extends from the lowest point of the transect at 2275 m up to 2675 m (median = 2480 m). The range of C. pax, which is the ‘upper’ species, extends from 2575 m up to 2975 m (median = 2780 m) (U23,54 = 32.5, P < 0.0001). The zone where the ranges of the two species overlap is about 100 m in elevational span.
In the case of a third pair of parapatric species, P. montagna and P. ferratilis (Fig. 5), the range of distribution of the first species extends from 2250 to 2775 m (median = 2530 m) and that of the second species from 2500 to 2950 m (median = 2780 m) (U174,141 = 1263.5, P < 0.0001). The zone of overlap between the two species spanned 280 m.
Discussion
Patterns of vertical distribution
Results from previous research on the elevational distribution of insects are somewhat controversial and remain insufficient to suggest a uniform model of species richness in relation to elevation (Rahbek, 1995). Generally, the observed patterns of species richness in mountains can be grouped into two different categories. Those showing a decrease in number of species with elevation would be in one category (Hagvar, 1976; Hebert, 1980; Sachan & Gangwar, 1980; Lawton et al., 1987; Wolda, 1987; Fernandes & Price, 1988); and those showing a diversity peak at middle elevations are the second. A middle elevational peak was found not only in insects, which are discussed below, but also for leaf litter invertebrates (Olson, 1994), birds (Terborgh, 1977; Rahbek, 1997) and mammals (Rickart et al., 1991; Md. Nor, 2001; Sánchez-Cordero, 2001). However, for some insects, e.g. psocids (Psocoptera) (Turner & Broadhead, 1974), diversity may increase with elevation.
The largest number of Pronophilini butterflies discovered at middle elevations along the Monte Zerpa transect provides an example of a distribution pattern reported by other authors for other groups of insects, including Lepidoptera. The mid-elevation pattern has been found, moreover, in mountains that are situated at lower and at higher latitudes. In tropical mountains of South-east Asia a middle-elevation peak was reported by Hanski (1983), for dung and carrion beetles. Such a pattern was explained as the result of increased availability of food resources at middle elevations. A mid-altitude peak in the richness of butterfly species in a northern Iberian mountain range was reported by Gutiérrez (1997), who explained it as being an effect of different but overlapping biogeographical elements, i.e. by the overlapping distributional ranges of lower and higher elevation species. Brown (1982) also explains the high species diversity on the foothills of the East Andes in the area called the Napo refugium as an effect of overlapping montane and lowland pools of species, called by him the ‘disturbance-vicariance’ phenomenon. Similarly, in Northern Sulawesi (Holloway et al., 1990), a peak in diversity of various groups of Lepidoptera occurs at an elevation of between 600 m and 1000 m in a zone of transition from lowland to the upper montane zone. A similar situation is seen in Borneo, where the diversity of Lepidoptera peaks at 1000 m (Holloway, 1987).
The largest number of pronophiline butterflies in Monte Zerpa, seen here at middle elevations, also seems to result from the overlapping ranges of species distributed over lower and upper parts of the cloud forest. It must be pointed out, however, that the climatic conditions in a cloud forest also favour high species diversity at higher elevations.
In the Cordillera de Merida, clouds cover the slopes for most of the day so that the conditions of relative humidity and temperature are relatively stable and change little with increased elevation. This enables a large number of plants (Veillon, 1989) and animals to live at elevations much greater than in mountains affected by drier climates. The effect of such a protective blanket of clouds on the distribution of Pronophilini butterflies is clearly visible on the slopes of Monte Zerpa, where diversity stays high up to an elevation of about 2700 m and then gradually decreases towards the upper timberline. Above, at an elevation of 3300 m, where clouds disappear, temperature and humidity fall abruptly and strong winds deepen the effect of adverse climatic conditions, only three species of Pronophilini occur (Adams & Bernard, 1981).
It should be mentioned that P. panyasis, which appeared to be a very rare species in the Cordillera de Mérida, was much more common in the Tambito Forest Reserve in the Columbian Western Cordillera, where we conducted similar observations in 1997 (Pyrcz & Wojtusiak, 1999). In that area, P. panyasis occurs commonly in a zone that extends from 2000 m to 2400 m. Another species, P. polla, also uncommon in Monte Zerpa, can be found in fair numbers in the southern part of Cordillera de Mérida (El Batallón), where it flies within an elevational zone ranging from 2600 m to 3000 m a.s.l.
The pattern of distribution of the Pronophilini fauna in Monte Zerpa, in which certain species are restricted to lower and certain to upper parts of the cloud forest, reflects historical processes. Montane biota in the Chama Valley were subjected to climatic oscillations during the Pleistocene so that during that period the vegetation and the fauna of the upper part of the cloud forest belt underwent several phases of partition and reconnection. Contact between populations living on the slopes directed towards the Chama Valley and those on the outside slopes of Cordillera de Mérida was interrupted and resumed as their habitats migrated up and down during warmer and colder intervals of the Pleistocene (Vuilleumier & Ewert, 1978; Van der Hammen, 1979; Whitmore & Prance, 1987; Colinvaux, 1993). This scenario was proposed by Vuilleumier (1970) for Andean birds and also by Adams (1985) to explain the existence of endemic species of Pronophilini in the upper part of the cloud forest in the northern Andes.
The parapatric species
A number of questions can be asked about the nature of factors responsible for partitioning pronophiline populations within the entire cloud forest. Those about the mechanisms of range formation in populations of closely related, parapatric species and about the mechanisms underlying their altitudinal separation seem to be the most intriguing.
Parapatric distributions among Pronophilini are not limited to the Monte Zerpa area in the Cordillera de Mérida. Working on these butterflies in the Northern Andes, Adams (Adams & Bernard, 1979; Adams, 1985) was the first to show that some closely related species can occupy well-defined ranges at different elevations. He distinguished ‘uppermost forest species’, ‘parapatric duos’ and so on, but without giving precise data on their ranges. We also reported another pair of parapatric species when investigating the vertical distribution of Pronophilini in the Tambito reserve on the western slopes of Cordillera Occidental in Colombia (Pyrcz & Wojtusiak, 1999). Current data suggest that such parapatric distributions must be quite a common phenomenon in Pronophilini, a pattern awaiting confirmation by means of more precise data from surveys in other regions.
Is it possible to explain the mechanism underlying this phenomenon? Differentiation in the distribution of species restricted to certain habitats, such as the forest–páramo ecotone, secondary areas, or extremely humid biotopes along water courses, can be easily explained in terms of habitat-dependent selection (Jiggins et al., 1996). However, the partitioning of species into well-defined faunal pools in an apparently ‘homogeneous’ cloud forest environment is difficult to explain on a purely ecological basis, even if we cannot entirely rule out the possibility that subtle environmental changes may have some effect. Other factors are likely to be relevant, including historical factors and interspecific relationships among these butterflies.
The vertical distribution of parapatric species in the lower zones of Monte Zerpa, such as L. obsoleta, P. montagna and C. chelonis, might be affected by the presence of their upper, closely related counterparts, L. dietzi, P. ferratilis and C. pax. For example, we found that in the adjacent valley of Santo Domingo where L. dietzi does not occur, the entire cloud forest zone is occupied by the population of L. obsoleta, so that the upper range of the species almost reaches the forest–paramo border at 3000–3100 m. L. obsoleta (Westwood) is a widespread species and occurs throughout the Andes. In the Valley of Chama, L. dietzi, which is endemic to that area, occurs above L. obsoleta (Adams & Bernard, 1981). The upper distribution limit of L. obsoleta (Fig. 4) in Monte Zerpa is therefore at much lower elevation, so that this species reaches an elevation of only 2700 m. This suggests that the presence of the population of the high-elevation species may somehow limit the distribution of the lower-elevation species.
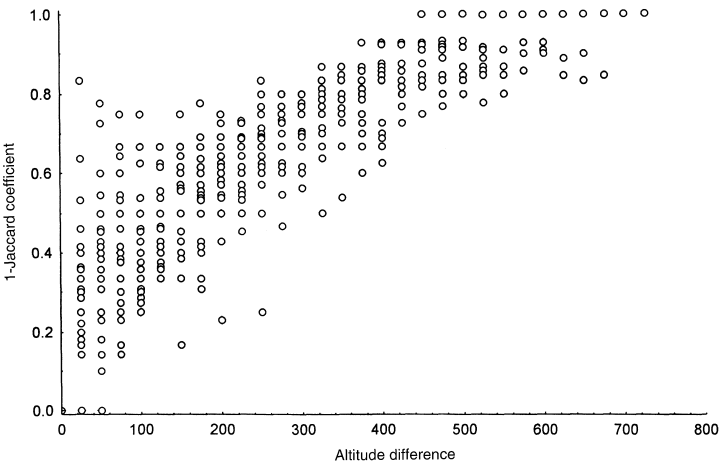
Differences between species composition in relation to elevation. To find out whether elevation is positively associated with species compositional differences, we calculated Mantel’s correlation coefficient. It is used to calculate correlation between the two square matrices containing information about the distance between pairs of objects. The first matrix contained information about differences in species composition between any two elevational sites being compared and was calculated as 1-Jaccard’s coefficient. The cells of the second matrix contained differences in altitude between any two sites being compared. The figure shows that an increase in elevation was associated with an increase in species composition difference (Mantel correlation coefficient, r= 0.86, P < 0.001).
The second parapatric pair of species, C. chelonis and C. pax, is another example of two closely related taxa, in which C. chelonis occupies the lower part of Monte Zerpa and C. pax its upper part. From another survey carried out in the El Tamá range we know that C. chelonis is not replaced at higher elevations by any parapatric ally and, as we could expect, its population can reach as high as the upper tree-line. Adams (1985) was the first to suggest that in mountains with a small number of pronophiline species, the average depth range of the vertical distributions for a given species is significantly greater than in areas with a larger number of species. He points out that for 22 species that occur in the isolated massif of Sierra Nevada de Santa Marta the mean width of an altitudinal zone occupied by a given species is 867 m, whereas for 36 species known from Cordillera de Mérida the figure is 537 m. As we have mentioned above, the spatial pattern of the overlapping zones shows noticeable similarities between the three pairs of species. Their zones form narrow, 100–200-m-wide bands, where the two contacting populations are at their lowest densities.
Working on the structure of the hybrid zone of two Heliconius (Heliconiidae) species in Southern Ecuador, Jiggins et al. (1996) suggest that habitat-dependent selection could be the key factor responsible for parapatric distribution phenomena. However, such an explanation cannot explain the parapatric distribution of our three pairs, L. obsoleta and L. dietzi, P. montagna and P. ferratilis, C. chelonis and C. pax. In zones where the pairs of populations contact, there are no evident ecological boundaries, abrupt changes of climatic condition, or changes in vegetation. However, we cannot rule out the possibility that some very subtle climatic changes (water stress, or atmospheric pressure) can play a role in forming the parapatric distribution.
Hanski (1983) and Hanski & Niemelä (1990) suggest that competition, which occurs in south-east Asian dung and carrion beetles as well as in birds (Diamond, 1972, 1977;Terborgh, 1977), can be a major factor responsible for maintaining the parapatric distribution of beetles populations. We can accept the limitation in the availability of suitable nesting sites for birds and the scarcity of dung for dung beetles as obvious factors that can generate competition, and hence act in favour of parapatric distributions. However, the resource competition hypothesis cannot be used in the case of Pronophilini butterflies, because there is no scarcity of food in the cloud forest and their oligophagous larvae can feed on various species of Chusqea bamboo widely distributed throughout the cloud forest.
A possible factor could also be the limited availability of perching sites for territorial males that could generate competition between populations of two neighbouring species, assuming of course that their males exhibit territorial behaviour. The lack of ethological observations, especially of information about the mating systems in these species, makes it difficult to judge the importance of this factor. On the other hand, territorial butterflies can co-exist in very large numbers of species and individuals through the partitioning of territories in time and space (Rutowski, 1991).
Finally, we can assume that if sexual isolation mechanisms are not completely efficient, for example because of imperfection in the mate recognition systems, then hybridization between parapatric species could be expected. In fact, as quoted by Jiggins et al. (1996), interspecific hybridization is a common phenomenon in the wild and is found in approximately 9% of bird species (Grant & Grant, 1992). It is similarly or even more frequent in butterflies (Guillaumin & Descimon, 1976). However, in Monte Zerpa no hybrids were recognized, although we should acknowledge that such specimens in the Pronophilini group are difficult to identify because of the high degree of phenotypic similarity between parapatric species. By contrast, brightly coloured hybrids in the genus Heliconius are easily identified in the population. The explanation that sexual isolation mechanisms are not complete is thus theoretically plausible and, if hybrids were at least partly viable, their dispersal ability would have allowed them to spread over the entire vertical range of each species. Given the partition of distributions we see, there is reason to suggest that post-mating isolation may be the primary factor responsible for maintaining the parapatric distributions of species occupying the upper and lower zones of the Andean cloud forest slopes, including Monte Zerpa, and for preventing their respective distributions from expanding.
At the present stage of our research it is still too early to propose any convincing hypothesis to explain the observed patterns of parapatric distribution of the three pairs of Pronophilini butterflies in the Monte Zerpa area. It is also too early to adopt any model from those which were discussed by Bull (1991) in his extensive review of the literature on parapatric distributions. It thus remains for future investigation to shed light on this problem. In view of the results we obtained in present and previous work (Pyrcz & Wojtusiak 1999) we can conclude that pronophiline butterflies constitute a perfect model group to study geographical distribution of insects along the Andes and to identify biodiversity hot spots for the purpose of protecting the biodiversity of this geographical region.
Acknowledgments
The authors would like to thank to J. Weiner, Angel L. Viloria and David Spencer-Smith for useful comments on earlier drafts and David Carter for critical reading of the manuscript. Field studies in Venezuela have been supported by DS and BW research grants of the Institute of Zoology of the Jagiellonian University.




