Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system
Summary
The skin commensal and opportunistic pathogen Staphylococcus epidermidis is the leading cause of nosocomial and biofilm-associated infections. Little is known about the mechanisms by which S. epidermidis protects itself against the innate human immune system during colonization and infection. We used scanning electron microscopy to demonstrate that the exopolysaccharide intercellular adhesin (PIA) resides in fibrous strands on the bacterial cell surface, and that lack of PIA production results in complete loss of the extracellular matrix material that has been suggested to mediate immune evasion. Phagocytosis and killing by human polymorphonuclear leucocytes was significantly increased in a mutant strain lacking PIA production compared with the wild-type strain. The mutant strain was also significantly more susceptible to killing by major antibacterial peptides of human skin, cationic human β-defensin 3 and LL-37, and anionic dermcidin. PIA represents the first defined factor of the staphylococcal biofilm matrix that protects against major components of human innate host defence.
Introduction
Staphylococcus epidermidis is a commensal microorganism of human skin and the most frequent cause of hospital-acquired infections. S. epidermidis causes infection only in immunocompromised individuals or after damage to the epithelium. Most infections with S. epidermidis occur on indwelling medical devices and typically involve the formation of biofilms ( Vuong and Otto, 2002 ). A biofilm represents a structured, high-density population of cells embedded in a heterogeneous matrix, which protects sequestered bacteria from antibiotics and the effects of the human immune system ( Costerton et al., 1999 ). Although this general picture of biofilm-mediated protection is widely accepted, the specific factors and mechanisms contributing to protection from innate host defence are mostly unknown ( Stewart and Costerton, 2001 ). In S. epidermidis , the extracellular matrix, often called ‘slime’, has been shown to mediate protection against polymorphonuclear leucocyte (PMN) phagocytosis ( Johnson et al., 1986 ). However, the antiphagocytic properties have not been attributed to a specific molecule.
Staphylococcus epidermidis produces an extracellular polysaccharide named polysaccharide intercellular ad-hesin (PIA), which is a positively charged homopolymer of β-1,6-linked N-acetylglucosamine (NAG) residues ( Mack et al., 1996 ). PIA is produced by the ica gene cluster, which comprises the icaA , icaD , icaB , icaC and icaR genes ( Heilmann et al., 1996a ). It is an essential factor for staphylococcal biofilm formation and causes haemagglutination and bacterial aggregation ( Heilmann et al., 1996a; Mack et al., 1999 ). PIA contributes significantly to virulence in animal models of catheter infection ( Rupp et al., 1999a, b ). In an epidemiological study, the presence of the genes responsible for PIA production correlated significantly with infection originating from indwelling medical devices ( Galdbart et al., 2000 ). These previous studies led us to the hypothesis that PIA is involved in the protection against innate host defences.
Results
PIA is an integral and essential factor of the extracellular matrix of S. epidermidis
Previous macroscopic studies have shown that PIA is essential for intercellular adhesion and biofilm formation (Heilmann et al., 1996a). However, little is known about the subcellular distribution of PIA and its role in the formation of extracellular matrix at a submacroscopic level. We first determined PIA content in subcellular fractions of S. epidermidis wild-type and ica– mutant strains to determine whether PIA is retained on the cell surface in the wild-type strain and to confirm its absence in the mutant strain (Fig. 1). As expected, the wild-type strain produced PIA, which was located on the cell surface and not in the cell supernatant. Moreover, PIA was not produced by the ica– mutant strain (Fig. 1). We note that there was decreased intercellular adhesion and clumping in the ica– mutant, consistent with previous studies (Heilmann et al., 1996b; data not shown). We next used scanning electron microscopy (SEM) to visualize ultrastructural differences between the wild-type and mutant strains (Fig. 2). Importantly, we discovered that wild-type S. epidermidis produced extracellular fibrous material not present on the ica– mutant (Fig. 2A and C). We also performed immunoelectron microscopy and labelled PIA on the wild-type and mutant strains (Fig. 2B and D). PIA co-localized specifically with the fibrous material on the bacterial cell surface and is therefore an integral component of that structure (Fig. 2B and D). Collectively, our results demonstrate that PIA is an essential, surface-located component of the S. epidermidis extracellular matrix.
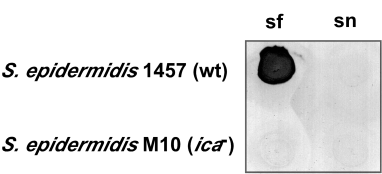
Immuno dot-blot of PIA production. PIA produced in S. epidermidis wild-type and ica – mutant strains was detected on the cell surface (sf) and in the supernatant (sn) of cultures with an immuno dot-blot using PIA-specific antiserum.
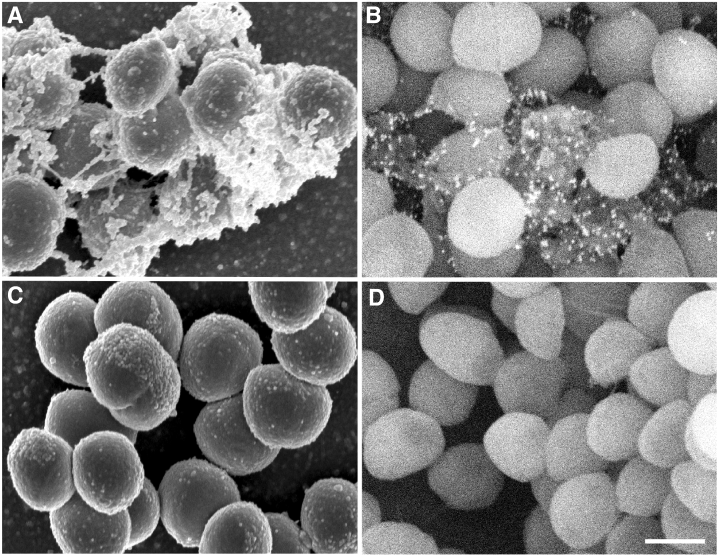
Ultrastructural analysis of S. epidermidis . Scanning electron and immunoelectron microscopy images of the wild-type (A and B) and ica – mutant (C and D) strains of S. epidermidis . PIA was immunogold labelled using αPIA-antisera (B and D). Magnification 25 000×. The scale bar represents 0.6 µm.
PIA increases resistance to cationic and anionic human antibacterial peptides
Antibacterial peptides such as cathelicidin/hCAP18 (LL-37), human β-defensin 3 (hBD3) and dermcidin are secreted by skin epithelial cells or sweat glands (Turner et al., 1998; Harder et al., 2001; Schittek et al., 2001). Thus, these peptides represent the first form of innate host defence against bacterial infection. In addition, cathelicidins and defensins are part of the oxygen-independent bactericidal mechanism used by human PMNs (Schroder, 1999). To determine whether PIA produced by S. epidermidis confers resistance to antibacterial peptides, we tested the susceptibility of the wild-type and ica– mutant strains to LL-37, hBD3 and dermcidin (Fig. 3). All peptides had significantly higher bactericidal activity towards the ica– mutant strain at physiological salt concentration and mildly acid pH, resembling the conditions on human skin (Fig. 3). Importantly, these findings demonstrate that PIA protects S. epidermidis from the microbicidal effects of antibacterial peptides (Fig. 3). Although our finding that these peptides had significant antistaphylococcal activity is consistent with previous studies (Harder et al., 2001), these data are the first to demonstrate that each kills S. epidermidis. We previously identified mechanisms based on electrostatic repulsion used by Staphylococcus aureus to protect against antibacterial peptides, namely d-alanylation of teichoic acids and incorporation of a specific cationic phospholipid in the cellular membrane (Peschel et al., 1999; 2001). Electrostatic repulsion may also contribute to PIA-mediated protection against the cationic peptides LL-37 and hBD3. This assumption is supported by the finding that the efficacies of hBD3 and LL-37 are higher at low salt conditions, which are favourable to electrostatic repulsion, than those of anionic dermcidin (Fig. 3D–F). On the other hand, PIA also protected against anionic dermcidin with an increased level of protection detected at physiological compared with low salt concentration. These findings, together with the anionic nature of dermcidin, suggest that the mechanism of protection towards dermcidin is not based on electrostatic repulsion. The observed increased protection against dermcidin is of special interest, as the anionic characteristics of dermcidin are very unusual for an antibacterial peptide. Anionic dermcidin might be specifically targeted against microbial factors that confer resistance against the more common cationic antibacterial peptides (Peschel, 2002). Its microbicidal activity appears to be favourably adapted to conditions of human skin (i.e. high salt), which represents the natural habitat of S. epidermidis and the environment in which dermcidin functions (compare conditions with and without NaCl in Fig. 3C and F). In providing protection not only against cationic peptides, but also against dermcidin, PIA seems to be suited specifically to combat the antibacterial peptides found on human skin.
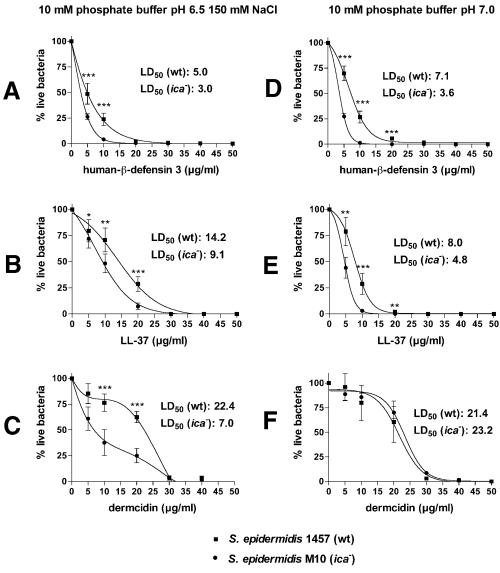
Killing of S. epidermidis by LL-37, dermcidin and hBD3. Antibacterial activity of each peptide (hBD3, A and D; LL-37, C and E; dermcidin, C and F) was determined by incubating wild-type and ica – mutant strains of S. epidermidis with the indicated concentrations of peptide for 2 h at 37°C in 10 mM phosphate buffer, pH 6.5/150 mM NaCl (left column, A–C) or 10 mM phosphate buffer, pH 7.0 (right column, D–F). S. epidermidis survival was determined with the formula 1 – (cfu H2O2 /cfu Control ) × 100. Results are the mean ± SD of six measurements.
PIA inhibits phagocytosis and killing by human PMNs
To determine whether PIA facilitates evasion of cellular components of innate host defence, we compared phagocytosis and killing of the wild-type and ica– mutant strains of S epidermidis by human PMNs (Fig. 4). Compared with the wild-type strain, phagocytosis of the ica– mutant was significantly increased (12.2 ± 7.2% increase, P < 0.001, n = 5), demonstrating that PIA contributes significantly to protection against PMN phagocytosis (Fig. 4A). Consistent with that finding, human PMNs killed ≈ 18% more of the ica– mutant strain (42.6 ± 9.7% and 60.5 ± 15.5% killed for the wild-type and mutant strains respectively; P = 0.04, n = 6) (Fig. 4B). The varied susceptibilities of these two strains to PMN killing probably results from the observed differences in phagocytosis (Fig. 4A) and/or effects of antibacterial peptides (Fig. 3), as susceptibility to hydrogen peroxide, a key proximal reactive oxygen species produced by PMNs, did not differ between wild-type and ica– mutant strains (Fig. 4C).
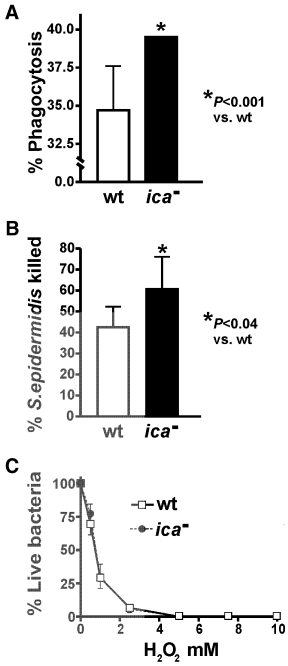
PIA promotes S. epidermidis resistance to phagocytosis and killing by human PMNs. A. Phagocytosis of wild-type and ica– mutant strains of S. epidermidis by human PMNs. Results are the mean ± SD of five experiments.B. Killing of S. epidermidis by human PMNs. At 2 h, PMNs were lysed and S. epidermidis were plated on tryptic soy agar. Per- centage S. epidermidis killed was calculated using the equation 1 – (cfuPMN+/cfuPMN–) × 100. Results are the mean ± SD of six experiments.C. Sensitivity of S. epidermidis to hydrogen peroxide. S. epidermidis parental wild-type and ica– mutant strains were incubated with varied concentrations of H2O2 for 1 h at 37°C. S. epidermidis killed was determined by cfu counting and calculated by the formula 1 – (cfuH2O2/cfuControl) × 100.
Discussion
It has remained unclear until now how S. epidermidis evades destruction by the human innate immune system during colonization and infection. We demonstrate here that PIA is essential for the formation of extracellular material, which protects against destruction from critical components of the innate immune response. Specifically, our results indicate that PIA inhibits killing of S. epidermidis by skin-associated antibacterial peptides and human PMNs. PIA probably serves to sequester bacteria and associated proinflammatory products, acting as a mechanical barrier to block the effects of antibacterial peptides and inhibit PMN phagocytosis. Electrostatic repulsion between cationic antibacterial peptides and PIA probably also contributes to the protective effect of PIA. It is tempting to speculate that the specific characteristics of PIA contribute to the fact that S. epidermidis is the predominant microbe on human skin and in nosocomial infections. Our findings underline the importance of PIA as a virulence factor of S. epidermidis that is involved in the formation of biofilms and protection against innate host defence. Of note, PIA is the first exopolymer of the S. epidermidis cell surface shown to mediate such protection.
The ica genes responsible for the production of PIA in other staphylococcal species have been identified (Allignet et al., 2001), although they have yet to be implicated in evasion of host defence. Several other bacterial pathogens express genes homologous to those within the S. epidermidis ica gene cluster, including hms of Yersinia pestis, which is also involved in biofilm formation (Darby et al., 2002). Thus, it is likely that production of PIA represents a general mechanism used by bacteria to protect against the human innate immune system.
Experimental procedures
Bacterial strains and growth conditions
Staphylococcus epidermidis 1457 and its isogenic ica – mutant S. epidermidis M10 ( erm R ) ( Mack et al., 1994 ) were grown in tryptic soy broth (TSB) supplemented with 0.5% glucose. Bacterial cultures for each experiment were inoculated from precultures grown overnight at a dilution of 1:100 and incubated at 37°C with shaking at 120 r.p.m. for 16 h, unless otherwise noted. Erythromycin was used at a final concentration of 5 µg ml −1 to select for S. epidermidis M10 in precultures.
Production of αPIA antiserum
Staphylococcus epidermidis cultures were harvested by centrifugation at 3000 g , and pellets were washed with phosphate-buffered saline (PBS buffer: 10 mM sodium phosphate, pH 7.0, 150 mM NaCl). Surface-associated PIA was extracted by incubating the cells in 0.5 M EDTA, pH 8.0 (final volume: 1:50 of bacterial cultures) for 5 min at 100°C. PIA-containing extracts were dialysed against distilled water for 24 h and subsequently digested with DNase (0.5 mg ml −1 final concentration; Sigma), RNase (0.5 mg ml −1 final concentration; Sigma), lysostaphin (0.5 mg ml −1 final concentration; Biosynexus) and lysozyme (0.5 mg ml −1 final concentration; Sigma) at 37°C for 16 h, followed by incubation with proteinase K (4 mg ml −1 final concentration; Qiagen) at 37°C for 16 h. Samples were centrifuged at 28 000 g at 4°C for 30 min. Clarified supernatants were concentrated about fivefold by centrifugal concentrators (Amicon Centriprep YM-10; Millipore) and injected in 10 ml aliquots on to a HiLoad 26/60 Superdex 200 gel filtration column (Amersham Pharmacia Biotech). Sodium phosphate (20 mM), pH 7.0, containing 150 mM sodium chloride was used as a buffer at a flow rate of 3 ml min −1 . Most PIA eluted shortly after the exclusion volume. PIA-containing fractions were dialysed against water, lyophilized, dissolved in concentrated hydrochloric acid, neutralized with sodium hydroxide and buffered by 100 mM sodium phosphate buffer, pH 7.0. A solution containing 2 mg of PIA was used to produce rabbit antisera using a standard protocol (Sigma Genosys). To block non-specific binding, αPIA antiserum was diluted 1:100 in TBS (Tris-buffered saline: 10 mM Tris-HCl, pH 7.4, 150 mM NaCl) and incubated with several extracts isolated from S. epidermidis M10 for 16 h with gentle shaking. Precipitated material was sedimented by centrifugation (30 min, 28 000 g , 4°C), and 1 mM sodium azide was added to the clear supernatant used for further investigation. The following extracts of S. epidermidis M10 were used to block the αPIA antiserum: (i) extract isolated by boiling cells with 0.5 M EDTA for 5 min at 100°C; (ii) extract isolated by boiling cells in 1% SDS for 5 min at 100°C; (iii) extract isolated from cells treated with lysostaphin; (iv) crude cell extract prepared by breaking cells with glass beads; and (v) extract obtained from culture medium precipitated by trichloroacetic acid. Extracts were prepared from 50 ml of S. epidermidis M10 cultures or from 200 ml of bacterial supernatant respectively.
Immuno dot-blot assay
To quantify PIA production, corresponding amounts of S. epidermidis cells and culture supernatants were used. Surface-located PIA was extracted by incubating the cells in 0.5 M EDTA, pH 8.0 (final volume: 1:50 of cultures) for 5 min at 100°C. Staphylococcal supernatants were concentrated about 50-fold by centrifugal filter devices (Amicon Ultrafree-MC, YM-10). Aliquots (3 µl) of the samples were spotted on a nitrocellulose membrane, air dried, and PIA was detected using αPIA antiserum as described previously (Vuong et al., 2000).
Peptide synthesis and analysis
Human β-defensin 3 and dermcidin were synthesized by J. Lukszo at the Peptide Synthesis and Analysis Unit of the National Institute of Allergy and Infectious Diseases (NIAID), Bethesda, MD, USA. Purity of the peptides was assessed by reversed-phase high-performance liquid chromatography (HPLC) and matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry. The peptides proved to be 100% pure. Both peptides have been shown previously to be active when synthesized by conventional solid-phase synthesis (Harder et al., 2001; Schittek et al., 2001). LL-37 was purchased from Phoenix Pharmaceuticals.
Isolation of human PMNs
PMNs were isolated from heparinized venous blood of healthy individuals with a standard method (Voyich et al., 2003). All studies were performed in accordance with a protocol approved by the Institutional Review Board for Human Subjects, NIAID. Cell preparations contained > 99% PMNs, and all reagents used contained < 25.0 pg ml−1 endotoxin.
Phagocytosis experiments and PMN bactericidal activity
Phagocytosis of S. epidermidis by human PMNs was analysed by flow cytometry by a previously described method (Hoe et al., 2002; Voyich and DeLeo, 2002; Voyich et al., 2003). Killing of S. epidermidis by human PMNs was determined by a previously described method (Voyich et al., 2003). Briefly, PMNs (106) were combined with ≈ 106S. epidermidis in 96-well plates (ratio of 1 PMN:1 S epidermidis), centrifuged at 400 g for 5 min and incubated at 37°C for 2 or 4 h. PMNs were lysed with 0.1% saponin (20 min on ice), and S. epidermidis were plated on TSB agar. Colonies were enumerated the following day, and percentage S. epidermidis killed was calculated using the equation 1 – (cfuPMN+/cfuPMN–) × 100.
Hydrogen peroxide sensitivity assay
Staphylococcus epidermidis were washed in PBS and resuspended in 10 mM sodium phosphate buffer, pH 7.0. Approximately 10 5 bacteria were incubated with 0.5, 1.0, 2.5, 5.0, 7.5 and 10.0 mM H 2O2 for 1 h at 37°C. Aliquots of each assay were plated on TSB agar, incubated at 37°C for 24 h, and surviving cells were enumerated. The percentage of killed S. epidermidis was calculated using the equation 1 – (cfuH2O2/cfuControl) × 100.
Peptide bacterial killing assays
Staphylococcus epidermidis cultures were harvested, washed with PBS buffer and resuspended in 10 mM sodium phosphate buffer, pH 7.0, or in 10 mM sodium phosphate buffer/150 mM sodium chloride, pH 6.5. Bacterial killing assays were performed using a final concentration of ≈ 10 5 S. epidermidis cells in each sample. Antimicrobial peptides were dissolved in the following solutions: human β-defensin 3, 10 mM acetic acid, dermcidin, 50 mM sodium phosphate buffer, pH 6.5, and LL-37, 10% acetonitrile with 0.1% trifluoroacetic acid. The bacteria were exposed to a range of antimicrobial peptide concentrations (0, 5, 10, 20, 30 and 40 µg ml −1 ). An equal volume of the respective peptide dilution buffer was applied to control samples. Samples were incubated at 37°C for 2 h, and appropriate dilution series of the samples were plated on TSB agar. Survivor S. epidermidis cells were enumerated after 24 h incubation at 37°C. The percentage of killed S. epidermidis was calculated using the equation 1 – (cfu H2O2 /cfu Control ) × 100.
Scanning electron microscopy
For ultrastructural preservation of the PIA structure, samples were prepared as described by Fassel and Edmiston (1999) with the following modifications. Staphylococcal cells in suspension were allowed to settle on 0.1% poly l-lysine-coated thermanox coverslips for 30 min and then fixed for 2 h with 75 mM lysine acetate in 0.075% (w/v) alcian blue, 2% paraformaldehyde, 2.5% glutaraldehyde, buffered with 0.1 M sodium cacodylate. Samples were washed 3 × 5 min with 0.1 M sodium cacodylate and post-fixed with 1% osmium tetroxide in dH2O for 1 h. Samples were washed 1 × 5 min with 0.1 M sodium cacodylate and 2 × 5 min with dH2O before dehydration with a graded ethanol series of 50%, 75%, 95% and 3 × 100%. Samples were critical point dried under CO2 with a Bal-Tec model cpd 030 drier (Balzers) mounted on aluminium studs and sputter coated with 125 angstroms of iridium in a model IBS/TM200S ion beam sputterer (South Bay Technologies) before viewing at 5 kV on a Hitachi S-4500 field emission scanning electron microscope (Hitachi). Images were processed using Adobe photoshop version 7.0 software (Adobe Systems).
Scanning immunoelectron microscopy
Aliquots (50 µl) of S. epidermidis cultures were washed with PBS buffer, and cells were resuspended in 200 µl of αPIA-antiserum and incubated at 37°C with agitation at 400 r.p.m. for 12 h. Samples were washed with PBS buffer, and pellets were subsequently incubated with goat anti-rabbit IgG conjugated with 20 nm gold (BB International) at 37°C with agitation at 400 r.p.m. for 2 h. After antibody labelling, the cell suspensions were attached to coverslips, fixed and dried as described above. After mounting on aluminium studs, the samples were coated with 75 angstroms of chromium and viewed at 10 kV in either backscatter or secondary imaging modes with the same instruments as before.
Statistics
Statistics were performed with a Student's t-test using GraphPad prism version 4.0.
Acknowledgements
We thank D. Mack, Hamburg, Germany, for providing S. epidermidis wild-type and ica mutant strains.




