Gene expression patterns of epithelial cells modulated by pathogenicity factors of Yersinia enterocolitica†
In memory of our colleague and co-author Joerg Lauber who has passed away.
Summary
Epithelial cells express genes whose products signal the presence of pathogenic microorganisms to the immune system. Pathogenicity factors of enteric bacteria modulate host cell gene expression. Using microarray technology we have profiled epithelial cell gene expression upon interaction with Yersinia enterocolitica. Yersinia enterocolitica wild-type and isogenic mutant strains were used to identify host genes modulated by invasin protein (Inv), which is involved in enteroinvasion, and Yersinia outer protein P (YopP) which inhibits innate immune responses. Among 22 283 probesets (14 239 unique genes), we found 193 probesets (165 genes) to be regulated by Yersinia infection. The majority of these genes were induced by Inv, whose recognition leads to expression of NF-κB-regulated factors such as cytokines and adhesion molecules. Yersinia virulence plasmid (pYV)-encoded factors counter regulated Inv-induced gene expression. Thus, YopP repressed Inv-induced NF-κB regulated genes at 2 h post infection whereas other pYV-encoded factors repressed host cell genes at 4 and 8 h post infection. Chromosomally encoded factors of Yersinia, other than Inv, induced expression of genes known to be induced by TGF-β receptor signalling. These genes were also repressed by pYV-encoded factors. Only a few host genes were exclusively induced by pYV-encoded factors. We hypothesize that some of these genes may contribute to pYV-mediated silencing of host cells. In conclusion, the data demonstrates that epithelial cells express a limited number of genes upon interaction with enteric Yersinia. Both Inv and YopP appear to modulate gene expression in order to subvert epithelial cell functions involved in innate immunity.
Introduction
Pathogenic microorganisms have evolved mechanisms to subvert and manipulate host cell functions in order to establish infection. Most of the microbial pathogenicity factors involved in these processes are required to establish the extracellular or intracellular habitat of the pathogen or to evade or subvert host defence mechanisms. Yersinia enterocolitica is an enteropathogenic bacterium that causes a broad spectrum of diseases ranging from gastrointestinal disorders such as enteritis, enterocolitis and mesenteric lymphadenitis, as well as systemic manifestations including reactive arthritis, erythema nodosum, uveitis and septicaemia (Smego et al., 1999; Koornhof et al., 1999). Host factors such as age, HLA B27 or immune status, and the concerted action of a number of Yersinia pathogenicity factors, determine whether Y. enterocolitica infection is restricted to the local site of the gut or whether the pathogen causes systemic disease.
The pathogenicity of Y. enterocolitica depends on a virulence plasmid (pYV) that encodes a type three secretion system (TTSS), secreted Yersinia outer proteins (Yops) as well as a major outer membrane protein, Yersinia adhesin A (YadA). In particular, the yop virulon enables Yersinia to overcome the innate immune system of their host and to survive in the lymphoid tissues. The TTSS enables extracellularly located yersiniae to translocate at least six toxic effector proteins (Yops) directly into the cytosol of the host cells (Cornelis, 2002) where YopE (Rosqvist et al., 1991) An (Andor et al., 2001), YopT (Shao et al., 2003) and YopO (Barz et al., 2000) cause destruction of the actin microfilament structures by modifying small Rho GTPases (Galyov et al., 1993; Iriarte and Cornelis, 1998; Black and Bliska, 2000; Juris et al., 2000; Pawel-Rammingen et al., 2000; Shao et al., 2003). YopH is a protein tyrosine phosphatase, which acts on various eukaryotic proteins such as the focal adhesion kinase, paxillin and p130Cas thereby inhibiting phagocytosis (Black and Bliska, 1997). YopM is distributed within the nucleus of host cells (Skrzypek et al., 1998; Hines et al., 2001; Skrzypek et al., 2003) and stimulates the activity of two kinases (McDonald et al., 2003). YopP affects eukaryotic cells by interfering with NF-κB signalling pathways via inhibition of the activation of IκB kinase and MAPK kinases resulting in reduced cytokine production and apoptosis in macrophages (Ruckdeschel et al., 1998; 2001a; Orth et al., 1999; Denecker et al., 2001). Another important plasmid-encoded virulence factor is YadA, a non-fimbrial adhesin that mediates adherence to cells and extracellular matrix proteins, as well as resistance against defensins, phagocytosis and complement (El Tahir and Skurnik, 2001).
Chromosomally encoded factors including yersiniabactin, invasin, SodA and Irp-1 have been demonstrated to be important in virulence of the pathogen (Roggenkamp et al., 1997; Pelludat et al., 1998; Cornelis 2000; Carniel 2001). The outer membrane protein invasin (Inv) is expressed at low temperature and at early stationary phase of growth (Revell and Miller, 2000; Nagel et al., 2001), mimicking environmental conditions during which Yersinia are ingested along with contaminated food or water. Inv appears to be important in the early phase of the infection by promoting intestinal translocation of the pathogen and colonization of Peyer's patches (Pepe and Miller, 1993a,b; Schulte et al., 2000a). Molecular studies revealed that Inv is a high-affinity ligand for β1 integrins thereby inducing a zipper-like uptake into host cells (Isberg and Leong, 1990; Schulte et al., 2000a). Additionally, Inv may promote proinflammatory host cell responses (Kampik et al., 2000). In fact, Inv triggers activation of the nuclear factor (NF)-κB and production of the proinflammatory chemokine interleukin (IL)-8 in intestinal epithelial cells (Schulte et al., 2000b) in conjunction with Rac-1 and MAP kinase cascades (Grassl et al., 2003). Furthermore, Inv stimulates signalling pathways which appear to be linked to host cell responses involved in the control of innate immunity (Juris et al., 2002).
Yersinia enterocolitica was demonstrated to be taken up by M cells located within the follicle-associated epithelium of Peyer's patches (Autenrieth and Firsching, 1996; Clark et al., 1998), accomplished by interaction of Inv with apical β1 integrins of M cells (Schulte et al., 2000a). Subsequently, yersiniae colonize the Peyer's patches and may disseminate to lymph nodes and spleen. Histological studies suggest that innate host defence mechanisms including phagocytes are involved in control of Yersinia in Peyer's patches (Autenrieth et al., 1996). In addition, clearance of infection involves NK cells, cytokines and the activation of an adaptive immune response (Autenrieth and Heesemann, 1992; Autenrieth et al., 1993; 1994; Bohn and Autenrieth, 1996a,b; Bohn et al., 1998a,b), suggesting that T cell-activated macrophages are the effector components in pathogen control.
Epithelial cells have been recognized to play an important role in mucosal immunity and therefore exhibit a number of immunological functions including expression of adhesion molecules, secretion of effector molecules such as defensins, and expression of cytokines and cytokine receptors (Kagnoff and Eckmann, 1997; O’Neil et al., 1999). Therefore epithelial cells are an integral part of the mucosal immunity network which by secretion of inflammatory cytokines and chemokines may signal the presence of pathogenic bacteria to the mucosal immune system and inflammatory cells (Eckmann et al., 1995; Kagnoff and Eckmann, 1997). The inflammatory reaction at mucosal sites can assist pathogen control, however, it may also be subverted by the pathogen to promote its dissemination in the host.
In this study we wanted to explore the roles of two major pathogenicity factors of Y. enterocolitica, namely Inv and YopP. Inv appears to be involved in the early phase of infection with Yersinia at the intestinal mucosal epithelium and confers enteroinvasion and proinflammatory host responses. In contrast, YopP may suppress inflammatory processes thereby inhibiting anti-Yersinia immune responses and promoting tissue colonization of the pathogen (Orth, 2002). Although a number of host cell reactions due to these two pathogenicity factors have previously been described, we wanted to perform a genome wide transcriptional profiling of epithelial cells using DNA microarrays to analyse the epithelial cell gene expression programs initiated by Y. enterocolitica and to identify gene expression pathways manipulated by Inv and YopP.
Results and discussion
General considerations
To investigate early events in Y. enterocolitica infection we analysed differential gene expression of HeLa human epithelial cells at 0, 2, 4 and 8 h post infection. Yersinia enterocolitica wild type and isogenic mutant strains were used to investigate the effects of Yersinia pathogenicity factors on the host cell transcriptome. The mutant strains Y. enterocolitica WA-C (pYV–) and Y. enterocolitica WA-C Δ inv (pYV–inv–) were selected to address gene regulation in epithelial cells modulated by Inv and/or other chromosomally encoded pathogenicity factors. The wild-type strain Y. enterocolitica WA-P (pYV+) and the mutant strain Y. enterocolitica WA-P Δ yopP (pYV+yopP –) were used to investigate YopP-specific modulation of gene regulation in epithelial cells. We performed three independent infection experiments with each Yersinia strain for each point in time. The infection experiments were validated by determination of IL-8 mRNA expression levels as well as levels of secreted IL-8 (Supplementary material Fig. S1). The data show that IL-8 mRNA expression and IL-8 secretion is highly induced only in pYV– and pYV+yopP – infected cells. This data confirms previous results indicating that Inv triggers expression of IL-8 (Schulte et al., 1996) whereas YopP may suppress IL-8 production (Denecker et al., 2002).
Analysis of the expression profiles of infected cells was performed using Affymetrix HG_U133A microarrays containing 22 283 different probesets corresponding to 14 239 independent Unigene clusters (Unigene build 155) as described in Experimental procedures.
Gene expression in HeLa cells regulated by Yersinia infection
A total of 193 probesets corresponding to 165 unique genes were found to be differentially expressed at least twofold compared to uninfected cells upon infection with at least one of the four Yersinia strains at least at one of the three time-points investigated. Such genes or probesets were also termed regulated genes. The functional categories of proteins encoded by differentially expressed genes are depicted in Supplementary material Table S2. The most abundant functional categories of differentially expressed gene products upon Yersinia infection were nucleic acid binding proteins (transcription factors, DNA/RNA binding/modifying proteins), receptors and signalling components, growth factors, chemokines and cytokines involved in innate immune responses and inflammation, as well as proteins involved in cytoskeletal organization, cell–cell interaction and cell proliferation. At least 24 of the genes are known to be regulated by NF-κB, which plays a key role in inflammatory cell reactions and innate immunity (Caamano and Hunter, 2002).
According to the applied filters, 26.6% of probesets (44 genes) were found to be regulated at 2 h post infection, 83.6% (138 genes) at 4 h post infection and 23% (38 genes) at 8 h post infection (Fig. 1A). The majority of these genes were regulated by pYV– and the smallest percentage was regulated by pYV+ and pYV–inv–. The wild-type strain pYV+ and pYV+yopP– mutant strain only modulated expression of a small number of genes at 2 and 4 h post infection (8 and 16% of probesets, respectively; Fig. 1A), whereas the plasmid-cured strain pYV– was the most potent modulator of host gene expression (73% of probesets) with a peak at 4 h post infection. This suggests that Inv plays a major role in modulation of host cell gene expression by Yersinia.
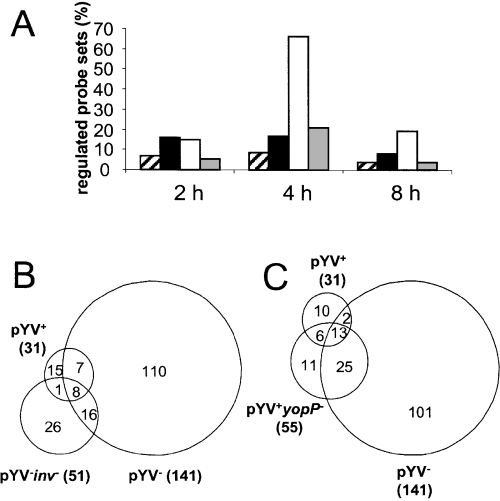
Regulation of probesets upon infection with Y. enterocolitica wild type and mutant strains. A. Percentage of probesets whose expression was regulated by Y. enterocolitica pYV+ (hatched), pYV+yopP– (black), pYV– (white), and pYV–inv– (grey) at least twofold compared to uninfected cells. B and C. Venn diagram of all probesets differentially regulated at least twofold between non-infected cell and cells infected with at least one of the indicated Y. enterocolitica strains. Numbers in bold face at the sides of the Venn diagram report the total number of genes induced or suppressed. Numbers within the regions of the Venn diagrams represent the genes uniquely regulated or regulated in common by infection with the various Y. enterocolitica strains.
Venn diagram analysis (Fig. 1B) illustrates that 117 out of 141 probesets regulated by pYV– were no longer regulated by pYV–inv –. Only 24 probesets were regulated in common by pYV– and pYV–inv– indicating that chromosomally encoded factors other than Inv are involved. The 15 probesets commonly regulated by pYV+ and pYV– reflect that plasmid-encoded factors repress expression of the majority of those genes induced by chromosomally encoded Yersinia factors. Comparison of expression profiles following infection with pYV+ and pYV+yopP – revealed that the presence of YopP caused a small, but constant, reduction in the number of regulated genes. Infection for 2 h with either pYV+yopP – or pYV– resulted in a similar percentage of regulated genes suggesting that YopP accounts for repression of Inv-induced genes (Fig. 1A). In contrast, at 4 h post infection pYV-encoded factors distinct from YopP repressed host cell genes regulated by Inv. Venn diagrams (Fig. 1B) also indicate that a few probesets are exclusively regulated by pYV+ encoded factors. Sixty-one per cent of pYV+ and 34.5% of pYV+yopP – regulated genes were regulated in common indicating that expression of such probesets is independent of YopP, whereas all others may be dependent upon YopP.
In contrast to data from Yersinia-infected murine macrophages in which 857 out of 6657 genes were regulated by Yersinia at 2.5 h post infection (Sauvonnet et al., 2002a) only a small number of genes (n = 165) were regulated upon Yersinia infection in epithelial cells. There may be several reasons for the relatively small number of regulated genes upon Yersinia infection in our study. First it might reflect the simple pattern of gene expression of epithelial cells in inflammatory reactions. In fact, in Helicobacter pylori-infected epithelial AGS cells only 127 out of 22 571 probesets were found to be regulated over a 12 h period (Guillemin et al., 2002). Second, the lack of response of HeLa epithelial cells to LPS compared with macrophages or dendritic cells may explain these observations. For example, 685 genes were found to be regulated in dendritic cells during E. coli infection (Huang et al., 2001). Third, the Yersinia O9 strain used for expression profiling of Yersinia infected macrophages (Sauvonnet et al., 2002a) is less virulent than the Yersinia O8 strain used in this study. Thus, for example because of differences in the amino acid sequence, YopP of Y. enterocolitica O9 strains is less effective in suppressing IL-8 and IL-6 compared to highly virulent O8 strains (Ruckdeschel et al., 2001b; Denecker et al., 2002).
Our data suggests that pathogenicity factors of Y. enterocolitica O8 strains are highly effective in silencing epithelial cells and consequently the immunological ‘watchdog’ function of epithelial cells is effectively subverted by such factors.
Clustering of commonly expressed genes during the course of infection with Y. enterocolitica
All probesets whose expression changed significantly upon infection with Y. enterocolitica were identified and subjected to cluster analysis. Self-organizing map (SOM) algorithms (Genecluster 2.1, Whitehead Institute for Genomic Research, Cambridge, MA) were used to identify genes, which were regulated in common during the course of infection with Y. enterocolitica. The results (Supplementary material Fig. S2, Table S3) show that most probesets were transiently expressed during Y. enterocolitica infection. Most genes were upregulated at 4 h post infection and expression declined again at 8 h post infection (e.g. clusters 6–8). Other clusters revealed genes that were expressed at 2 h, peaked at 4 h and declined at 8 h post infection (clusters 3–5). Probesets included in these clusters typically comprised NF-κB-regulated genes such as IL-8 (cluster 4). The genes of cluster 1 were repressed at 2 and 4 h, but not at 8 h, post infection, whereas the genes in cluster 2 were expressed at 2 h and repressed at 4 and 8 h post infection. Probesets mainly upregulated by plasmid-encoded factors of Y. enterocolitica showed maximal expression at 2 h (cluster 2), whereas genes primarily upregulated (clusters 3–8) as well as downregulated (cluster 2) by chromosomally encoded factors were maximally expressed at 4 h post infection. Irrespective of the fact that distinct pathogenicity factors of Yersinia modulated gene expression in HeLa cells, the clusters depicted in Supplementary material Fig. S2 may reflect the coordinated expression of genes as part of general regulatory pathways of epithelial cells induced by bacterial infections.
Clustering of genes regulated in common by Y. enterocolitica wild-type and mutant strains
To identify genes, which were regulated in common by distinct Y. enterocolitica virulence factors, SOM clustering was used at each point in time (Fig. 2 and Supplementary material Table S4). Clustering revealed five clusters (1, 4–6, 8) at 2 h, eight clusters (1–7, 9) at 4 h and five clusters (1, 2, 4, 5, 7) at 8 h post infection. At each time-point only cluster 5 showed repressed probesets. One major pattern of gene regulation is represented by cluster 1 comprising genes that are upregulated by Inv and repressed by YopP. Many of these genes encode proinflammatory cytokines and chemokines (e.g. IL-8, IL-1α) and are known to be regulated by NF-κB (Ruckdeschel et al., 1998; Schulte et al., 2000b). Similarly, cluster 2 includes probesets which are induced by chromosomally encoded factors and repressed by pYV, although the roles of Inv and YopP are less defined by this cluster (e.g. intercellular adhesion molecule 1, ICAM-1; small Maf protein F, MafF). Moreover, cluster 6 includes probesets that are upregulated by chromosomally encoded factors other than Inv and repressed by plasmid-encoded factors other than YopP (e.g. thrombospondin 1, THBS1; cysteine rich angiogenic inducer 61, CYR61). Cluster 4 is composed of probesets that were induced by all Yersinia strains investigated and therefore primarily represents chromosomally induced genes that are not repressed by plasmid-encoded factors (e.g. B cell translocation gene 1, BTG1; secreted and transmembrane 1, SECTM1). Cluster 8 (2 h post infection) is unique as it is the only cluster in which upregulation of genes is mediated by pYV-encoded factors other than YopP. One of these genes, glucocorticoid-induced leucine zipper (GILZ), is believed to inhibit activation of NF-κB (Ayroldi et al., 2001). Cluster 7 (e.g. Cbp/p300-interacting transactivator, CITED2) and 9 (e.g. a disintegrin-like and metalloprotease 5, ADAMTS5) comprise probesets in which the lack of Inv results in gene upregulation. Taken together, the cluster analyses illustrates that the majority of genes regulated by Yersinia infection are upregulated by Inv and other chromosomally encoded proteins, and that plasmid-encoded factors have a strong impact on gene repression in epithelial cells.
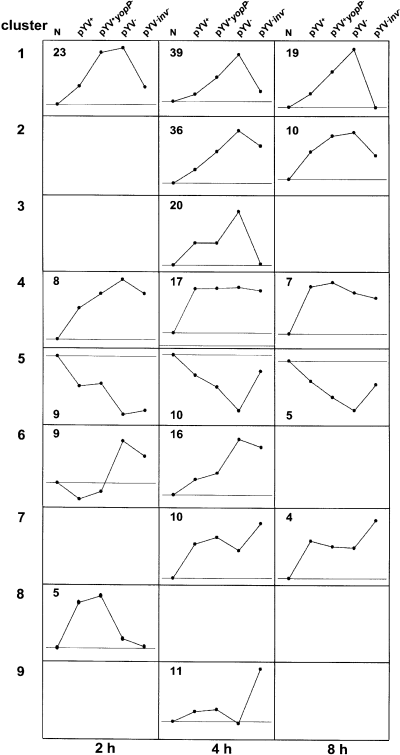
Comparison of gene expression patterns upon infection with different Yersinia strains by SOM cluster analysis. The SOM cluster analysis was performed of probesets (n = 54 at 2 h, 159 at 4 h and 45 at 8 h) whose expression was at least twofold different between non-infected cells and cells infected with at least one of the indicated Y. enterocolitica strains. Each graph represents the mean expression profile of all probesets in each single cluster with arbitrarily scales derived from the signal log ratios. N is given as a reference point defining the base line (no regulation), which is shown in each cluster. Points above or below the baseline refer to probesets upregulated or downregulated by Yersinia strains respectively.
As one main focus of this work was to identify genes upregulated by Inv and YopP in epithelial cells, the first microarray experiments and cluster analysis were performed by growing the bacteria for the final 2.5 h prior to cell stimulation at optimal temperature for Inv expression (pYV–, pYV–inv– at 27°C) and YopP expression (pYV+, pYV+yopP– at 37°C, see Experimental procedures.) To address whether the different temperatures might have an effect on regulation of those genes the cluster analysis was applied to, HeLa cell gene expression profiles where also determined upon infection with pYV– grown at 27°C or pYV– grown at 37°C. We found that 4 h post infection expression of only three genes was affected by the different growth conditions of the bacteria (Supplementary material Fig. S4). RIG-1 was upregulated upon infection with pYV– grown at 27°C but downregulated upon infection with pYV– grown at 37°C. At present it is unclear which bacterial factor accounts for upregulation or repression of RIG-1. In addition, COX2 and BIRC2 were induced to a lesser extent upon infection with pYV– grown at 37°C compared with pYV– grown at 27°C.
Gene expression regulated by Y. enterocolitica pYV +
Heat maps were prepared of the probesets that were up- or downregulated at least twofold upon pYV+ infection compared with uninfected HeLa cells (Supplementary material, Fig. S3). The data demonstrate that 27 genes were significantly regulated by pYV+. Unc-51 like kinase 1, RhoB and transcriptional regulators such as nuclear receptor NR4A2, KLF2 and GILZ were only upregulated at 2 h post infection whereas cytochrome P450 polypeptide I subfamily Ib (CYP1B1) was upregulated at 2, 4 and 8 h post infection. NR4A2 and CYP1B1 were also upregulated by pYV– infection, therefore all other factors are exclusively upregulated by pYV+.
It is interesting to note that KLF2, RhoB and GILZ are reported to be involved in silencing of gene transcription (Engel et al., 1998; Ayroldi et al., 2001; Buckley et al., 2001; Fritz and Kaina, 2001; Mittelstadt and Ashwell, 2001; Banerjee et al., 2003) which is consistent with the fact that pYV-encoded Yops may inhibit immune responses (Juris et al., 2002). Three NF-κB-activated proinflammatory molecules namely immediate early response 3, TNF-alpha induced protein 3 (A20) and cyclooxygenase 2 (COX-2) were expressed 4 h after infection with Y. enterocolitica pYV+. Cystein-rich angiogenic inducer 61 (CYR61), an extracellular matrix protein involved in cell survival and angiogenesis (Chen et al., 2001), and serum induced kinase (SNK) which plays a role in cell division, were both repressed by pYV+ infection.
Our data suggest that pYV+ infection promotes weak transcriptional responses in epithelial cells including proinflammatory responses, antiproliferative responses and genes whose products sustain silencing of host responses.
Inv-dependent epithelial cell gene expression
Genes whose expression changed at least twofold upon pYV– or pYV–inv– infection compared with uninfected cells and which showed a twofold differential expression between pYV– and pYV–inv– infected cells were defined as significantly Inv regulated genes. Figure 3A and Supplementary material Table S5 indicate 50 genes which were significantly regulated by Inv including cytokines, chemokines and molecules involved in cytokine signalling such as IL-8, GRO-1, MCP-1 (SCYA 2), IL-6, IL-1α, IL-6ST (gp130) as well as other proinflammatory molecules including COX-2. The mRNA expression levels of several Inv-induced genes were confirmed by RT-LightCycler PCR or Northern blot analysis (Fig. 4). Furthermore, Inv was shown to have an impact on adhesion molecules (e.g. syndecan-4, ICAM-1, claudin-1 and CD83) and transcriptional regulators (e.g. ATF3, CEBPD, NFKB1, NFKB2, IκB, RelB and Smad3). This is consistent with previous data illustrating that IL-8, IL-1α and MCP-1 are upregulated upon pYV– infection (Kampik et al., 2000). However, TNF-α which was found to be weakly upregulated by a more sensitive RT-PCR (Kampik et al., 2000) was not detected in microarray experiments.
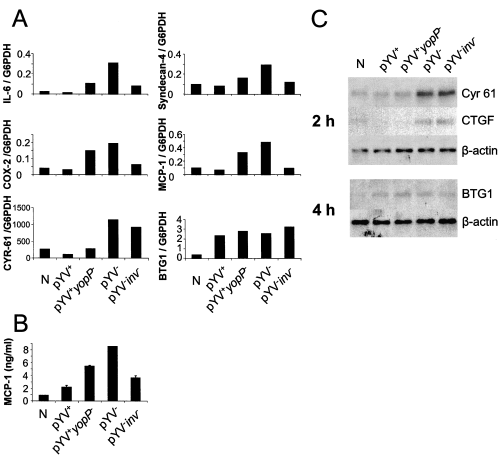
Quantification of mRNA expression. A. Yersinia-infected HeLa cells by semiquantitative real-time RT-PCR. IL-6, syndecan-4, Cox-2, MCP-1, CYR-61 and BTG at 4 h post infection. mRNA expression was normalized to glucose-6-phosphate dehydrogenase expression levels. The data is representative for at least three independent experiments. B. Quantities of MCP-1 in culture supernatants of Yersinia-infected HeLa cells at 4 h post infection as determined by ELISA. The values are the means ± standard deviations. C. Northern blot analysis of Cyr 61, CTGF, BTG1 mRNA expression. Total RNA was isolated from HeLa cells 2 or 4 h after infection with Yersinia strains as indicated, or from uninfected cells (N). Ten µg RNA was separated by gel electrophoresis and transferred onto nylon membranes (See Experimental procedures). Probes for Cyr61, CTGF, BTG1 and β-actin were used for hybridization.
The large number of Inv-regulated genes confirms that interaction of Inv with β1 integrins is an effective pathway for induction of genes in epithelial cells, and the downstream involvement of NF-κB signalling is demonstrated by upregulation of molecules such as NFKB1 (p50), NFKB2 (p52), RelB, IκB and A20, whereas ATF-3 expression suggests JNK activation (Witzenbichler et al., 1998; Schulte et al., 2000b). Moreover, upregulation of Smad 3 may indicate links between integrin and TGF-β receptor signalling as previously suggested (Weinstein et al., 2001). The Inv-induced genes are also found to be regulated by infections with other pathogens such as Toxoplasma (Blader et al., 2001), H. pylori (Guillemin et al., 2002) or RSV (Tian et al., 2002) and therefore represent typical innate immune responses controlled by NF-κB regulated genes. The signalling events triggered by Inv activate pathways including activation of MAP kinases and NF-κB, which are also activated by LPS-stimulated Toll-like receptor signalling. However, intestinal epithelial cells lack CD14 and therefore are hyporesponsive to LPS-induced TLR signalling in the absence of soluble CD14 (Cario et al., 2000).
Recent published data showed that Nod1 detects a unique muropeptide motif from Gram-negative bacterial peptidoglycan resulting in activation of NF-κB and production of IL-8 (Girardin et al., 2003) and that Nod1 is involved in intracellular sensing of bacteria (Girardin et al., 2001; 2003) To address whether Inv-induced gene expression might be due to intracellular signalling events rather than due to β1 integrin activation, we compared gene expression upon pYV– infection for 4 h with or without addition of wortmannin. Wortmannin has been demonstrated to inhibit Inv-triggered internalization by 70–90% without affecting IL-8 production (Schulte et al., 1998; 2000b; Kampik et al., 2000). We found that in pYV– infected HeLa cells in the presence of wortmannin compared to pYV– infected HeLa cells without wortmannin only a few invasin-induced genes (Smad3, NFKB2, ATF3 and claudin 1) were induced at a lower level whereas gene expression levels of all other Inv-induced genes was comparable or even slightly higher (Supplementary material Fig. S4). These data suggest that Inv-triggered gene expression profiles are predominantly triggered by engagement of β1 integrins at the host cell membrane rather than to the internalization and subsequent signalling via Nod1. However, we cannot rule out that Nod1, in addition to β1 integrins, might be involved in this process.
Induction of gene expression by chromosomally encoded factors other than Inv
To identify genes which are up- or downregulated by chromosomally encoded factors distinct of invasin, probesets were selected which changed at least twofold upon infection with pYV– or pYV–inv– compared to uninfected control and showed no significant difference comparing pYV– and pYV–inv– infections (0.5 > SLR > −0.5). A group of 30 genes fulfilled these criteria and appeared to be regulated by factors encoded by the Yersinia chromosome besides Inv or by unspecific stress provided by the bacteria (Fig. 3 and Supplementary material Table S6). Several of these genes are involved in TGF-β receptor signalling: thrombospondin may activate latent TGF-β1 into its bioactive form (Harpel et al., 2001; Yevdokimova et al., 2001), early growth response 1 (EGR-1) may regulate cell interaction with the extracellular matrix by coordinated induction of TGF-β1, fibronectin, and PAI-1 (Liu et al., 2000). CTGF (Stratton et al., 2002), Cyr61 (Denton and Abraham, 2001) and PAI-1 (Doroshow, 1986) are induced by TGF-β receptor signalling. mRNA expression of CTGF, Cyr61 (and BTG1) upon Yersinia infection was confirmed by RT-LightCycler PCR or Northern blots (Fig. 4). The upregulation of TGF-β receptor signalling effector genes upon Yersinia infection might reflect the hosts attempt to restore cell integrity or to avoid cell destruction. The actual function(s) of these genes in bacterial infections is unknown. pYV-encoded factors other than YopP repress expression of these genes. However, the Yops that are affecting TGF-β receptor signalling have to be further elucidated.

Regulation of genes in HeLa cells upon infection with Yersinia 2 h and 4 h post infection. Genes which are displayed fulfilled the following criteria: (A) genes whose SLR between uninfected and pYV– infected cells or uninfected and pYV–inv– infected cells was >0.99 or < −0.99 and whose SLR between pYV– and pYV–inv– infected cells was >0.99 or < −0.99, indicating Inv regulated genes; (B) genes whose SLR upon infection with pYV– versus pYV–inv– was 0.5 > SLR > −0.5 and pYV– versus uninfected cells was >0.99 or > −0.99 indicating chromosomally regulated genes; (C) genes whose SLR upon infection with pYV+ versus pYV+yopP– was >0.99 or < −0.99, indicating YopP regulated genes; and (D) genes whose SLR comparing pYV+yopP– or pYV+ infected cells with pYV– infected cells was >0.99 or < −0.99 and showed no significant difference comparing pYV+ and pYV+yopP– infections (0.5 > SLR > −0.5) were defined as regulated by pYV+ distinct from YopP.
Taken together, the early gene expression pattern upon pYV– infection consists predominantly of Inv-induced NF-κB regulated genes and genes most likely induced by TGF-β receptor mediated signalling.
YopP-dependent host gene expression
Genes regulated by YopP were defined as genes, which were at least twofold changed upon infection with pYV+yopP – or pYV+ compared to uninfected cells and additionally showed a twofold difference when comparing pYV+ and pYV+yopP – infections. Eighteen genes fulfilled these criteria. At 2 h post infection, YopP repressed predominantly those genes that were upregulated by Inv (Fig. 3 and Supplementary material Table S7). It is known that NF-κB is involved in transcription of most of these genes (e.g. ATF3, IL-8, COX2, NFKB1A, SCYA20, SCYA2, ICAM-1) indicating that YopP predominantly inhibits NF-κB induced genes and this confirms earlier reports that YopP interferes with MAP kinase kinases (MKK) and IκB kinase (Orth et al., 1999; Orth, 2002). The majority of Inv induced genes was not repressed by YopP 4 h post infection suggesting that the YopP-mediated repressive effect is transient. This might be explained by other transcription factors besides NF-κB directly induced by Inv or induction of autocrine host cell factors. There is evidence that Yops other than YopP, e.g. YopE, YopH and YopT, might affect Yersinia induced gene expression (Sauvonnet et al., 2002a; Viboud et al., 2003).
Comparison of YopP-modulated gene expression in epithelial cells and macrophages (Sauvonnet et al., 2002a) reveals striking differences. Only a few genes (A20, IκB) were similar regulated by YopP upon Yersinia infection of macrophages or epithelial cells. Whether this is a result of the different host species, cell type, or Yersinia strain differences is not clear.
MCP-1 which was reported to be repressed by YopH, but not by YopP, upon Yersinia O9 infection in macrophages (Sauvonnet et al., 2002b), was found to be YopP regulated in epithelial cells upon Yersinia O8 infection. To evaluate these contradiction MCP-1 expression was investigated by LightCycler RT-PCR and ELISA (Fig. 4) confirming the microarray data presented herein. YopP of Yersinia O9 is less efficient in repressing gene expression compared with Yersinia O8, which may explain that YopP affects MCP-1 expression only upon Y. enterocolitica O8 infection. Because the lack of YopP restores MCP-1 expression only partially, we speculate that besides YopP, possibly YopH, may also play a role in repressing MCP-1 in epithelial cells. Furthermore, YopP-dependent gene regulation was confirmed by RT-LightCycler PCR for IL-8 and COX-2 (Fig. 4 and Supplementary material Fig. S1). IL-6 did not fulfil the criteria in microarray experiments for being YopP regulated but was clearly found to be YopP regulated by RT-LightCycler PCR (Fig. 4). Similarly, YopP clearly contributes to repression of syndecan-4 mRNA, although in microarray experiments as well as in LightCycler RT-PCR difference in gene expression upon infection with pYV+ and pYV+yopP– was less than twofold (Fig. 4).
Genes whose SLR comparing pYV+yopP– or pYV+ infected cells with pYV– infected cells was > 0.99 or < −0.99 and showed no significant difference comparing pYV+ and pYV+yopP– infections (0.5 > SLR > −0.5) were defined as regulated by pYV+ distinct from YopP. Those genes that were significantly regulated by pYV+ infection but not repressed by YopP 2 h post infection (Fig. 3D and Supplementary material Table S8) can be divided in two major groups. Group 1 consists of genes associated with TGF-beta receptor mediated effects such as EGR-1, Cyr-61, CTGF and GADD45A, whereas group 2 consists of three putative transcriptional silencing genes namely RhoB, GILZ and KLF2.
Conclusions
Genome-wide expression profiling of epithelial cells infected with Yersinia may provide new insights into the pathways involved in gene regulation, modulation and subversion of host responses upon bacterial infection. Validation of several regulated genes by real-time RT-PCR as well as Northern blots confirmed the validity of the microarray analysis.
Inv is the most prominent factor in upregulating proinflammatory factors in epithelial cells. Virulence plasmid encoded factors delivered by translocation of effector Yops into the host cell cytoplasm counteract the Inv-induced proinflammatory host cell reaction by largely silencing host gene expression of epithelial cells. This study provides some novel candidates induced by pYV+ infection such as GILZ, KLF2 and RhoB which were reported to possess negative regulatory function on transcription and might be involved in autocrine repression of gene expression (Ayroldi et al., 2001; Buckley et al., 2001; Fritz and Kaina, 2001; Mittelstadt and Ashwell, 2001).
YopP was found to transiently repress Inv-induced NF-κB regulated early after infection and thereafter, other pYV-encoded factors are important for counteracting Inv-induced gene expression in epithelial cells. A number of candidate genes have to be further investigated in order to elucidate the molecular pathogenesis of Yersinia infections. The most obvious gene expression pattern induced by chromosomally encoded factor other than Inv was found to be dependent on TGF-β receptor signalling. Because TGF-β is known to have an important function in wound repair the induction of this pathway reflect maintenance of tissue integrity.
In summary transcriptional profiling by microarrays is a powerful tool to uncover novel host gene expression pathways upon bacterial infections and their dynamic modulation by microbial pathogenicity factors.
Experimental procedures
Bacterial strains, culture and infection of HeLa cells
The following Yersinia enterocolitica strains were used in this study: Y. enterocolitica WA-P of serotype O8 wild type carrying the virulence plasmid pYV (designated as pYV+); a YopP-deficient mutant (pYV+yopP–; kindly provided by K. Ruckdeschel, Munich); a plasmid-cured Y. enterocolitica WA-C (pYV–), and a plasmid-cured Inv-deficient mutant strain (pYV–inv–). All strains of Y. enterocolitica were grown overnight in LB media at 27°C. A 1:50 dilution of the overnight Yersinia culture was prepared and incubated for additional 2.5 h either at 37°C (pYV+, pYV+yopP– and pYV–) or at 27°C (pYV– and pYV–inv–). The bacteria were washed twice with 5 ml PBS (Invitrogen, Karlsruhe, Germany) and the OD600 was determined. HeLa epithelial cells (5 × 106) were grown in 15 ml RPMI 1640 medium (Biochrom, Berlin, Germany) supplemented with 10% FCS (Sigma, Taufkirchen, Germany) and 2 mM glutamine (Invitrogen, Karlsruhe, Germany) under standard conditions in an 80-mm2 culture flask and then infected at an MOI of 20. Expression of Inv was controlled by Western blotting as described previously (Wiedemann et al., 2001) showing that Inv expression was comparable on all strains with exception of pYV–inv– independent of growth conditions (Supplementary materialFig. S4). The bacteria were spun onto the cells at 300 g for 5 min. After 1 h of infection the media was removed and the cells were washed with PBS. The cells were then cultured for a further 1, 3 or 7 h in fresh media containing 0.1 mg gentamycin per ml to kill the extracellular bacteria. In single experiments 50 nM wortmannin (Sigma) was added to the culture media as described previously (Schulte et al., 1998) in order to address the role of bacterial internalization for host cell gene expression.
Isolation of total RNA from HeLa cells
Total RNA was isolated from uninfected or infected cell culture with 5 ml TrizolLS (Invitrogen) after washing twice with PBS following the manufacturer's instructions.
Reverse transcription cDNA synthesis was performed with 2 µg of total RNA using a Biometra thermocycler (Biometra, Göttingen, Germany). The RNA was denatured together with 0.5 µg oligo-d(pT)18 primer (NewEngland Biolabs, Frankfurt A.M., Germany) in a total volume of 10 µl at 65°C for 10 min and afterwards rapidly cooled to 4°C. At 42°C, 10 µl of a mix containing 200 U Superscript II RNaseH–, 4 µl 5× 1st strand buffer, 2 µl DTT, 1 µl RNaseOUT and 2 µl 10 mM dNTPs (all Invitrogen) was added. The cDNA-synthesis was performed for 1 h at 42°C. After a final inactivation step at 70°C for 10 min, the cDNA was diluted with RNase-free water to 200 µl.
Real-time quantitative PCR
We used the SYBR-green Faststart LightCycler system (Roche, Mannheim, Germany) to verify infection experiments and microarray data. To control the success of the individual infection experiments we used IL-8 mRNA expression as a marker and glucose-6-phosphate dehydrogenase (G6PDH) as a housekeeping control. For quantification of IL-8, G6PDH, IL6, COX2 and MCP-1 mRNA we used the corresponding LightCycler kit (SearchLC, Heidelberg, Germany) as described in the manufacturer's manual. All other quantifications were carried out using a cloned RT-PCR product as an external standard. A PCR product was generated from cDNA using the primers in Supplementary material Table S1, and was then cloned into the pCR 2.1 TOPO TA vector (Invitrogen). 102−105 copies of the plasmid vector carrying the standard were then used in LightCycler PCR.
DNA microarray hybridization and analysis
Quality and integrity of the total RNA was controlled by running all samples on a 2100 Bioanalyzer (Agilent Technologies). Preparation of the biotinylated RNA probe was performed as described elsewhere (Affymetrix GeneChip Expression Analysis Manual, Affymetrix). Briefly, 5 µg total RNA was converted to dsDNA using 100 pmol of an oligo-dT primer containing a T7 promoter. The cDNA was then used directly in an in vitro transcription reaction in the presence of biotinylated nucleotides and 12.5 µg of the resulting cRNA were fragmented and used for hybridization onto HG_U133A Genechips (Affymetrix, High Wycombe, UK). Following a 16 h incubation, Genechips were washed, stained with streptavidin-phycoerythrin and read using an Affymetrix Genechip scanner and accompanying gene expression software at the array facility of the German Research Center for Biotechnology, Braunschweig.
Analysis of microarray data
Analysis of microarray data was performed using the Affymetrix Microarray Suite 5.0, Affymetrix MicroDB 3.0 and Affymetrix Data Mining Tool 3.0. All array experiments were scaled to a target intensity of 150, otherwise using the default values of the Microarray suite. Filtering of the results was performed as follows: the median of signals and signal log2 ratios (SLR) of the three independent experiments for each mutant and time-point was calculated. A median SLR of greater than 0.99 or less than − 0.99 was considered a significant change. The absolute detection calls and change calls were assigned using the median of either the detection P-values or the change P-values. A detection P-value of ≤ 0.065 was considered as present (P), a detection P-value of > 0.065 and ≤ 0.07 was considered medium (M) and a detection P-value > 0.07 was considered absent (A). A change call of increase (I) was assigned with a median change P-value of ≤ 0.006 and a change call of medium increase (MI) was assigned at a median change P-value > 0.006–0.007. Change calls of medium decrease (MD) were assigned at a median change P-value of ≥ 0.993 to < 0.994 and a change call of decrease (D) was assigned at a median P-value ≥ 0.994. All others were assigned no change (NC). Only those probesets with a significant SLR were retained that had a change call other than NC in comparison to uninfected and were not absent in both infected and uninfected cells. Probesets with an increase but a detection call of A in infected cells were also discarded. Of the remaining probesets only those with a signal at least three times higher than the average noise were used for further analysis.
The filtering results were validated by applying significance analysis of microarrays (SAM) (Tusher et al., 2001). All of the probesets, which were found by filtering, were also detected as regulated using SAM.
Cluster analysis
We used Genecluster 2.1beta (Whitehead Institute Center for Genome Research; http://www-genome.wi.mit.edu/cancer/software/genecluster2/gc2.html) to perform self-organizing map (SOM) clustering of the microarray data. Clustering was performed using the median SLR for all probesets which were found to be regulated at a specific time-point or with those found to be regulated across all time-points. Parameters used for clustering were as follows: row normalization to means of 0 and variance of 1, 50 000 iterations, seed range 42, random vectors initialization and gaussian neighbourhood. Internal clustering of each time-point was performed with a cluster range from 6 to 16, and for clustering across all time points a cluster range of 6–12 was used.
IL-8 and MCP-1 ELISA
The amount of IL-8 secreted by HeLa cells into the culture supernatant was determined as previously described (Schulte et al., 1998). MCP-1 ELISA was done using the human MCP-1 OptEIA ELISA kit (BD Pharmingen, Heidelberg, Germany) according to the manufacturers directions.
Northern blotting
Ten µg of total RNA was separated on a 0.8% agarose gel at 5 V cm−1 for 3 h in TAE buffer. The RNA was blotted on HybondN + membrane (Amersham Biosciences, Freiburg, Germany) using the downward alkaline procedure (Chomczynski, 1992). The probes were hybridized to the membrane at 50°C over night using a hybridization buffer of 0.5 M NaH2PO4 (pH 7.2) with 7% SDS. After hybridization the membrane was washed two times for 20 min at 55°C with washing buffer containing 0.1 M NaH2PO4 (pH 7.2) and 1% SDS. Restriction fragments of cloned RT-PCR fragments were used as probes. These fragments were labelled with α-[32P]-dCTP using the Rediprime II random labeling system (Amersham Biosciences) according to the manufacturer's instructions.
Acknowledgements
This work was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF) 01GS0114 TuebinGENome/Nationales Genomforschungsnetz, Deutsche Forschungsgemeinschaft, and Fortüne programme (University of Tübingen). We thank Pierre Kyme for critical reading of the manuscript.
Supplementary material
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/cmi/cmi346/cmi346sm.htm
Fig. S1. IL-8 production by HeLa cells upon infection with Y. enterocolitica. HeLa cells were infected with Y. enterocolitica wild type and mutant strains for indicated time points. (A) IL-8 mRNA expression was analyzed by quantitative LightCycler RT-PCR. (B) IL-8 protein in culture supernatants was detected by IL-8 ELISA. The indicated values are the means +/− standard deviations of triplicate samples. All experiments were performed at least three times and revealed comparable results.
Fig. S2. SOM cluster analysis of gene expression profile according to the time course was performed for all the probesets (n = 195) that were at least twofold differentially expressed between non-infected cells and cells infected with at least one of the indicated Yersinia strains. Each graph represents the mean expression profile of all probesets in each single cluster with arbitrarily scales derived from the signal log ratios. N is given as a reference point defining the base line (no regulation), which is shown in each cluster. Points above or below the baseline refer to probesets upregulated or downregulated by Yersinia strains respectively.
Fig. S3. Regulation of genes in HeLa cells upon infection with Y. enterocolitica pYV+. Those genes are displayed where the SLR upon infection with pYV+ compared with uninfected cells was >0.99 or <−0.99. The heat map indicates the levels of gene expression. Red, upregulated genes compared with uninfected cells. Black, no regulation. Green, repressed genes compared with uninfected cells. Scale of SLR, +3.0 to −3.0.
Fig. S4. A. Heat map of invasin regulated genes at 4 h postinfection using pYV- grown at either 27°C or 37°C. Alternatively wortmannin was added to the cells to inhibit internalization of the bacteria. Underlined genes were affected by wortmannin; non-underlined genes were affected by the different growth temperatures.
B. Western blot of pYV+ (37°C), pYV+yopP− (37°C), pYV− (27°C and 37°C) and pYV−inv− (27°C) cell extracts. Invasin protein was detected using a polyclonal rabbit anti-invasin antibody.
C. IL-8 ELISA of cell culture supernatant of infections using pYV- grown either at 27°C or 37°C or at 27°C with addition of wortmannin (wo).
Table S1. Primers used for RT-PCR and real-time quantitative PCR.
Table S2. Functional classification of regulated genes.
Table S3. Time course SOM clusters.
Table S4A. SOM clusters, 2h post infection.
Table S4B. SOM clusters, 4h post infection.
Table S4C. SOM clusters, 8h post infection.
Table S5. Genes regulated significantly by invasin.
Table S6. Genes regulated by factors encoded on the Yersinia chromosome.
Table S7. Genes regulated significantly by YopP.
Table S8. Genes regulated by pYV-encoded factors other than YopP.