Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory
Abstract
In conditioned taste aversion (CTA), a subject learns to associate a novel taste (conditioned stimulus, CS) with visceral malaise (unconditioned stimulus, US). Considerable evidence indicates that the noradrenergic system in the amygdala plays an important role in memory consolidation for emotionally arousing experiences. The specific aim of the present set of experiments was to determine the involvement of noradrenergic activity in the basolateral amygdala (BLA) during the US presentation and consolidation of CTA as well as during the consolidation of a nonaversive/incidental gustatory memory. Selective bilateral microinfusions of the β-adrenergic antagonist propranolol administered into the BLA immediately before intraperitoneal (i.p.) lithium chloride (LiCl) injections disrupted CTA memory. Additionally, propranolol infused into the BLA immediately after a pre-exposure to the saccharin (CS) significantly attenuated latent inhibition. The present findings indicating that alterations in noradrenergic function in the BLA affect taste memory formation, provide additional evidence that the BLA plays a critical role in modulating the consolidation of memory and that the influence is mediated by interactions with other brain regions that support memory for different kinds of experiences.
Introduction
Conditioned taste aversion (CTA) is a learning paradigm in which the novel taste of food or drink (conditioned stimulus, CS) is paired with visceral signals of malaise (unconditioned stimulus, US). After a single CS-US pairing, animals avoid consuming the food or drink previously associated with the US. The anatomical substrates of CTA have been extensively studied (Kiefer, 1985; Bures et al., 1998). The insular gustatory cortex is strongly involved in the mnemonic representation of taste and its functioning is disrupted by nucleus basalis magnocellularis (NBM) lesions (Miranda & Bermúdez-Rattoni, 1999; López-García et al., 1993) or cortical cholinergic antagonists (Naor & Dudai, 1996; Ramirez-Lugo et al., 2003).
Despite the substantial literature regarding the role of the amygdala during CTA, there is still a debate about the effects of amygdala lesions and pharmacological manipulations on CTA. In general, lesions of the entire amygdala disrupt CTA induced by a variety of agents serving as a US, including amphetamine, lithium chloride, and X-rays (McGowan et al., 1972; Grupp et al., 1976; Elkins, 1980; Bermudez-Rattoni et al., 1986). Functional inactivation of the amygdala with tetrodotoxin (TTX) before the gustatory stimulus presentation does not block CTA acquisition. However, TTX inactivation of the amygdala after the gustatory stimulus presentation disrupts CTA memory formation (Buresova & Bures, 1973; Lasiter et al., 1982; Gallo et al., 1992; Roldan, & Bures, 1994). Such evidence strongly suggests that the amygdala is involved in CTA, at least partly, through influences on the consolidation of the memory of the US.
Although these studies suggest the involvement of components of the amygdaloid complex in CTA, the contribution of the amygdala and its several nuclei during the discrete phases of CTA behaviour has not been clearly elucidated. The evidence suggests that the central amygdala and the basolateral amygdala (BLA) may have an important role in CTA. However, there is still a controversy about the role of the BLA that plays in CTA, which may stem from the kind of lesions and behavioural protocols used in those reports (see Lamprecht & Dudai, 2000). For example, some investigators have reported that partial or complete electrolytic or excitotoxic BLA lesions do not affect CTA (Fitzgerald & Burton, 1981:; Bermudez-Rattoni & McGaugh, 1991; Ferry et al., 1995; Hatfield & Gallagher, 1995), whereas others have concluded that electrolytic but not excitotoxic can affect taste aversion (Dunn & Everitt, 1988) meanwhile others reported that an intact BLA is essential for normal CTA (Yamamoto & Fujimoto, 1991; Morris et al., 1999) when a nonfamiliar taste is used as the gustatory stimulus.
There is extensive evidence that the BLA plays an important role in the consolidation of memory for emotionally arousing experiences (Cahill & McGaugh, 1991; McGaugh, 2000). The finding that the memory-modulating effects of peripheral epinephrine, released during emotionally arousing learning tasks, are blocked by intra-amygdala infusions of β-adrenergic receptor antagonists indicates that epinephrine influences on memory consolidation require noradrenergic activation in the BLA (Liang et al., 1986; Introini-Collison et al., 1989; Liang et al., 1990; Quirarte et al., 1997). Several findings indicate that the BLA is the key nucleus responsible for the amygdala's noradrenergic modulatory influence on memory consolidation (McGaugh, 2002). Infusions of norepinephrine (NE) or β-adrenoreceptor agonists administered selectively into the BLA after training enhances memory consolidation in inhibitory avoidance, contextual fear conditioning and spatial tasks, whereas β-adrenoreceptor antagonists impair consolidation (Ferry & McGaugh, 1999; Ferry et al., 1999; Hatfield & McGaugh, 1999; LaLumiere et al., 2003).
The specific aim of the present set of experiments was to determine the involvement of the noradrenergic system in the BLA during the US presentation and during the consolidation of CTA as well as during the memory consolidation of a nonaversive/incidental gustatory memory. The first experiment examined the effects of bilateral infusions of the β2-adrenergic agonist clenbuterol and the β-adrenergic antagonist propranolol into the BLA immediately before i.p. lithium chloride (LiCl) injections during CTA acquisition. The second experiment investigated the effects of infusing the adrenoceptor antagonist propranolol into the BLA immediately after a pre-exposure to the saccharin CS to examine the role of β-adrenergic receptors in the BLA during ‘incidental’ taste memory formation.
Materials and methods
Animals
One hundred and eight male Sprague–Dawley rats (Charles River) weighing approximately 300 g at the time of surgery were used. They were individually housed under 12-h light : 12-h dark cycle, with food and water ad libitum, and given 7–8 days to acclimatize to the vivarium before undergoing surgery. All methods used were in compliance with NIH guidelines for care of laboratory animals and were approved by the UC Irvine Institutional Animal Care and Use Committee.
Guide cannulae implantation
The rats were anaesthetized with sodium pentobarbital (50 mg/kg, i.p.) and given atropine sulphate (0.1 mg, i.p.) to prevent respiratory congestion as well as 3.0 mL of saline (s.c.) to prevent dehydration during surgery. The rats were then placed in a small animal stereotaxic instrument (Kopf Instruments, Tujunga, CA, USA). Two surgical screws were implanted into the skull as anchors and guide cannulae aimed at the basolateral amygdala (BLA) were implanted bilaterally, 2.5 mm posterior and 5.0 mm lateral and 7.0 mm ventral to Bregma (Paxinos & Watson, 1998). The nose bar was maintained at −3.5 mm relative to the interneural line. The guide cannulae were constructed of 23-gauge stainless steel tubing cut to a length of 15.00 (± 0.02) mm. The cannulae and the screws were affixed to the skull with dental cement. Insect pins (15-mm long 00 insect dissection pins) were inserted into the cannulae to maintain patency and were removed only for the infusions.
Conditioned taste aversion
One week after surgery, the rats were deprived of water for 24 h and then habituated to drink water from a spout inserted in a graduated cylinder for 20 min per day for 5 days, until a stable water consumption baseline was reached. On day 6, the rats were randomly separated into groups and the acquisition of CTA was performed. The water was replaced by a 0.1% sodium saccharin solution and the rats were permitted to drink for 20 min. Thirty minutes after the presentation of saccharin the animals were injected i.p. with LiCl (3 mL, 0.4 m), which induces a robust CTA. For the next three days, water consumption baselines were recorded. On day 10, the water was again substituted with a 0.1% of saccharin solution to test for taste aversion (see Fig. 1A).
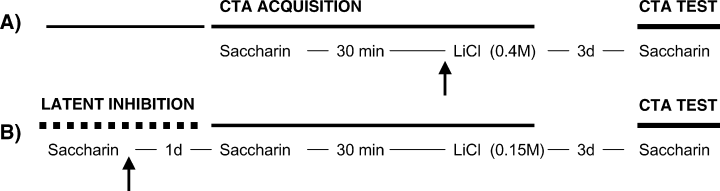
Schematic representation of microinjection procedure used in (A) CTA and (B) latent inhibition experiments. Arrows indicate the time of injection in bilateral BLA.
Latent inhibition
The rats were deprived in the same way as in the above CTA protocol. After five days of baseline recording, on day 6, they received the saccharin solution. The next day, saccharin was given again and 30 min later the animals were injected i.p. with LiCl (3 mL, 0.15 m). Baseline levels of water consumption were taken on days 8 and 9. On day 10, the animals were given the saccharin solution to test for taste aversion (see Fig. 1B).
Statistical analysis
The saccharin consumption volume during test day in both protocols was calculated as a percentage of the baseline (water) consumption volume from the two days immediately before the first saccharine presentation, and taken as the taste aversion score. For each experiment, a one-way anova was performed with the percentage of baseline consumption of each subject (% of baseline = consumption test × 100/consumption baseline), followed by posthoc pair-wise Fisher tests, where appropriate.
Bilateral microinfusions
For CTA experiments the animals were randomly separated in six groups: no cannula (CTA-CONTROL); infusions of isotonic saline (CTA-SALINE); infusions of 0.3 µg propranolol (Prop-3); infusions of 1.0 µg propranolol (Prop-1), infusions of 10 ng of clenbuterol (clen-10) and 100 ng of clenbuterol (clen-100). Propranolol and clenbuterol were obtained from Sigma (St. Louis, MO). Both drugs were dissolved in an isotonic saline solution. The BLA-bilateral infusions were administered just before the injection of LiCl (Fig. 1A).
For latent inhibition experiments the animals were randomly separated in two groups: Pre-exposure infusions of isotonic saline (SALINE) and infusions of 1.0 µg propranolol (Prop). The infusions for these groups were administered just after the first presentation of saccharin (Fig. 1B). An additional group given infusions of isotonic saline (CTA-CONTROL) was added as a control. The infusions were given to animals without pre-exposure to the saccharin.
The microinjections were made through the intracerebral cannula by using 17 mm dental needles (30 gauge, which protrude from the tip of the guide cannulae 2.0 mm) attached to clear polyethylene tubing, backfilled with distilled water. The tubes were connected to 10-µL Hamilton syringes, which were connected to a microinfusion pump. Infusions were performed in a 0.2-µL volume delivered over 60 s per hemisphere of the drug corresponding to each group. The infusion needles were left in position for an additional 30 s to allow for diffusion.
Histology
One day after behavioural testing, animals were deeply anaesthetized with pentobarbital and perfused transcardially with saline solution followed by a 4% (v/v) solution of formaldehyde. The brains were placed overnight in formaldehyde and transferred to a 20% buffered sucrose solution and stored at 4 °C until they were cut. Coronal sections (50 µm thick) were taken through the areas of the infusion needle. The slide sections were stained using cresyl violet.
Results
Verification of cannula placement. Figure 2 shows a schematic diagram of the location of the cannulae in the BLA. Any infusion needle tracks not located within the complex of the BLA were excluded from analysis. In some cases (15%), the needle tips were located on the border of central and lateral amygdala nuclei. Fifteen animals were discarded from further analysis due to incorrect cannulae placements.
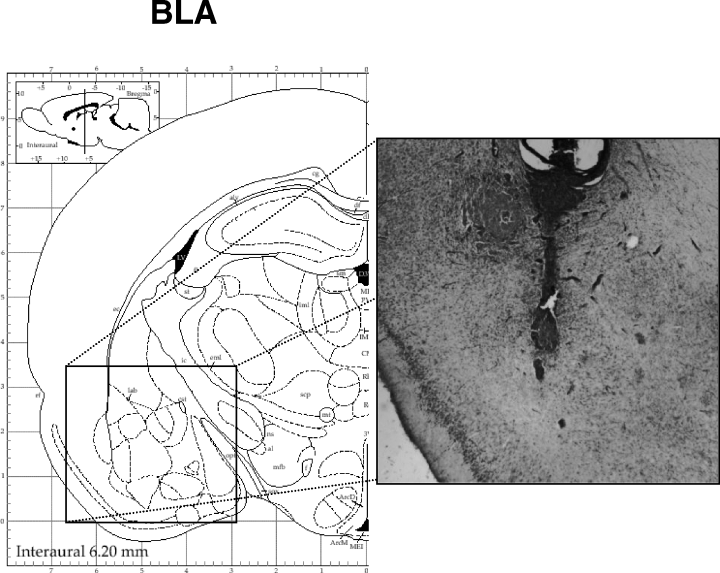
Diagram of right coronal sections and micrograph of the localization of cannula tip aimed to the BLA.
Effects of clenbuterol and propranolol during taste aversion acquisition
There were no significant differences among groups in baseline water intake (the average consumption was 16.8 mL) or in saccharin consumption on the CTA acquisition day (the average consumption was 15.2 mL). There was, however, a significant difference between groups in saccharin consumption on the test day, as a percentage of baseline consumption, as revealed by a one-way anova (F5,43 = 4.281; P < 0.01). Figure 3 shows the percentage consumption of saccharin during the test by rats given clenbuterol or propranolol infusions. Infusions of the β-adrenergic agonist clenbuterol into the BLA did not significantly affect CTA retention as assessed by saccharin consumption (P > 0.05). However, infusions of the β-adrenoreceptor antagonist propranolol impaired the memory for CTA. Posthoc analysis showed that percentage consumption of the group given propranolol (1.0 µg) differed significantly from that of the sham, intact, and propranolol (0.3 µg) groups (P < 0.01 for all comparisons). The saccharin consumption of the 0.3 µg propranolol group did not differ from that of the control groups (P > 0.1).
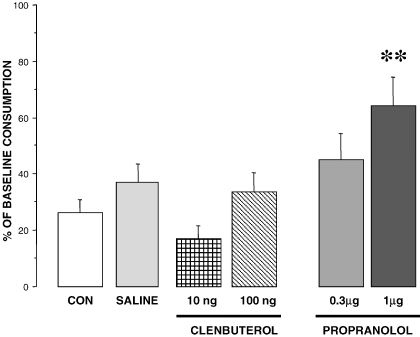
Percentages of base line consumption of saccharin during the CTA test by animals given clenbuterol or propranolol infusions just before LiCl during the acquisition day. Infusions of the β-adrenoreceptor antagonist propranolol (1.0 µg) impaired the memory for CTA. Intact animals (CTA-CON). **P < 0.01. Groups: no cannula (CTA-CONTROL, n = 8); infusions of isotonic saline (CTA-SALINE, n = 13); infusions of 0.3 µg propranolol (Prop-3, n = 6); infusions of 1.0 µg propranolol (Prop-1, n = 8), infusions of 10 ng of clenbuterol (clen-10, n = 6) and 100 ng of clenbuterol (clen-100, n = 8).
Effects of propranolol on latent inhibition of taste memory
There were no significant differences among groups in baseline water intake (the average consumption was 16.2 mL) or during pre-exposure or acquisition saccharin consumption (the average consumption was 15.6 mL) (P > 0.05). On the test for CTA (Fig. 4), there was a significant difference among the groups in the percentage of saccharin consumed as revealed by a one-way anova (F2, 41 = 8.721 P < 0.001). Posthoc analysis revealed a significant difference between latent inhibition (SALINE) and the CTA control groups (P < 0.01), indicating that preexposure of saccharin impaired the later association of the saccharin with the LiCl-induced visceral illness.
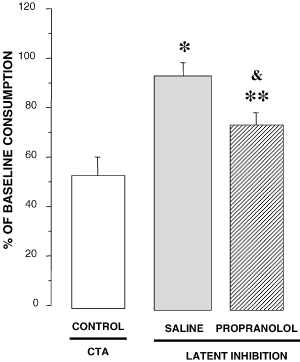
Percentages of base line consumption of saccharin in CTA and latent inhibition groups. There was a significant difference between latent inhibition (SALINE n = 12) and the CTA control groups, indicating that preexposure of saccharin impaired the later association of the saccharin with the LiCl-induced visceral illness. Propranolol infusions (Prop, n = 23) attenuated, but did not completely block, the latent inhibition. **P < 0.01 compared with pre-exposed saline group (SALINE): *P < 0.001 and &P < 0.05 compared with the control group not pre-exposed to saccharin (CONTROL, n = 9).
Figure 4 also shows that propranolol infusions attenuated, but did not completely block, the latent inhibition. The percentage of saccharin consumed by the group that received propranolol 1.0 µg was significantly lower than that of the pre-exposed saline group (P < 0.01) but was also significantly higher than that of the control group not pre-exposed to saccharine (P < 0.05).
Discussion
The results of the present experiments provide evidence that β-adrenoreceptors in the BLA are involved in regulating memory for taste and taste-malaise association. In the first experiment, infusions of 1.0 µg of the β-adrenergic antagonist propranolol into the BLA 30 min after a novel taste, but just prior to injection of LiCl, impaired CTA retention. Although infusions of the adrenoreceptor agonist clenbuterol appeared to enhance CTA memory, that effect was not statistically significant. In the second experiment, intra-BLA propranolol infusions administered immediately after the first exposure to saccharin attenuated the latent inhibition of CTA seen in controls given pre-exposure to the novel taste that was subsequently used as a CS. That finding suggests that β-adrenergic activation in the BLA is also important for the consolidation of incidental memory for taste.
These findings provide further evidence that the amygdala, particularly the BLA, is part of a system that serves to modulate gustatory memory formation. The evidence that propranolol, infused into the BLA prior to the LiCl injections, dose-dependently disrupted the acquisition of CTA suggests that noradrenergic receptors in the BLA are involved in modulating the visceral US input during the consolidation of taste aversion memory. Glutamate release from the vagus nerve terminating in the nucleus of the solitary tract (NTS) appears to be one mechanism by which the vagus influences neural activity and memory in limbic structures. Bilateral infusions of glutamate administered into the BLA just before a mild US presentation enhance taste aversion (Miranda et al., 2002). Miyashita & Williams (2002) reported that microinfusions of glutamate administered into the NTS induced significant and long-lasting increase in amygdala NE concentrations and significantly enhanced inhibitory avoidance memory. It remains to be determined if this glutamatergic activity depends on NE activation into the BLA.
The present findings are consistent with those of other reports suggesting that the amygdala is engaged during the association of the gustatory and visceral stimuli in CTA acquisition as well as consolidation of the association (Buresova & Bures, 1973; Gallo et al., 1992; Roldan & Bures, 1994; Yamamoto et al., 1994; Tucci et al., 1998; Yasoshima et al., 2000). More specifically, our findings suggest that BLA noradrenergic activation is involved in enabling the formation of CTA. The finding that infusions of the β-adrenergic agonist clenbuterol did not significantly increase CTA (reduce saccharin consumption, compared to controls) may reflect a ‘floor’ effect in the consumption measure.
Previous studies, reporting that destruction of NE terminals within the amygdala induced by 6-hydroxydopamine block taste-potentiated odour aversion but not taste aversion, have suggested that catecholamines in the amygdala are more important for the acquisition of odour aversion (Fernandez-Ruiz et al., 1993). However, the compensatory mechanisms following the induction of permanent lesions could explain the apparent contradiction with the current results.
In this regard, a recent study (Kobayashi & Kobayashi, 2001) reported that mice heterozygous for the mutation of the gene encoding tyrosine hydroxylase, the initial and rate-limiting enzyme for the biosynthesis of catecholamines, exhibit reduced TH activity and a moderate reduction in NE accumulation and release in several brain regions. These mice also displayed long-term memory deficits in learning of active avoidance, cued fear conditioning, and conditioned taste aversion. These memory deficits were attenuated by injecting an NE reuptake inhibitor, desipramine, which stimulates noradrenergic activity. These provide additional evidence that the central noradrenergic system is important for long-term memory consolidation.
Despite the considerable evidence that the BLA modulates the consolidation of emotionally arousing or aversive memory (McGaugh, 2000), there is relatively little evidence concerning the role of the amygdala in the consolidation of memory for nonaversive experiences. Latent inhibition provides a method of investigating memory for nonaversive information. Re-exposure to a stimulus diminishes the ability of the same stimulus to serve as an effective cue in subsequent learning. Thus, latent inhibition combined with subsequent CTA is used as a convenient quantitative test for the incidental acquisition of a gustatory memory (Rosenblum et al., 1993; Naor & Dudai, 1996).
The current latent inhibition experiment clearly assesses incidental taste memory. It is unlikely that the latent inhibition observed can be attributed to the incidental memory of other stimuli (e.g. to the context), as the presentations of the gustatory stimulus occurred under the same environmental and context conditions on each exposure. Our results demonstrate that noradrenergic activity is necessary after CS preexposure for normal taste memory. It is known that propranolol acts for approximately 4 h, and thus, that its half-life is relatively short (Walle et al., 1988). As the propranolol infusions in the CTA experiment were administered 30 min after the saccharin presentation and immediately before the US presentation, it is also possible that the propranolol-induced retention impairment reported in the CTA experiments may have been due to impairment of the gustatory trace (CS) as well as to propranolol interference with the visceral stimulus (US).
It is unlikely that the effects obtained in these studies were due to state-dependency as the drugs were administered after the CS presentation. Consequently, our results suggest that the action of propranolol is limited to the processes involved just after the exposure to the taste; i.e. during the consolidation of the taste memory.
Previous studies have reported that lesions of the BLA reduce the effect of preexposure, that is, disrupt latent inhibition, in an appetitive conditioning experiment in which an auditory CS was paired with a food US (Coutureau et al., 2001) and that infusions of the NMDA receptor antagonist AP5 (d,l-2-amino-5-phosphonopentanoic acid) into the BLA before pre-exposure of rats to the neutral stimulus impair latent inhibition of fear-conditioning (Schauz & Koch, 2000). Such findings, taken together with the current results, suggest that BLA activity can influence memory for neutral, or relatively nonaversive, experiences. The present finding that propranolol infused into the BLA immediately after the pre-exposure to a new taste attenuated CTA clearly indicates that noradrenergic activity in the BLA participates in the consolidation of memory of the novel taste.
Extensive evidence indicates that the amygdala modulation of memory storage involves the release of NE within the amygdala (McGaugh et al., 1993). Post-training infusions of propranolol into the amygdala impair retention of inhibitory avoidance (Gallagher et al., 1977; Liang et al., 1995) and water-maze spatial learning (Hatfield & McGaugh, 1999. Activation of β-adrenergic mechanisms in the amygdala (in particular in the BLA) induce dose-dependent memory enhancement for training in those tasks (Liang et al., 1986, 1990; Introini-Collison et al., 1989, 1991; Ferry & McGaugh, 1999; Ferry et al., 1999; Hatfield & McGaugh, 1999). Additionally, recent evidence indicates that post-training intra-BLA infusions of NE enhance memory for contextual fear conditioning, a clearly Pavlovian task, like CTA, based on CS–US associations (LaLumiere et al., 2003). Combined with the current findings, these studies suggest that the BLA noradrenergic system plays a general memory-modulatory role in many kinds of tasks.
Phillips & LaPiane (1980) reported that electric stimulation of the amygdala at the onset of toxicosis disrupts CTA. Further, in vivo tetanic stimulation of the basolateral nucleus of amygdala induces LTP in the insular cortex of adult rats (Escobar et al., 1998; Jones et al., 1999). The induction of LTP in the BLA-insular cortex projection before CTA training enhances the retention of this task (Escobar & Bermúdez-Rattoni, 2000). These findings suggest that noradrenergic activation within the BLA may influence taste memory by modulating neuroplasticity in the BLA-insular cortex projection.
The present findings indicating that alterations in noradrenergic function in the BLA affect CTA and latent inhibition of a taste and provide additional evidence that the BLA plays a critical role in modulating the consolidation of memory and that the influence is mediated by interactions with other brain regions that support memory for different kinds of experiences (McGaugh, 2002).
Acknowledgements
Supported by NIH Grant NIMH12526 (JLM). We thank Linda Nguyen, Broc Mushet, Kelly Lei and Arthur Han for technical assistance and Nancy Collett for editorial assistance.
Abbreviations
-
- BLA
-
- basolateral amygdala
-
- CS
-
- conditioned stimulus
-
- CTA
-
- conditioned taste aversion
-
- NE
-
- norepinephrine
-
- NTS
-
- nucleus of the solitary tract
-
- US
-
- unconditioned stimulus,