Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer's disease)
Abstract
Previous group studies using positron emission tomography to assess resting cerebral glucose metabolism in very early Alzheimer's disease and mild cognitive impairment have identified the posterior cingulate and adjacent cingulo-parietal cortex as the first isocortical area to develop hypometabolism. We studied the profile of resting cerebral glucose metabolism in individuals with mild cognitive impairment to assess whether more specific and stereotyped regional hypometabolism would be evident across subjects. The study found that the most consistently hypometabolic region between individual subjects was a subregion of the posterior cingulate, the retrosplenial cortex (BA 29/30). This result is discussed in the context of regional connectivity, focal lesion evidence and functional activation studies of episodic memory paradigms in both normal and Alzheimer's disease groups. We propose that the retrosplenial cortex may represent a key junction between prefrontal areas involved in implementing retrieval strategies for episodic memory and hippocampal-based mnemonic processing; we therefore interpret the retrosplenial hypometabolism as a probable contributor to the memory impairment seen in mild cognitive impairment by disconnecting these two anatomical networks.
Introduction
18Fluorodeoxyglucose positron-emission tomography (FDG-PET) analysed by the voxel-based method has identified posterior cingulate and adjacent cingulo-parietal hypometabolism as the earliest cortical abnormality in early stage Alzheimer's disease (AD) (Minoshima et al., 1997). In a recent study (Nestor et al., 2003), this finding was extended to a group of subjects with mild cognitive impairment (MCI), the amnesic prodrome of AD in which patients have progressive memory symptoms, confirmed on formal neuropsychological tests, in the absence of the pervasive cognitive deficits indicative of dementia (Petersen et al., 2001). Using a method where regions of interest were defined upon coregistered volumetric MRI, posterior cingulate hypometabolism was found to be part of a network of abnormal structures that included the hippocampal formation, mammillary bodies and anterior thalamic nuclei (Nestor et al., 2003). Nevertheless, the most significant metabolic deficit was in the posterior cingulate region. As the area designated ‘posterior cingulate’ encompasses several architectonic fields [Brodmann areas (BA) 23, 31, 30 and 29] with varying neural connectivity, we were interested in whether a specific subregion of hypometabolism would be consistently identified across individuals with MCI. Given the emergent activation study literature concerning the role of the posterior cingulate cortex in episodic memory (Cabeza & Nyberg, 2000) this, in turn, could offer a complimentary insight into the neural network supporting human episodic memory.
Patients and methods
Ten patients with MCI (age 63.3 ± 5.9 years) were recruited with a mean Mini-Mental State Examination (MMSE) (Folstein et al., 1975) score of 28.4 ± 1.5. The MCI cases presented with memory symptoms of insidious onset and were defined, neuropsychologically, by the finding of impairment on a battery of tests of delayed recall [score ≥ 1.5 SD below mean for elderly controls on at least two of the following four tests: (i) Story recall (Wechsler, 1987), (ii) Rey-Osterreith complex figure (Osterreith, 1944), (iii) Doors and people visual version, and (iv) Doors and people verbal version (Baddeley et al., 1994)]. They did not, however, display significant impairment in attention, visuospatial, semantic or executive function (for details, see Nestor et al., 2003) and no alternate explanation for their amnesia was evident. Since scanning, the cases have remained under review (mean follow-up period 19.3 ± 7.6 months); in that time there has been a significant decline in mean MMSE to 26.0 ± 2.1 (paired t-test, P < 0.001). Two cases have converted to meeting National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria (McKhann et al., 1984) for probable AD (Cases I and IV). Cases were compared to an aged matched control group of 15 healthy subjects (MMSE 29.7 ± 0.5, age 60.8 ± 7.1). Written informed consent was obtained from each subject; the study had the approval of the Local Regional Ethics Committee and the Administration of Radioactive Substances Advisory Committee, UK, and was carried out in accordance with the Declaration of Helsinki.
Each subject underwent a volumetric, spoiled-gradient, 3T Bruker MRI scan for coregistration followed by fasting FDG-PET; scans were obtained 35–55 min after intravenous bolus of 74 MBq of FDG on a General Electric Advance scanner in 3D mode. Subjects' PET images were normalized to the metabolic rate for glucose of cerebellar vermis (to minimize effects of normal interindividual variability in cerebral metabolism) and then coregistered to MRI using Statistical Parametric Mapping (SPM99; Wellcome Department of Cognitive Neurology, London, UK). The coregistered images were then spatially normalized to the T1-MRI template of SPM99, using the MRI to define the normalization parameters, and smoothed with a 16-mm Gaussian filter. Each patient's scan was analysed individually in comparison to the control group at a statistical threshold of Puncorrected < 0.001. Scans showing no abnormality at that threshold were also analysed at Puncorrected < 0.01. The hypometabolic clusters for each subject were then projected back onto that individual's spatially normalized MRI scan (i.e. FDG-PET and MRI scans for each case were in the same standardized space). Anatomical localization of hypometabolic clusters was assessed both by comparing the stereotaxic co-ordinates of cluster peaks to the coplanar stereotaxic atlas (Talairach & Tournoux, 1988) and by visual comparison of the MRI-projected sections with corresponding slices from the Duvernoy (1999) brain atlas. In this article the term ‘posterior cingulate’ refers to BA 23/31/29/30 while ‘retrosplenial cortex’ refers to the subregion of BA 29/30.
Results
The results are summarized in Fig. 1 and Table 1. At Puncorrected < 0.001, eight of the 10 cases had posterior cingulate hypometabolism. Of these eight cases, the cluster peak in seven (Cases I–VII in Fig. 1 and Table 1) localized maximally to the retrosplenial cortex (BA 29/30). When the cluster for each individual was projected onto their normalized MRI scan and compared to the Duvernoy sections, these clusters lay subjacent to the splenium where the retrosplenial cortex narrows to become the isthmus of the gyrus fornicatus. In the remaining subject with posterior cingulate hypometabolism at Puncorrected < 0.001 (Case IX), the cluster peak localized to the dorsal posterior cingulate (BA 23, T = 4.75, x = 6, y = −28, z = 32). The two cases in whom the posterior cingulate was not identified at Puncorrected < 0.001 were re-analysed at Puncorrected < 0.01. At this threshold both (Case VIII and Case X) had small hypometabolic clusters in the retrosplenial region similar in location to that described above. Case IX, whose posterior cingulate peak localized to BA 23, was also re-analysed at Puncorrected < 0.01. At this statistical threshold a second peak also emerged in the retrosplenial region.
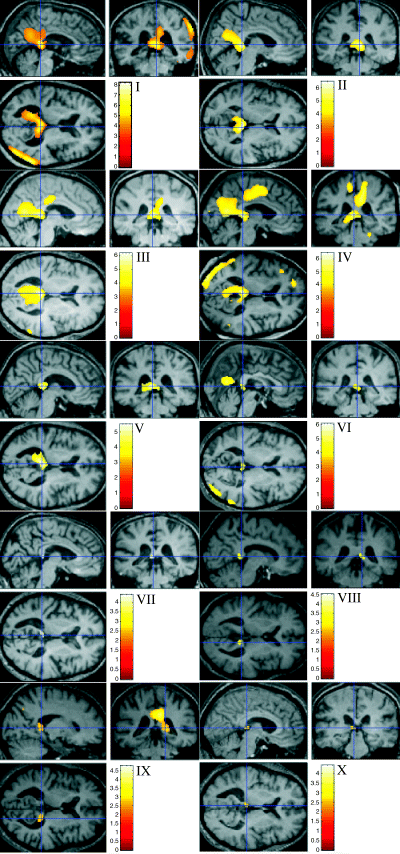
SPM analysis of hypometabolic clusters for each case projected onto their spatially normalized MRI. Cases I–VII are at Puncorrected < 0.001 and Cases VIII–X at Puncorrected < 0.01; in each the cross-hair is located on the retrosplenial peak.
| Case | Age (years) | MMSE score | T-value | Lateralization of retrosplenial cluster | Co-ordinates of retro- splenial cluster peak | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| I | 67.7 | 28 | 6.91 | Bilateral | 4 | −36 | 6 |
| II | 59.9 | 29 | 6.45 | Bilateral | 6 | −38 | 4 |
| III | 57.5 | 30 | 6.13 | Bilateral | −4 | −38 | 6 |
| IV | 64.3 | 26 | 5.99 | Bilateral | −4 | −36 | 6 |
| V | 65.9 | 28 | 5.51 | Bilateral | −4 | −36 | 6 |
| VI | 65.7 | 30 | 4.63 | Bilateral | 2 | −36 | 4 |
| VII | 61.9 | 29 | 4.36 | Left | −4 | −36 | 6 |
| VIII | 75.7 | 28 | 3.48 | Right | 10 | −40 | 4 |
| IX | 57.6 | 26 | 2.99 | Right | 18 | −38 | 6 |
| X | 56.7 | 30 | 2.81 | Left | −4 | −30 | 4 |
Cases I and IV had clear evidence of additional hypometabolic changes in lateral temporo–parietal association cortex; in Case I the statistical peak of these changes in the right inferior parietal lobule/angular gyrus (x = 50, y = −72, z = 24) was more significant than that seen in the retrosplenial region (T = 8.19 and T = 6.91, respectively). Cases V, VI and IX also showed some minor lateral association cortex changes which were much less significant than their posterior cingulate abnormalities and which were not topographically consistent between cases. No hypometabolic clusters were identified beyond the posterior cingulate region in the remaining five cases (Cases II, III, VII, VIII and X). In summary, retrosplenial hypometabolism was the most significant abnormality in eight cases and occurred in the absence of changes beyond the posterior cingulate region in five of these. The two cases whose most significant abnormality was elsewhere (Case I, right inferior parietal lobule/angular gyrus; Case IX, dorsal posterior cingulate, BA 23) also showed the retrosplenial defect.
Discussion
In this study, individual cases with MCI, the amnesic prodrome of AD, were found to have retrosplenial hypometabolism as a universal finding. The statistical effect at this location was not strong in three cases, in whom the threshold had to be lowered in order to identify the abnormality. However, with this procedure, retrosplenial hypometabolism in these three cases was seen in a similar location to that found in the rest of the group and without leading to the emergence of clusters elsewhere. We propose therefore that these changes are more likely to represent significant results than false positives. It is of interest that the two cases (I and IV) with most significant lateral temporo-parietal cortical hypometabolism were the first to progress to AD, consistent with a recent report that such changes are a predictor of early conversion (Chételat et al., 2003).
Since the seminal finding of Minoshima et al. (1994; 1997) of posterior cingulate hypometabolism in early AD, single photon emission computed tomography (SPECT) hypoperfusion in this region has been identified in MCI cases who subsequently converted to AD (Johnson et al., 1998; Kogure et al., 2000). Furthermore, serial MRI scans in presymptomatic carriers of autosomal dominant mutations for AD have identified an accelerated rate of posterior cingulate regional volume loss (Scahill et al., 2002). In these group studies, no more precise observation regarding lesion location within the posterior cingulate region was described. In the current analysis, however, a single highly localized area, the retrosplenial cortex, was identified in the absence of other anatomically consistent abnormalities. In interpreting the possible relevance of the retrosplenial cortex lesion, the findings of the present study will be discussed with respect to the anatomical connectivity of this region, the syndrome of retrosplenial amnesia and functional activation experiments of episodic memory in both healthy controls and AD patients.
A connection, via the cingulum bundle, from frontal to retrosplenial cortex and posterior parahippocampal area has long been established from work on rhesus monkeys (Goldman-Rakic et al., 1984). Recently, a more specific pathway was identified: the retrosplenial cortex (BA 29/30) sends afferent fibres to the dorso-lateral prefrontal cortex (BA 9, 9/46, 46) while this latter area plus medial BA 9 and 9/32 send fibres back to the retrosplenial cortex (Morris et al., 1999a; Petrides & Pandya, 1999). BA 30 is also a source of significant input to the entorhinal cortex (Insausti et al., 1987; Suzuki & Amaral, 1994). BA 29/30 therefore occupies a pivotal position between dorso-lateral prefrontal cortex and the mesial temporal lobe (Morris et al., 1999b). A key difference between the connectivity of the dorsal posterior cingulate (BA 23) and BA 30 is that the former connects more widely to the frontal cortex (dorso-lateral and orbital) and, compared to BA 30, does not connect strongly to the entorhinal area (Baleydier & Mauguiere, 1980; Morris et al., 1999b). This last observation accords with the entorhinal cortex being the earliest site of tau pathology in AD (Braak & Braak, 1991; Delacourte et al., 1999) and the results of the present study. In view of the proposed role of the prefrontal cortex in mnemonic processing, these connections are of considerable interest. The prefrontal cortex is not thought to store memories per se but to act in an executive capacity forming strategies for retrieval and monitoring output (Moscovitch, 1992), exemplified in lesion studies by poorer perfomance in free recall rather than cued or recognition tasks (Incisa della Rocchetta & Milner, 1993; Gershberg & Shimamura, 1995; Baldo et al., 2002).
The subjects presented here were previously shown to display hypometabolism in a network of structures implicated, from focal lesion studies, in human amnesia (Nestor et al., 2003). As such, the amnesic syndrome that defines MCI cannot be solely attributed to retrosplenial hypometabolism. However, focal lesion reports illustrate that retrosplenial damage alone can produce memory impairment (Kasahata et al., 1994; Valenstein et al., 1987; Takayama et al., 1991; Katai et al., 1992; Taniwaki et al., 1996; Takahashi et al., 1997; Sato et al., 1998; Katayama et al., 1999; Masuo et al., 1999; Maeshima et al., 2001; McDonald et al., 2001).
Numerous functional activation studies have implicated the posterior cingulate in mnemonic processing leading Cabeza & Nyberg (2000) to conclude, from a meta-analysis, that posterior cingulate activation was particularly associated with successful retrieval of episodic memory. One recent fMRI study is of particular interest to contrast to the current results as it also analysed the activation profile of individual subjects; in the test condition, subjects had to retrieve autobiographical episodes prompted by the names of personal acquaintances (Maddock et al., 2001). The only brain region universally activated in the test condition was the retrosplenial cortex with the next most common activation being the prefrontal cortex (BA 10/11).
Turning to AD, in a PET activation study of episodic memory, patients (but not controls) showed increased cerebral blood flow in a network which included dorso-lateral prefrontal cortex (BA 8/9/10) (Becker et al., 1996). A further PET study of cued recall found activation, both in controls and mild AD cases, in bilateral prefrontal cortex (BA 10/46 and 9/44). In addition, the AD group showed disproportionate activation of left prefrontal BA 46 (as well as posterior cingulate, BA23, and right middle temporal gyrus). In controls, left hippocampal activation was seen which was not present in the AD group (Backman et al., 1999). These two studies used different memory tasks and certainly further work is required to understand the activation profiles of AD; nevertheless, both tasks disproportionately engaged the dorsolateral prefrontal cortex. The finding that BA 23 was activated in the latter study supports the hypothesis that this area is hypometabolic in MCI through relative de-afferentation from the limbic network rather than local degeneration.
In summary, the current study offers evidence that the earliest area of cortical hypometabolism in MCI, the amnesic prodrome of AD, is the retrosplenial cortex. Case reports of focal lesions to this region are also associated with amnesia. Functional imaging studies of episodic memory have consistently shown activation in the posterior cingulate region in general and, in certain circumstances, the retrosplenial cortex in particular, usually in association with prefrontal activation. Speculating on its specific role, it is proposed that the retrosplenial cortex may form a critical link between frontal and mesial temporal structures with retrosplenial amnesics therefore having a disconnection syndrome. This would suggest that increased activation of prefrontal regions when patients with AD perform memory tasks is due to a relatively preserved prefrontal cortex being maximally recruited in an attempt to gain access to the failing limbic network. In support of this idea, hypermetabolism has been described in the ipsilateral frontal lobe of a retrosplenial amnesic (Heilman et al., 1990). This hypothesis also offers an explanation for the neuropsychological findings of the retrosplenial stroke case of McDonald et al. (2001) who was amnesic but additionally showed impairment of frontal strategic processes, such as poor release from proactive inhibition and impaired category clustering on word list recall, in spite of normal performance on frontal executive tasks not related to memory. Further imaging and neuropsychological studies in healthy volunteers, mild AD cases and retrosplenial amnesics would clearly be of interest in testing this hypothesis as well as further exploring whether subregions within the posterior cingulate (e.g. BA 29/30 vs. BA 23) and prefrontal cortex subserve different functions in mnemonic processing.
Abbreviations
-
- AD
-
- Alzheimer's disease
-
- BA
-
- Brodmann areas
-
- FDG-PET
-
- 18fluorodeoxyglucose positron-emission tomography
-
- MCI
-
- mild cognitive impairment
-
- MMSE
-
- Mini-Mental State Examination
-
- MRI
-
- magnetic resonance imaging.