Restricted daily consumption of a highly palatable food (chocolate Ensure®) alters striatal enkephalin gene expression
Abstract
Brain opioid peptide systems are known to play an important role in motivation, emotion, attachment behaviour, the response to stress and pain, and the control of food intake. Opioid peptides within the ventral striatum are thought to play a key role in the latter function, regulating the affective response to highly palatable, energy-dense foods such as those containing fat and sugar. It has been shown previously that stimulation of mu opiate receptors within the ventral striatum increases intake of palatable food. In the present study, we examined enkephalin peptide gene expression within the striatum in rats that had been given restricted daily access to an energy-dense, palatable liquid food, chocolate Ensure®. Rats maintained on an ad libitum diet of rat chow and water were given 3-h access to Ensure® daily for two weeks. One day following the end of this period, preproenkephalin gene expression was measured with quantitative in situ hybridization. Compared with control animals, rats that had been exposed to Ensure® had significantly reduced enkephalin gene expression in several striatal regions including the ventral striatum (nucleus accumbens), a finding that was confirmed in a different group with Northern blot analysis. Rats fed this regimen of Ensure® did not differ in weight from controls. In contrast to chronic Ensure®, acute ingestion of Ensure® did not appear to affect enkephalin peptide gene expression. These results suggest that repeated consumption of a highly rewarding, energy-dense food induces neuroadaptations in cognitive-motivational circuits.
Introduction
In recent years there has been increasing attention directed at understanding the neural control of food intake. Because of the current obesity epidemic in Western countries and its profound effect on health, lifestyle, and longevity, there is currently an urgent need to understand further the neural pathways involved in the control of body weight, and the motivation states underlying ingestive behaviour. Even in very recent years, obesity rates continue to grow at alarming rates in many developed countries (Hill et al., 2003). Recent research has provided some remarkable advances in understanding the complex and intricate control of eating and satiety, particularly at the level of regions within the hypothalamus, such as the arcuate nucleus, paraventricular nucleus, dorsal and ventromedial nuclei, and lateral hypothalamus. For example there is now fairly good understanding of the roles of neuropeptides such as neuropeptide Y, proopiomelanocortin (POMC), Agouti-related protein (AGRP) in these regions as well as their regulation by such peripheral signalling factors as leptin, grehlin, and insulin (Sawchenko, 1998; Woods et al., 1998; Baskin et al., 1999; Elmquist et al., 1999). Overall, energy homeostasis is achieved through the brain's ability to sense both short-term and long-term changes in energy stores and availability.
While theories of internal energy homeostasis and regulation of metabolism are an important piece of the puzzle of ingestive behaviour, short-term motivational states drive the immediate desire to eat and to continue eating. Indeed, the affective state associated with food intake, depending on the palatability of the food and the state of deprivation, can be associated with powerful positive states of reward or pleasure. In this regard, ventral striatal regions, particularly the nucleus accumbens, may play a prominent role in certain aspects of ingestive behaviour, particularly in sensory processes, learning, anticipatory, and incentive mechanisms. In particular, opioid peptides and opiate receptors are believed to mediate the affective response to highly palatable food such as fat and sugar (Cooper & Kirkham, 1993; Cole et al., 1995; Giraudo et al., 1999; Kelley et al., 2002), and the nucleus accumbens, which contains dense levels of opioids (Pickel et al., 1980; Haber & Lu, 1995), is one region where this may occur (Majeed et al., 1986; Bodnar et al., 1995; Pecina & Berridge, 2000; Kelley et al., 2002). For example, direct mu opioid stimulation of the nucleus accumbens results in a selective enhancement of fat intake vs. carbohydrate (Zhang et al., 1998), and also preferentially increases sucrose, saccharin, and salt intake over water (Zhang & Kelley, 2002). Although there is clear evidence for opioid modulation of palatable food intake, there is little direct evidence for alterations in striatal opioid-containing neurons in association with feeding behaviour. We sought to address this question by examining striatal enkephalin gene expression in rats that consumed a highly palatable liquid food, chocolate Ensure®, during a 3-h session daily for two weeks. We report here a down-regulation in expression of the gene encoding enkephalin peptides, preproenkephalin (PPE), in a broad expanse of striatum.
Materials and methods
Animals and housing
A total of 49 male Sprague–Dawley adult rats (Charles River Laboratories) weighing approximately 300 g at the time of purchase were used throughout these experiments. Rats were housed in pairs in the animal colony in clear polycarbonate cages with ad libitum access to food (Purina rat chow) and water. The animals were maintained on a normal 12-h day-night cycle with lights on at 07:00 h. The rats were handled daily to minimize stress.
Experimental procedure
Experiment 1
Starting approximately one week after arrival in the laboratory, the rats (n = 12) were given daily 3-h access to a highly palatable liquid, chocolate Ensure® (Ross Products Division, Abbott Laboratories, Columbus, Ohio, USA) otherwise being maintained ad libitum on rat chow and water the remainder of the day. For 16 consecutive days, the rats were brought into test cages in a different environment from the animal cages (a different room and hanging wire test cages). Attached to these cages were bottles of either Ensure® or water. Half the rats were given access to water (n = 6) and half to Ensure® (n = 6). Intake was measured daily following the 3-h session. Testing was always conducted between 14:00 and 17:00 h. Twenty-four hours following the last exposure, the rats were killed and their brains processed for in situ hybridization (see below). In a second group of rats (n = 13; seven water and six Ensure®), the exact same procedure was followed and on the day of killing the brains were removed and dissected for Northern blot analysis. In these rats, the nucleus accumbens was punch-dissected with a 3-mm dissection tool (without differentiating core and shell) and bilaterally pooled; other regions were also taken for future analysis. Samples were quickly frozen and stored for analysis (see below).
Experiment 2
In order to investigate the effects of acute exposure to the highly palatable liquid, rather than chronic daily exposure, and to measure weights over the course of chronic exposure, an additional group of rats (total n = 24) was given either Ensure® (n = 12) or water (n = 12) for 16 days in a manner similar to that described above. All rats were weighed every day. On days 14 and 15, the water group was given 20-min access to Ensure® in order to adapt the rats to the novel taste. On the day of killing (day 17), the chronic Ensure® rats were euthanized and their brains were not used. The former water group (now the ‘acute Ensure®’ group, n = 12) was allowed access to 30 min of water (n = 6) or Ensure® (n = 6). Thus, all the rats in the experimental group had some experience with the Ensure®, but on the test day half the group was exposed to Ensure® and half to the water. These rats were anaesthetised, and perfusion-fixed (see below) 2.5 h following the 30-min test.
Preproenkephalin in situ hybridization
Tissue preparation
Home cage pairs were processed at the same time. Rats were deeply anaesthetized with sodium pentobarbital (50 mg/kg) and transcardially perfused with 300 mL of 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) in 0.1 m phosphate buffer, pH 7.4. Brains were removed and equilibrated in 10% and 20% sucrose in 0.1 m phosphate buffer, pH 7.4. Fifty-micron sections were cut on a cryostat into 12 compartments and stored at −20 °C in a cryoprotectant solution consisting of 30% sucrose and 30% ethylene glycol.
A 48-mer oligonucleotide probe was synthesized (Genosys Biotechnologies, Inc., The Woodlands, TX, USA) complementary to the mRNA encoded by bases 388–435 of the rat preproenkephalin cDNA, and was labelled with 35S dATP (1250 Ci/mmol; New England Nuclear Research Products, Boston, MA, USA) with terminal deoxynucleotidyl transferase (TdT; Boehringer Mannheim Biochemica, Indianapolis, IN, USA).
Sections were processed for in situ hybridization free-floating in gently agitating solutions. Probe was diluted to 0.1 × 107 c.p.m./mL in buffer [4× SSC (0.15 m sodium chloride, 0.015 m sodium citrate) 50% formamide, 10× Denhardt's, 100 µg/mL denatured salmon sperm DNA, 100 µg/mL polyadenylic acid, 50 mm dithiothreitol, and 10% dextran sulphate] and sections were hybridized overnight at 37 °C. After hybridization, sequential rinses in 2× SSC, 1× SSC, and 0.5× SSC were carried out at 25 °C. A final, 2-h rinse in 0.5× SSC was carried out at 54 °C. Sections were mounted on gel-subbed slides, dried, and exposed to Hyperfilm B-max film (Amersham Corp., Arlington Heights, IL, USA) for a 2-day exposure. The sense probe was substituted for the antisense probe to confirm specificity of hybridization, and showed no hybridization signal.
Autoradiographic films were scanned into the computer, and analysed using Scion Image software. Mean optical densities for preproenkephalin mRNA hybridization were determined for seven striatal regions (see Fig. 1), at two rostro-caudal levels. Pseudocolor images were generated using Scion Image and Adobe Photoshop.
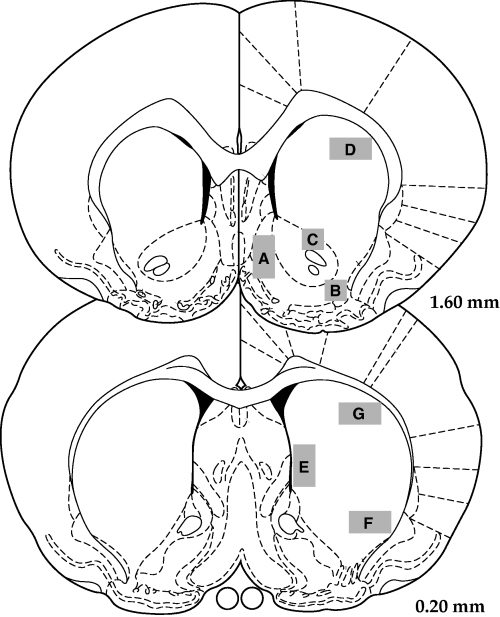
Analysed areas of the rostral and caudal regions (based on drawings from Paxinos & Watson, 1998). A, medial accumbens; B, lateral accumbens shell; C, accumbens core; D, anterior dorsal striatum; E, medial striatum; F, ventrolateral striatum; G, dorsolateral striatum.
Northern blot analysis
RNA was isolated using the Clontech NucleoSpin RNA kit II (Clontech, Palo Alto, CA, USA) using the manufactures suggested protocol. Briefly, tissue punches from brain were homogenized in GITC solution using a tissue tearror™ (Biospec Products, Inc., Bartlesville, OK, USA). Nucleic acids where ethanol precipitated, resuspended in GITC, and then bound to a NucleoSpin™ column (Clontech, Palo Alto, CA, USA). GITC was removed by centrifugation and samples were DNase treated on the column. DNase treatment was halted with a GITC wash. RNA was then washed twice with 100% ethanol and eluted from the column with RNAse-free water. The concentration of RNA was determined using the RiboGreen (Molecular Probes, Eugene, OR, USA) method and RNA quality was assessed using agarose electrophoresis.
One microgram of total RNA from each rat was electrophoresed on a 1.2% agarose/formaldehye/1XMOPS gel. The gels were transferred to GeneScreen™ plus (Perkin Elmer Life Sciences, Inc., Boston, MA, USA) using Stratagene's PosiBlotter™ (Stratagene, Inc., La Jolla, CA, USA). 32P labelled cDNA (2 × 106 c.p.m./mL) was hybridized to the membrane in Hybisol™ I (Intergen, Inc., Norcross, GA, USA). Northern blots were washed and exposed to a phosphorimager screen for 1 day. Screens were scanned on a Storm™ phosphorimager (Molecular Dynamics Inc., Sunnyvale, CA, USA). Quantification was performed using ImageQuant™ software (Molecular Dynamics Inc., Sunnyvale, CA, USA).
Probes used for Northern blotting were generated from rat nucleus accumbens cDNA using PCR amplification. The following primers were used to generate PCR product for PPE: forward primer 5′-ACCTTGTCAGAGACAGAACGG-3′ (274–294), reverse primer 5′-CCTCGTGGTCTGGATAACTGC-3′ (1248–1268). Numbers in parentheses after the primer sequence represent the base number as defined by the gene sequence in the Unigene database.
Data analysis
The in situ hybridization results (optical density readings for striatal sites) were analysed using two-factor analysis of variance (anova), with treatment (Ensure® vs. water) as the between subjects factor and site as the within subjects factor. Body weights in Experiment 2 were analysed by anova, with treatment as the between- and day as the within-subjects factor.
Results
Experiment 1
As is shown in Fig. 2, rats quickly learned to like the taste of Ensure® and avidly consumed it, with their intake steadily increasing in the first several days and then leveling off to a steady rate. Water intake was negligible. The results of the in situ hybridization autoradiography of PPE expression for Experiment 1 are shown in 3, 4. It can be observed from the quantitative analysis of the optical density readings that prior consumption of Ensure® resulted in a clear and significant reduction of PPE mRNA expression in most striatal regions analysed. Overall anova indicated a significant effect of treatment (F1,10 = 10.06, P < 0.01). There was also a strong effect of region (F6,60 = 41.1, P < 0.0001) but no treatment-region interaction. This profile indicates that there exists normal variation in PPE expression throughout striatum, and that intake of Ensure® is associated with a relatively uniform down-regulation of PPE expression throughout the striatum. However, it can be noted that this decreased expression was relatively more apparent in certain regions, such as the lateral shell of accumbens compared with the medial shell. For example, further one-way anova on the medial shell data alone revealed no significant difference (P < 0.14), whereas for the lateral shell the analysis revealed a difference at P < 0.01. Fig. 4 shows a pseudocolor image of PPE mRNA expression in a representative water-exposed and Ensure®-exposed rat, showing the reduced levels of PPE mRNA in the striatum of the Ensure®-exposed animal.
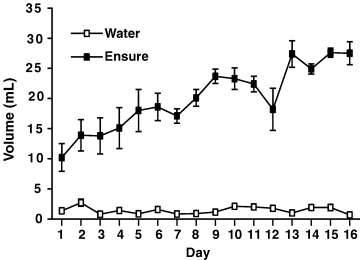
Average daily intake during each 3-h session for water and Ensure® subjects for the16 day treatment period.
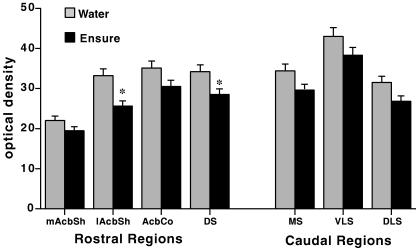
Striatal PPE mRNA following chronic Ensure® or water. Values represent means ± SEM (six rats per group) and are expressed as optical density (OD, mean pixel density) for the target regions depicted in Materials and methods. Abbreviations: mAcbSh, medial accumbens shell; lAcbSh, lateral accumbens shell; AcbCo, accumbens core; DS, anterior dorsal striatum; MS, medial striatum; VLS, ventral lateral striatum; DLS, dorsal lateral striatum. Results of repeated measures anova: P < 0.01. *P < 0.05, posthoc one-way anova.
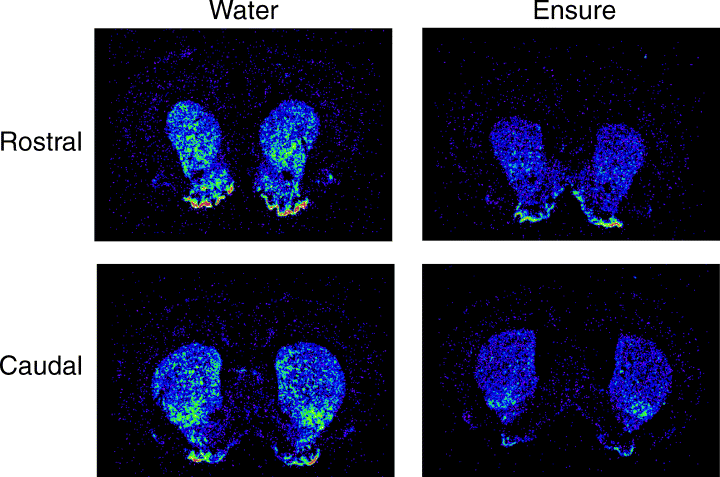
A pseudocolor image of PPE mRNA expression in a representative pair of subjects who received 3 h access to chronic water (left) and Ensure® (right) for16 days.
For the confirmation with Northern blot analysis, there was a clear down-regulation of PPE levels within the nucleus accumbens in animals that had received two weeks exposure to daily three-hour Ensure®, compared with water controls. Figure 5 shows the lanes of Ensure®-treated and water-treated rats as well as a graphical representation of the mean optical densities in each group.
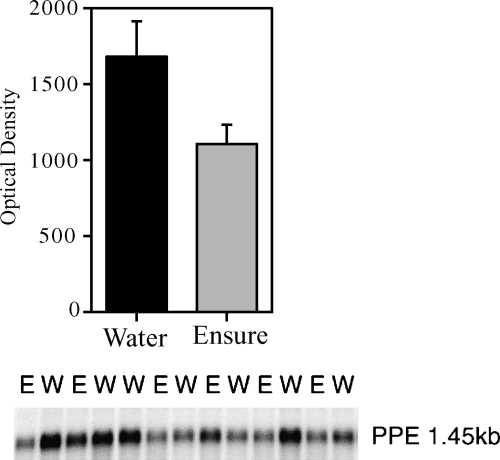
Preproenkephalin mRNA expression from a tissue punch of the nucleus accumbens shell and core regions following 16 days of 3 h access periods to either Ensure® or water.
Experiment 2
In the experiment that aimed to test the effects of acute intake of palatable liquid on PPE expression in striatum, no up-regulation or down-regulation was observed upon analysis of optical density levels for all striatal regions, which are shown in Table 1. (F1,10 = 0.383, P > 0.05). During the acute test, the mean intake of Ensure® was 15.1 ± 0.1.5 mL and the mean intake of water was 1.0 ± 0.14 mL. It was also found that rats given restricted, 3-h access to the Ensure® did not differ in body weight gain compared with their water-exposed counterparts (F1,22 = 1.2, P = 0.286, n.s.) (Fig. 6).
| mAcbSh | 1AcbSh | AcbCo | ADS | MS | VLS | DLS | |
|---|---|---|---|---|---|---|---|
| Water | 25.3 ± 3.1 | 37.4 ± 2.5 | 43.2 ± 3.4 | 29.3 ± 2.9 | 36.9 ± 3.6 | 43.1 ± 2.1 | 30.0 ± 2.6 |
| Ensure® | 23.3 ± 0.9 | 38.0 ± 3.3 | 38.3 ± 2.6 | 30.4 ± 3.0 | 31.7 ± 3.2 | 43.2 ± 4.3 | 26.7 ± 2.2 |
- mAcbSh, medial accumbens shell; 1AcbSh, lateral accumbens chell; AcbCo, accumbens core; ADS, anterior dorsal striatum; MS, medial striatum; VLS, ventral lateral striatum; DLS, dorsal lateral striatum.
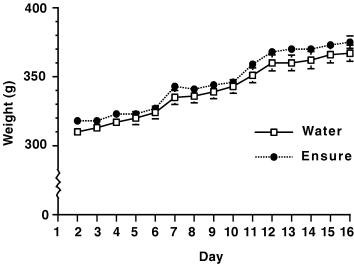
Weight change for both water and Ensure® treated subjects during the 16 day treatment period.
Discussion
The present results show that allowing rats to consume a highly palatable food, chocolate liquid Ensure®, for only a limited 3 h time period each day for 2 weeks induces a down-regulation of preproenkephalin gene expression in the ventral striatum and other striatal subregions. This effect is demonstrated with both the in situ hybridization approach as well as Northern blot analysis of the ventral striatum. In contrast to daily chronic intake of Ensure®, robust acute ingestion did not appear to alter striatal enkephalin gene expression, at least at the time point examined following ingestion. To our knowledge this is the first demonstration of altered gene expression in the striatum associated with food intake. The profile of findings, in conjunction with previous work demonstrating a clear role of striatal opioids in palatable food intake, suggests that consumption of calorie-dense food per se is enough to modify and regulate enkephalin gene expression, perhaps resulting in long-term motivational changes as a result of chronic intake of the highly rewarding food.
It is important to consider what factors or combination of factors are responsible for the gene expression alteration. Excessive weight gain is ruled out as a causative factor, as in the experiment where weight was measured, rats given daily exposure to Ensure® did not significantly gain weight above controls. Thus it is unlikely that a long-term adiposity signal is responsible for the down-regulated PPE, such as changes in leptin signalling (Levin & Dunn-Meynell, 2002b). Of course, if the rats had unlimited access to the chocolate liquid, there would likely have been weight gain. For example, rats given free access to a high calorie diet indeed gain more weight than those given access to rat chow only (Cole et al., 1995; Levin & Dunn-Meynell, 2002a). There are a number of nutrients in the Ensure®, including fats, protein, carbohydrates, sugars, vitamins and chocolate flavor. Any of these factors are candidates for inducing the effect, and further experiments will have to be carried out to determine the role of various components. However, our current hypothesis is that it is both the chronic aspect and the high palatability (high sugar, fat or both) that is driving the gene expression change. Evidence supportive of this notion is provided by the report that repeated excessive sugar intake is associated with increased mu opiate receptor binding in several brain regions (including accumbens shell) (Colantuoni et al., 2001). These researchers also found that either treatment with naloxone or withdrawal from the ‘binge’ sugar diet results in opiate withdrawal-like symptoms, suggesting a dysregulation of endogenous opiate systems at some level (Colantuoni et al., 2002). Thus, it is possible that long-term sugar consumption, the taste of sugar, or the neural changes induced by the affective response to highly palatable food triggers homeostatic alterations in endogenous opioids.
Further evidence for clear changes in the endogenous opioid system induced by food is found in the work of Kanarek and colleagues, who have found that chronic consumption of a palatable diet alters responsiveness to opiate drugs in a number of ways. For example, chronic exposure (approximately three weeks) of rats to a diet with freely available sucrose or fat results in enhanced morphine-induced analgesia (D'Anci et al., 1996; Kanarek et al., 1997a; Kanarek et al., 1997b). Several aspects of these studies suggest that the long-term alteration is due to caloric intake rather than taste per se; for example, a chronic diet including freely available saccharin fails to alter morphine sensitivity (D'Anci et al., 1997; Kanarek & Homoleski, 2000). Although these studies do not provide direct evidence for a change in gene expression within the endogenous opioid system, they strongly suggest that the consumption induces plasticity and cellular alterations at some level of the system. It is noteworthy that four-week access to a diet consisting of chow and 32% sucrose results in increased whole brain opiate receptor binding (Marks-Kaufman et al., 1989).
In the current study, acute intake of Ensure® did not appear to alter striatal PPE gene expression, which is somewhat surprising given the hypothesis that consumption of palatable food is associated with increased enkephalinergic activity. This negative effect should be interpreted cautiously, however. Originally we hypothesized that PPE gene expression would be increased in response to acute drinking of the palatable liquid; this was not confirmed. However, only one time point was measured here (2 h post consumption); it is possible that relatively small changes occur, or that they occur before or after this time point. What is clear from other experiments in the literature is that acute intake of palatable food is strongly reduced by treatment with naloxone or naltrexone, including naltrexone injections into accumbens (Bodnar et al., 1995; Kelley et al., 1996; Echo et al., 2001) and enhanced by treatments that stimulate opiate receptor function (Drewnowski et al., 1992; Cooper & Kirkham, 1993). Although to our knowledge direct measurement of enkephalin release within the nucleus accumbens during food intake has not been accomplished (due to considerable difficulties conducting microdialysis studies of peptides), evidence from several laboratories strongly implicates ventral striatal opioids and mu opiate receptors in modulation of palatable food intake (Zhang & Kelley, 1997; Pecina & Berridge, 2000; Ragnauth et al., 2000; Kelley et al., 2002). These studies assume a model in which striatal enkephalins are released from axon collaterals arising from medium spiny neurons (Pickel et al., 1980). However, other mechanisms that may not produce marked changes in PPE mRNA levels could occur; for example it is possible that activation of endorphins contributes to the neural response to palatable food intake. In support of this latter notion, several studies show increased release of endogenous beta-endorphin in response to acute sucrose and saccharin consumption (Dum et al., 1983; Yamamoto et al., 2000), and it has long been known that beta-endorphin infusion causes feeding (Morley et al., 1983), including when infused into the nucleus accumbens (Majeed et al., 1986). Moreover, mice lacking beta-endorphin or enkephalin show motivational deficits in instrumental responding for sweet food (Hayward et al., 2002). There is a little-cited endorphin-containing pathway originating from arcuate nucleus and projecting to nucleus accumbens (Finley et al., 1981; Sim & Joseph, 1991), which may play a role in the response to stress (Zangen & Shalev, 2003). Thus, either enkephalins or endorphins could be a principal initiator of long-term changes, and further experiments will be needed to clarify this.
It is interesting to consider the regional distribution of PPE mRNA decreases across the striatum. Although down-regulation was found to occur throughout widespread striatal regions, the effects were greatest in relatively rostral regions and particularly apparent in the lateral accumbens shell. This is an area with dense levels of PPE, enkephalin, and mu and delta opiate receptors (Pickel et al., 1980; Fallon & Leslie, 1986; Mansour et al., 1987; Haber & Lu, 1995). Also, it is the primary striatal site reached by afferents from gustatory cortex (Beckstead, 1979; McGeorge & Faull, 1989), and also receives dense input from the basolateral amygdala (Kelley et al., 1982; McDonald, 1991). Moreover, in our hands it is this site, along with the lateral accumbens core, that is most sensitive to feeding induced by low doses of DAMGO (Zhang & Kelley, 2000). Thus, perhaps relatively more ventral and rostral areas of striatum are affected preferentially by palatable food intake. However, it is important to note that rather widespread down-regulation was found throughout the striatum; this change was definitely not confined to ventral striatal regions. This profile suggests the possibility that opioid systems in many brain regions are affected by high-calorie food intake. In support of this idea, rats fed a chronic Ensure® diet showed a significant down-regulation of dynorphin mRNA in the ventromedial hypothalamic nucleus (Levin & Dunn-Meynell, 2002a), although increased levels of prodynorphin mRNA arcuate nucleus have also been reported following palatable food intake (Welch et al., 1996). Kanarek and colleagues have recently found that chronic sucrose intake alters opiate sensitivity, in a pain model, in the periaqueductal grey region (Kanarek et al., 2001). Thus, it appears that chronic intake of highly palatable food can affect opioid signalling in a variety of brain regions, beyond the obvious ones clearly implicated in food intake. In the present study, we did not analyse other regions but it would be of interest to do so in the future.
It is relevant to consider other treatments that are able to alter striatal enkephalin gene expression, in order to postulate possible underlying mechanisms. One injection (10 mg/kg) of morphine strongly decreases PPE expression in the nucleus accumbens, an effect that lasts at least 24 h (Yukhananov & Handa, 1997). Moreover, chronic treatment with morphine is found to result in a very similar down-regulation of striatal enkephalin gene expression (Uhl et al., 1988; Georges et al., 1999). In the latter study, the authors found that rats treated for morphine pellets for at least a week showed substantially less expression of PPE mRNA in caudate and accumbens. This parallel is particularly significant as it suggests common underlying mechanisms that link plasticity induced by natural rewards and to that induced by addictive drugs. It may be that repeated over stimulation of opiate receptors, whether with an exogenous drug or by palatable food intake, elicits long-lasting changes in receptor function or transduction mechanisms that subsequently result in compensatory down-regulation of enkephalin transcription. It is interesting to note that another addictive drug, ethanol, which increases accumbens PPE expression when given acutely (Li et al., 1998), decreases its expression when given to rats chronically (Cowen & Lawrence, 2001), again paralleling the profile shown here. Lewis rats, which more readily self-administer opiates and ethanol, have a decreased basal level of enkephalin gene expression (Martin et al., 1999).
It is well established that striatal enkephalin gene expression is highly regulated by dopaminergic tone (e.g. (Honore et al., 1988; Jaber et al., 1992; Caboche et al., 1993; Asselin et al., 1994; Campbell & Walker, 2002; Perier et al., 2002). Thus, although not directly studied here, it is useful to consider whether enkephalin gene expression changes induced by palatable food may be indirectly caused by dopaminergic influences. Consumption of highly palatable food and exposure to food-related cues has been shown to cause increased dopamine release in several forebrain areas, including ventral striatum and prefrontal cortex (e.g. Hoebel et al., 1992; Martel & Fantino, 1996; Bassareo & Di Chiara, 1997; Tanda & Di Chiara, 1998; Bassareo & Di Chiara, 1999; Datla et al., 2002). Constant exposure to a highly rewarding situation in which the animal is allowed to consume the palatable food could result in over activation of the dopamine system and consequent down-regulation of the enkephalin production in medium spiny neurons. Although we suspect that opioid-induced feeding is not directly dependent on DA release, as opioid-induced feeding is not blocked by DA antagonists (Kelley et al., 2000), it is likely that the concomitantly associated increased DA release modulates the general motivational state of the animal. Whether long-term striatal changes are induced directly by endogenous opioid activation, dopaminergic stimulation, or perhaps other mechanisms remains to be determined.
The data described here suggest that palatable food consumption, in animals that are not food-deprived and that do not show any noticeable changes in weight, is able to induce significant plastic changes in striatal enkephalin gene expression, changes which in some ways resemble those observed when this system is exposed to chronic drugs such as morphine and alcohol. While more clear understanding of the mechanisms leading to this phenomenon awaits further investigation, these results suggest that repeated intake of highly rewarding food induces neuroadaptive changes in corticostriatal networks controlling cognition and emotion.
Acknowledgements
The authors would like to acknowledge the support of grant DA09311 from the National Institute on Drug Abuse. M.J Will was supported by a National Research Service Award (F32 DA14751).
Abbreviations
-
- PPE
-
- preproenkephalin.