Ectopic activity in cutaneous regenerating afferent nerve fibers following nerve lesion in the rat
Abstract
Spontaneous activity, and mechanical and thermal sensitivity were investigated in regenerating afferent nerve fibers within 4–21 days post sural nerve lesion (crush or transection and resuturing) in anaesthetized rats. About 33–40% of the myelinated (A) and 22–27% of the unmyelinated (C) fibers excited by electrical nerve stimulation exhibited at least one of these ectopic discharge properties. In total 177 A- and 169 C-fibers with ectopic activity were analysed. Most A-fibers (161/177) were mechanosensitive. Spontaneous activity (median 1 imp/s) was present in 23/177 and thermosensitivity in 14/177 A-fibers (13 of them being activated by heat stimuli). Almost all A-fibers (159/177) exhibited only one type of ectopic discharge property. Most C-fibers (94/169) were thermosensitive responding either to cold (n = 45) or to heat stimuli (n = 33) or to both (n = 16). Eighty-four of 169 C-fibers were spontaneously active (median 0.3 imp/s) and 75/169 C-fibers were mechanosensitive. Both the proportion and the discharge rate of spontaneously active C-fibers were significantly higher after crush than after section and resuturing of the nerve. About 60% of the C-fibers (101/169) had only one ectopic discharge property and 40% two or three. In conclusion, regenerating cutaneous afferent A- and C-fibers may develop mechano- and/or thermosensitivity as well as spontaneous activity. We suggest that spontaneous and evoked ectopic activity in regenerating cutaneous afferents are a function of the intrinsic functional properties of these neurons and of the interaction between the regenerating nerve fibers and non-neural cells during Wallerian degeneration in the nerve distal to the nerve lesion.
Introduction
Following nerve lesion some axotomized afferent neurons develop ectopic spontaneous and evoked activity that may originate at the lesion site (see Jänig, 1988; Devor & Seltzer, 1999), in the cell bodies of muscle afferent neurons in the dorsal root ganglion (Michaelis et al., 2000) and possibly also proximal to the lesion site in the nerve (although this has never been systematically tested). The ectopic activity originating at the lesion site, e.g. after a nerve has been ligated and sectioned, consists of spontaneous activity as well as mechano-, thermo- and chemosensitivity (Michaelis et al., 1995, 1997, 1998; Blenk et al., 1996; Rivera et al., 2000). The type and pattern of ectopic activity depends on the time after nerve lesion, probably on the type of lesioned nerve (e.g. a skin or muscle nerve), on the type of nerve lesion (e.g. crush, transsection with or without microsurgical repair or nerve ligation and transection), and finally on the type of lesioned afferent fiber (e.g. myelinated or unmyelinated afferent fiber) (Blumberg & Jänig, 1984; Jänig, 1988; Johnson & Munson, 1991; Proske et al., 1995; Devor & Seltzer, 1999; Yates et al., 2000).
In the rat, even at 6–30 h after sural nerve lesion about 18% of the axotomized unmyelinated and about 30% of the myelinated nerve fibers develop ectopic activity (Blenk et al., 1996; Michaelis et al., 1995, 1998, 1999). The unmyelinated units became responsive to mechanical, cold or heat stimuli and could develop spontaneous activity. Post lesion large-diameter myelinated muscle and skin afferents may develop their original response characteristics and respond phasically or tonically to static mechanical stimuli applied to their novel axon endings. Few of them have spontaneous activity (Blumberg & Jänig, 1984; Johnson & Munson, 1991; Koschorke et al., 1991; Johnson et al., 1995; Michaelis et al., 1995; Proske et al., 1995; Bongenhielm & Robinson, 1998).
Most experimental investigations on ectopic activity in axotomized primary afferent neurons have been performed after nerve ligation and sectioning, preventing the axotomized nerve fibers from regenerating to their original target tissue. No systematic experimental investigations have been performed on the ectopic firing properties of lesioned afferent nerve fibers during the process of regeneration in a nerve. These data could be important because neuropathic pain develops particularly after partial nerve lesions or in diabetic neuropathy, which enable lesioned nerve fibers to regenerate. The ectopic activity of regenerating nerve fibers may differ from that in sectioned nerve fibers ending in a neuroma. Analysis of the pattern of this ectopic activity and its underlying mechanisms is important to understand the peripheral mechanism of neuropathic pain (Jänig, 1988; Devor & Seltzer, 1999).
In the present study we systematically investigated the ectopic firing properties of lesioned and regenerating afferent nerve fibers (both myelinated and unmyelinated) within 4–21 days post sural nerve crush or section and resuture in the rat. The ectopic spontaneous and the evoked activity (by mechanical, cold and heat stimulation) were quantitatively investigated.
Materials and methods
Nerve injury
Sural nerve lesion was performed on 40 male Wistar rats (body weight 250–370 g) at 4–21 days before the neurophysiological experiments. The surgery was done under general anaesthesia induced by pentobarbital sodium (Narkoren, 60 mg/kg injected, i.p.). Under aseptic conditions the left sural nerve was exposed 10–25 mm proximal to the ankle. In 18 rats the sural nerve was crushed with fine watchmaker's forceps; in 16 rats the sural nerve was sectioned and resutured. For the crush lesion the nerve was squeezed three times at the same location over 1–1.5 mm, leading to transparency of the nerve at the lesion site. For the second type of lesion the nerve was cut with the help of fine scissors and the peripheral stump was re-anastomosed to the proximal nerve end using two epineural fine sutures (Ethicon, Prolene, Germany). Fine nerve surgery was performed under a stereomicroscope; the lesion site was located 15–20 mm proximal to the ankle. The wound was closed in layers and recovery was uneventful. In six control experiments we tested whether nerve fibers can be activated by mechanical stimulation of the perineurium of the acutely sectioned sural nerve.
Anaesthesia and animal maintenance
On the day of the final experiment the rats were re-anaesthetized with pentobarbital sodium (Narkoren, 60 mg/kg i.p.), catheters were inserted into the tail artery for blood pressure recording (transducer LM-22, List, Darmstadt, Germany) and into the jugular vein for administration of fluid and drugs. Additional doses of pentobarbital were given regularly (10–20 mg/kg/h, i.v.) to maintain a sufficient level of anaesthesia as judged from the absence of spontaneous blood pressure fluctuations. Mean arterial blood pressure during the experiments was always above 70 mmHg.
The trachea was cannulated and the animals breathed spontaneously during the initial surgery. After finishing the initial surgical preparations the animals were paralysed with Pancuronium (Organon, initially 1 mg/kg, i.v., maintenance with 0.4 mg/kg) and artificially ventilated at 75 strokes per minute using O2-enriched room air. Blood gases were measured regularly throughout the experiment (ABL30, Radiometer Copenhagen) and pO2 always exceeded 70 mmHg. Rectal temperature was monitored and kept close to 37.0 °C by means of a servo-controlled heating blanket.
At the end of the experiments, the animals were killed under deep anaesthesia by an intravenous injection of a saturated potassium chloride solution. All experiments had been approved by the local animal care committee of the state administration and were conducted in accordance with German Federal Law.
Surgical procedure during neurophysiological experiments
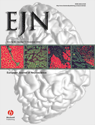
The left sural nerve was exposed from the ankle to its junction with the sciatic nerve. Seven to 5 mm distal to the lesion site the sural nerve was isolated from the surrounding tissue and mounted on a pair of platinum electrodes for electrical stimulation. In 13 experiments the sural nerve was isolated from surrounding tissue also proximal (5–7 mm) to the lesion site and put on a second pair of stimulating electrodes (Fig. 1A). At its junction with the sciatic nerve, the sural nerve was cut, isolated from connective tissue and placed on a rigidly fixed small black perspex platform. The exposure was covered with warm (30 °C) paraffin oil in a pool made from the skin flaps.
Nerve preparation and identification of nerve fibers by electrical stimulation. (A) Schematic presentation of the sural nerve preparation. Fiber activity was recorded in fine filaments teased from the sural nerve rostral to the lesion site and placed on a platinum recording electrode; the reference electrode was positioned on the nearby tissue. The nerve was placed on two pairs of electrodes proximal (p) and distal (d) to the lesion site for electrical stimulation of the nerve fibers. Natural (mechanical and thermal) stimuli were applied to the sural nerve dissected from surrounding tissue at the area of sprouting of fibers, both proximal and distal to the lesion site in order to excite the nerves. (B) Electrical identification of C-fibers. In this filament three C-fibers (marked 1, 2, 3) could be activated electrically both from proximal and distal stimulation electrodes. Electrical stimulation via the distal pair of electrodes excited the fibers at latencies d1, d2 and d3 (upper left record). Simultaneous electrical stimulation of the nerve via the proximal pair of electrodes with 5.7, 8.6 and 8.9 V excited the fibers at latencies p1, p2 and p3 and the action potentials collided with those elicited from the distal pair of stimulation electrodes (lower left and right records).
Recording and electrical stimulation
The proximal cut end of the sural nerve was split with sharpened watchmaker's forceps under a stereomicroscope. The fine filaments were teased out and put on electrodes for recording. Activity of single fibers was recorded unipolarly using a pair of platinum wire electrodes with the indifferent electrode connected to nearby tissue. The signals were differentially amplified (input resistance 10 MΩ), filtered with a bandwidth of 120 Hz to 1.0–1.2 kHz (unmyelinated fibers) or with a bandwidth of 120 Hz to 40 kHz (myelinated fibers), and fed through window discriminators. Spike discrimination was controlled by means of a delay circuit: the action potentials were delayed by 5 ms and displayed on a storage oscilloscope using the output of the window discriminator as a trigger.
The sural nerve was electrically stimulated with square wave pulses of 0.1 ms (A-fibers) or 0.5 ms (C-fibers) duration at a repetition rate of 3 Hz and 0.3 Hz, respectively, with variable intensities up to 40 V. Conduction velocity was calculated from the latency of the responses to electrical stimulation and the distance between recording and stimulation sites (18.1 ± 0.6 mm from the proximal pair of electrodes; 27.3 ± 0.7 mm from the distal pair of electrodes, Fig. 1A). No systematic discrimination of A-fibers into Aβ and Aδ was attempted because: (1) the distances between recording and stimulation sites were short and (2) lesioned afferent fibers shrink leading to a reduction in their conduction velocity (Michaelis et al., 1996, 2000). The afferent fibers were considered to be A- or C-fibers when their conduction velocities were > 2 m/s and ≤2 m/s, respectively. Some 10% of the fibers that could not be electrically identified were offline classified as A- or C-fibers according to the shape and height of their action potentials. This procedure allows us to classify about 95% of the nerve fibers into A- or C-fibers (see Michaelis et al., 1994).
Experimental procedure
Electrophysiological experiments were conducted 4–21 days after sural nerve lesion. We analysed only those filaments that contained at least one A- or C-fiber that exhibited spontaneous activity or/and mechanical or/and thermosensitivity. We first tested whether a filament isolated from the sural nerve contained fibers with at least one of these types of ectopic activity. If a filament contained a spontaneously active fiber, this discharge was recorded first for 3–5 min in order to determine its rate and pattern. Then a series of mechanical and thermal stimuli was applied along the lesioned sural nerve from 10 mm proximal up to 18 mm distal to the lesion site in order to determine the number of ectopically active fibers within the filament tested and to localize the receptive fields of the mechano- and/or thermosensitive afferents distributed along the nerve. The quantitative responses of the fibers to mechanical and thermal stimuli were analysed offline.
Natural stimulation
Mechanical stimulation
Mechanical stimuli were applied manually to the nerve with a fine-tipped blunt glass rod or with von Frey filaments under visual control using a stereomicroscope. The stimuli were applied either repetitively at 1 Hz (phasic stimulation) or continuously over about 10 s (tonic stimulation). To identify the size and location of receptive field(s) for individual afferent fibers, the mechanical responsiveness was tested in 1-mm increments starting 10 mm proximal to the lesion site up to the most distal point available on the exposed sural nerve (13–18 mm distal from the lesion site) using the fine-tipped glass rod. The receptive spots were located and carefully documented. Then the threshold of mechanical activation was measured in subsets of afferent nerve fibers. For this purpose a set of calibrated von Frey fiberglass filaments with circular plain tips of 0.5 mm diameter and forces of 0.45–100 mN (MARSTOCKnervtest, Fruhstorfer, Marburg, Germany) was used. First, stimuli of 7.5 mN were applied. In case of activation (at least three impulses in 10 s) the next weaker filament was used; when there was no response after three 10-s stimuli the next stronger filament was used. The strength of the finest filament that either evoked at least three action potentials in silent fibers or increased the activity by 100% in spontaneously active fibers was defined as the threshold strength.
Thermal stimulation
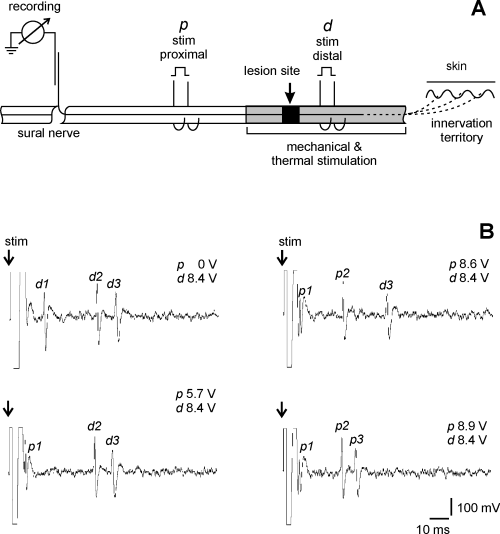
Thermal stimuli of 3.5–50 °C were applied by a water-perfused thermode (Fig. 2, see legend for characteristics of the thermode). The surface of the thermode tube contacting the nerve was 2 mm long. As a result of thermal conduction the stimulus covered a distance of 4 mm along the nerve. To test the thermosensitivity the thermode was therefore placed at five sites of the nerve: the lesion site, 4–6 mm proximal to the lesion site as well as 4–6 mm, 9–11 mm and 14–16 mm distal to the lesion site. In this way the area from 7 mm proximal to 17 mm distal to the lesion site was covered. For identification of cold-sensitive fibers the thermode was initially perfused with ice water; the lowest temperature reached was in the range 3.5–5 °C. If a fiber was activated before the minimal temperature was reached, a series of testing stimuli at higher temperatures (10–19 °C) was applied. For initial testing of heat-sensitive fibers, stimuli of 47–50 °C were applied (duration, 3–5 s; longer stimuli were avoided to prevent nerve damage). If a fiber was activated before peak temperature was reached, a series of testing stimuli at lower temperatures (30–42 °C) was applied. These temperature stimuli were maintained for approximately 15 s and started from a baseline temperature of about 22–24 °C. A fiber was regarded as thermosensitive if more than three action potentials were generated during increase or decrease of the temperature in silent fibers and fibers with rates of spontaneous activity below 0.5 imp/s or if the activity increased by ≥ 100% in fibers with rates of spontaneous activity > 0.5 imp/s.
Device used for thermal stimulation of the nerve. The thermode consisted of a U-shaped metal tube in which water at different temperatures was perfused. At its convexity (the contact area with the nerve) the temperature isolation of the U-tube was interrupted at 1 mm × 2 mm to allow optimal thermal conduction to the nerve. A small thermoelement about 1 mm in diameter (KTY 21–7, Siemens, Germany; time constant 1.5 s) was placed in the concavity of the U-tube, opposite from the nerve, in order to measure continuously the temperature on the surface of the U-tube.
Data analysis
Neural activity, temperature of the stimulation thermode and arterial blood pressure were simultaneously fed into a computer (IBM-compatible) with ADC and counter interface (Burr-Brown PCI-20000, data acquisition software CARDS by S. Tiedemann) and stored on a digital tape recorder (DTR-2602, Biologic, Claix, France) for further off-line analysis (custom data acquisition software and template-matching program; Forster & Handwerker, 1990). Data are expressed as means ± SEM. For statistical analysis the chi-squared test or the non-parametric Wilcoxon signed-rank test were used.
Results
Overall description
Spontaneous activity and responses to mechanical, cold and heat stimuli of axotomized afferent nerve fibers in the sural nerve were investigated 4–21 days after crush (n = 18 rats) or after section and resuturing of the nerve (n = 16 rats). The focus of this study was to analyse the ectopic discharges generated in afferent nerve fibers that were in the process of regeneration but had not yet reached the target tissue. In total 177 A-fibers and 169 C-fibers with at least one type of ectopic discharge property were studied (Table 1). Within the second half of the time period chosen (mostly in the third week post lesion) 29 afferent fibers (24 A- and five C-fibers) had already re-innervated either the peripheral targets in the original sural nerve territory or could be excited from the sural nerve branches below the skin by mechanical stimulation. Four of these fibers (three A- and one C-fiber) had mechanosensitive receptive fields both in the nerve and in the peripheral target tissue. Most of these fibers (23/29 fibers) were found in rats with crush lesion. They are not included in this study and will be presented together with other data in a forthcoming paper that describes regenerating afferent nerve fibers >30 days post nerve lesion, many of them having regenerated to their target tissue.
| A-fibers | C-fibers | |||
|---|---|---|---|---|
| Crush | Section and resuturing | Crush | Section and resuturing | |
| Total number of fibers with ectopic properties | 77 | 100 | 79 | 90 |
| Individual ectopic firing properties | ||||
| Spontaneous activity | 20 (26%)a | 3 (3%)a | 51 (65%)b | 33 (37%)b |
| Mechanosensitivity | 65 (84%) | 96 (96%) | 26 (33%)c | 49 (54%)c |
| Thermosensitivity | 6 (8%) | 8 (8%) | 49 (62%) | 45 (50%) |
| Cold activated | 0 | 1 | 29d | 16d |
| Heat activated | 6 | 7 | 12 | 21 |
| Cold and heat activated | 0 | 0 | 8 | 8 |
| Combination of ectopic firing properties | ||||
| Mechanosensitive/spontaneously active | 54*/6 | 92*/0 | 5*/7f | 28*/3f |
| Cold sensitive/spontaneously active | 0/0 | 0/1 | 9*/12e,f | 6*/6e,f |
| Heat sensitive/spontaneously active | 0/1 | 2/1 | 7/0e | 10*/1e |
| Cold and heat sensitive/spontaneously active | 0/0 | 0/0 | 1/6 | 3/1 |
| Mechanical and cold sensitive/spontaneously active | 0/0 | 0/0 | 2/6e | 1/3e |
| Mechanical and heat sensitive/spontaneously active | 3/2 | 3/1 | 4/1e | 5/5e |
| Mechanical, cold and heat sensitive/spontaneously active | 0/0 | 0/0 | 0/1 | 4/0 |
| Spontaneous activity only | 11* (14%) | 0 | 18* (23%) | 14* (16%) |
- * Afferent fibers with only one ectopic discharge property. The letters a, b, c and d indicate the ectopic firing properties that are significantly different (χ 2-test, P < 0.05) between the two types of nerve lesion (crush vs. section and resuturing). aSpontaneous activity in A-fibers higher after crush lesion (P < 0.01). bSpontaneous activity in C-fibers higher after crush lesion (P < 0.005). cMechanosensitivity in C-fibers higher after section and resuturing lesion (P < 0.005). dCold sensitivity in C-fibers higher after crush lesion (P < 0.01). ePercentage of spontaneously active cold-sensitive C-fibers higher than for heat-sensitive C-fibers (27/45 vs. 7/33; P < 0.005). fPercentage of spontaneously active cold-sensitive C-fibers higher than for mechanosensitive C-fibers (18/33 vs. 10/43; P < 0.01).
The numbers of afferent A- and C-fibers with at least one ectopic discharge property were almost equally distributed over the three time periods: first, second and third weeks post lesion in both experimental groups (Table 2). The response characteristics of the afferent nerve fibers throughout these three time periods were the same. Furthermore, the incidence of every ectopic discharge characteristic was, with one exception, not statistically significantly different among the three time periods (P > 0.05, χ2 test). Only the incidence of C-fibers with thermosensitivity was significantly lower in the first week than in the third week post lesion in rats with sectioned and resutured sural nerve. Therefore, the data obtained in these three weeks were pooled together (Table 1). Differences in results between both types of nerve lesion are described below.
| Crush | Section and resuturing | |||||
|---|---|---|---|---|---|---|
| 1st week | 2nd week | 3rd week | 1st week | 2nd week | 3rd week | |
| A-fibers | ||||||
| Total (n) | 38 | 27 | 7 | 46 | 33 | 26 |
| Spontaneously active | 11 (29%) | 8 (30%) | 1 (14%) | 1 (2%) | 1 (3%) | 1 (4%) |
| Mechanosensitive | 35 (92%) | 19 (70%) | 6 (86%) | 44 (96%) | 31 (94%) | 26 (100%) |
| Thermosensitive | 3 (8%) | 3 (11%) | 0 (0%) | 4 (9%) | 3 (9%) | 1 (4%) |
| C-fibers | ||||||
| Total (n) | 25 | 20 | 34 | 25 | 34 | 31 |
| Spontaneously active | 18 (72%) | 15 (75%) | 18 (53%) | 13 (52%) | 9 (26%) | 11 (32%) |
| Mechanosensitive | 9 (36%) | 7 (35%) | 10 (29%) | 9 (36%) | 19 (56%) | 21 (68%) |
| Thermosensitive | 16 (64%) | 11 (55%) | 22 (65%) | 8 (32%) | 17 (50%) | 21 (68%) |
- With one exception, the incidence of ectopic discharge properties of myelinated (A-) fibers and unmyelinated (C-) fibers did not differ significantly over the 3 weeks of investigation after both types of injury (P > 0.05, χ2-test). Following section and resuturing the proportion of thermosensitive C-fibers was significantly lower in the first week than the third week post lesion.
Percentage of nerve fibers exhibiting ectopic activity after nerve lesion
In 20 experiments we investigated the percentages of ectopically active A- or C-fibers among the total number of electrically activated fibers 5–21 days after crush or after section and resuturing of the sural nerve. For this purpose we analysed only those filaments in which the number of electrically activated A- or C-fibers could be determined (Fig. 1). These filaments contained maximally seven A-fibers (mean three) or maximally ten C-fibers (mean five). Strands in which the nerve fibers could not be identified by electrical stimulation were discarded. All electrically excited fibers were tested for their spontaneous activity and whether they could be excited by mechanical and/or thermal stimuli applied to the nerve. In these experiments we did not test whether individual nerve fibers could be excited from both the proximal and the distal pair of stimulaton electrodes or only from the proximal pair of stimulation electrodes.
Overall, 33–40% (mean 36%) of the A-fibers and 21–27% (mean 23%) of the C-fibers could be excited by mechanical or thermal stimulation and/or exhibited spontaneous activity. No significant difference in the percentages of ectopically active A- or C-fibers was found between the two types of nerve lesion (crush vs. section and resuturing) and within the same group of nerve lesion between the populations of fibers activated from the distal vs. proximal pair of stimulation electrodes (Table 3).
| A-fibers | C-fibers | |||
|---|---|---|---|---|
| Proximal | Distal | Proximal | Distal | |
| Crush | 31/92 (34%) | 24/73 (33%) | 27/125 (22%) | 29/106 (27%) |
| Section and resuturing | 63/157 (40%) | 45/122 (37%) | 51/244 (21%) | 51/222 (23%) |
A-fibers
Most A-fibers (157/177, 89%) investigated for all properties were either mechanosensitive (n = 146) or spontaneously active (n = 11). Six fibers were both spontaneously active and mechanosensitive, six both heat-sensitive and mechanosensitive, and three fibers had all three ectopic discharge properties. Four A-fibers were only activated by heat stimuli, two of them being spontaneously active. One spontaneously active A-fiber was activated by cooling. Spontaneous activity in A-fibers was not depressed by cooling or heating.
Spontaneous activity
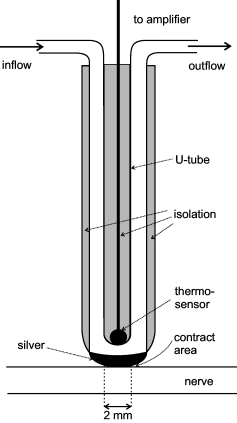
Twenty-three of 177 ectopically active A-fibers investigated were spontaneously active. Twenty of these spontaneously active A-fibers belonged to the crush group (P < 0.001, χ2-test), most of them in the first 2 weeks after nerve lesion. The remaining three spontaneously active fibers were observed after section and resuturing of the nerve. The rate of spontaneous activity measured at a temperature of the nerve of about 22–24 °C ranged from 0.03 to 10.6 imp/s (median 1 imp/s; mean 2.4 imp/s). The pattern of spontaneous activity was either irregular (14/23 fibers) or contained bursts (9/23 fibers) with intraburst frequencies being in the range 7.5–22.5 Hz (Fig. 3A and B). In 11 A-fibers the spontaneous activity was the only ectopic discharge property. In the remaining A-fibers the spontaneous activity was combined with mechano- or thermosensitivity (see Table 1).
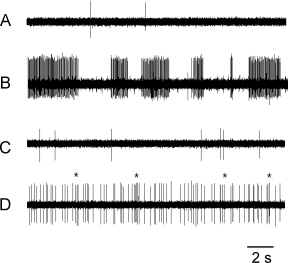
Original records of spontaneous activity in myelinated (A and B) and unmyelinated (C and D) nerve fibers. (A) Two myelinated nerve fibers (0.01, 0.05 imp/s). (B) Bursting activity in a myelinated nerve fiber (10.6 imp/s). The example presents an extreme case of spontaneous activity at a very high rate. (C) Unmyelinated nerve fiber (0.4 imp/s). (D) Unmyelinated nerve fiber exhibiting some bursting discharges, as indicated by asterisks (3.2 imp/s).
Mechanosensitivity
Mechanosensitivity was observed in 161/177 A-fibers. The responses to mechanical probing of the nerve with a fine-tipped blunt glass rod or with von Frey filaments were phasic (Fig. 4A and B); tonic responses to constant force stimuli were absent. The mechanical thresholds ranged from 0.45 to 45 mN (Fig. 4D, mean 5.9 ± 0.9 mN, n = 81). Most mechanical thresholds were in the non-noxious range; only a few fibers had thresholds ≥ 20 mN being probably in the noxious range (Fig. 4D). The maximal discharge rates during trains of phasic mechanical stimulation at 1 Hz intratrain frequency were in the range 42.5 ± 6.2 imp/s (n = 11). The receptive fields of the mechanosensitive A-fibers extended from 6 mm proximal to the lesion site to 15 mm distal to the lesion site depending on the time after nerve lesion and type of nerve injury (Fig. 5A). Following section and resuturing of the sural nerve, 38% of the mechanosensitive A-fibers could be activated from the lesion site. The other fibers could be activated from sites proximal (14%) or distal (48%) to the nerve lesion, the most distal point on the nerve being 9 mm. Following crush lesion only 6% of the mechanosensitive A-fibers were activated from the lesion site. Most other A-fibers were activated from sites distal to the nerve lesion (88%), the most distal point on the nerve being 15 mm. The size of the receptive fields of mechanosensitive A-fibers varied between 1 and 15 mm along the nerve (inset in Fig. 5A). The fields were frequently discontinuous and consisted of punctuate low threshold spots along the nerve. This is demonstrated for one A-fiber in Fig. 6 (large action potential) in which the mechanosensitivity was present 1 mm and 4–6 mm but not 2–3 mm distal to the nerve lesion.
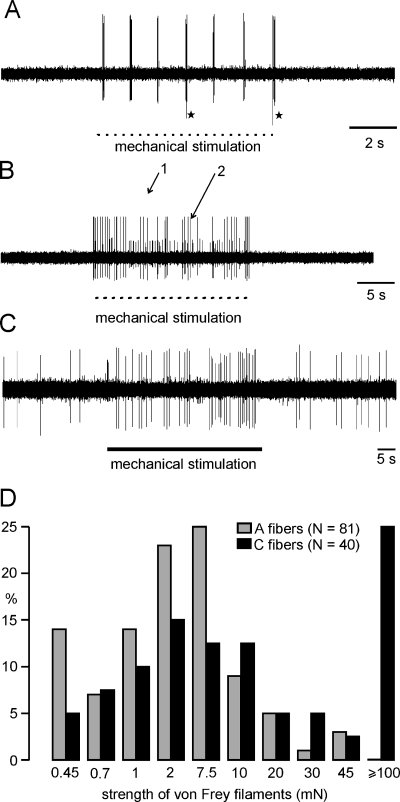
Ectopic mechanical sensitivity of regenerating nerve fibers. (A) Two silent A-fibers responding only at the beginning of the stimuli applied at about 1 Hz. Fibers marked with an asterisk rarely responded. The other fiber exhibited up to 100 Hz intraresponse frequency (6 days after nerve section and resuture). (B) Response of two silent A-fibers to repetitive mechanical stimuli (1 Hz) for 20 s (4 days after nerve crush). (C) Tonic response of a spontaneously active C-fiber to a mechanical constant force stimulus (7 days after nerve section resuture). (D) Distribution of mechanical thresholds of A- and C-fibers belonging to both groups of lesion. Mechanical thresholds were measured with calibrated von Frey filaments of different bending forces. C-fibers with thresholds > 100 mN were only excited by stimuli applied with a fine tipped glas rod.
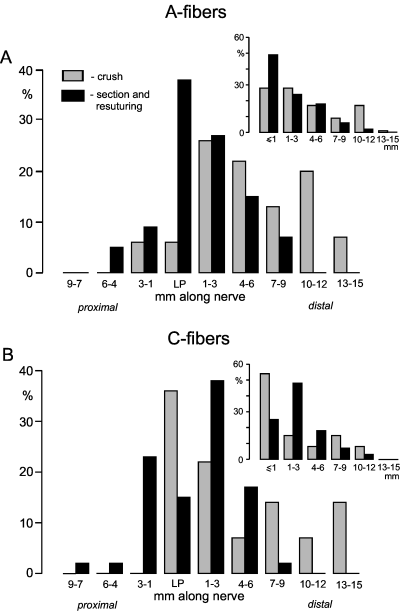
Distributions and size of receptive fields for mechanosensitive fibers under regeneration. Mechanical stimuli were applied at different sites along the nerve proximally and distally to the lesion site using a fine tipped glass rod. The main histograms exhibit the distribution of the most distal/proximal point of the receptive fields. The insets show the distribution of the receptive field size in millimetres. The smallest spatial resolution was 0.5 mm, and therefore fibers that could be excited only from the lesion point had a receptive field size of < 0.5 mm. (A) A-fibers. Location of the receptive fields (crush n = 53; section and resuturing n = 87). (B) C-fibers. Location of receptive fields (crush n = 14; section and resuturing n = 47). Size of receptive field (crush n = 13; section and resuturing n = 44).
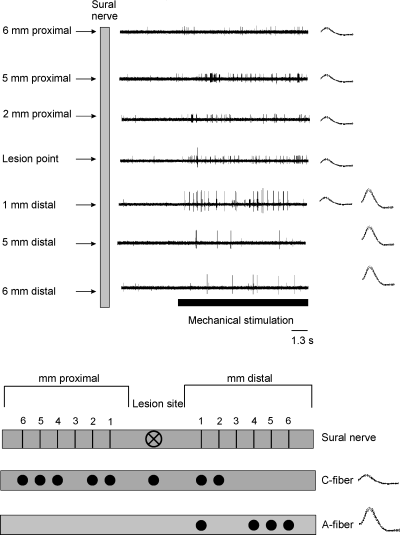
(A) Spatial distribution along the nerve of mechanosensitivity of one silent A-fiber and one spontaneously active C-fiber recorded in the same bundle. Repetitive mechanical stimuli were applied with a fine-tipped glass rod to the nerve from 6 mm proximal to 15 mm distal to the lesion site. (B) Schematic representation of the receptive field distribution of the same fibers. The A-fiber (large action potential) could be activated from 1 mm and 4 to 6 mm but not from 2 to 3 mm distal to the lesion point. The mechanosensitive C-fiber (small action potential) had receptive fields from 6 mm proximal up to 2 mm distal to the lesion site. Records on the left are superimposed action potentials (15 days after nerve section and resuture).
Thermosensitivity
Fourteen (8%) of the A-fibers in both groups of experimental nerve lesion were thermosensitive. Most of them (13/14 fibers) were activated by heat stimuli and had activation thresholds varying between 37 and 42 °C. One fiber was activated by cooling. The responses to heat stimuli were either phasic or phasic–tonic (Fig. 7A).
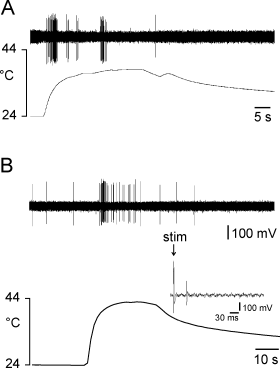
Responses of two regenerating afferent nerve fibers to heating of the nerve. (A) Silent myelinated fiber exhibiting some bursting activity. (B) Unmyelinated spontaneously active nerve fiber exhibiting a phasic–tonic response. Inset: identification of the C-fiber by electrical stimulation of the sural nerve.
C-fibers
The pattern of ectopic discharges in afferent C-fibers was significantly different from the pattern in A-fibers as far as the distribution of types of ectopic activity is concerned (see lower part of Table 1). Ninety-seven of 169 fibers (39/79 fibers [49%] in the crush group; 58/90 fibers [64%] in the section and resuturing group) were either spontaneously active or thermosensitive (responsive to cooling or heating) or mechanosensitive. The remaining responsive C-fibers had either two (45/169 fibers, 27%), three (26/169 fibers, 15%) or all four (one fiber) of these ectopic firing properties (Table 1).
Spontaneous activity
Eighty-four of 169 C-fibers (50%) were spontaneously active at a temperature on the nerve surface of about 22–24 °C (Fig. 3C and D). The proportion of spontaneously active C-fibers was significantly higher after crush lesion than after section and resuturing (51/79 vs. 33/90 fibers, P < 0.005; Table 1). The rate of spontaneous activity ranged from 0.01 to about 8 imp/s (median 0.3 imp/s, mean 0.86 ± 0.20, n = 84; Fig. 8). It was significantly higher in C-fibers after crush lesion than in C-fibers after nerve section and resuturing (1.23 ± 0.30 vs. 0.36 ± 0.06 imp/s, P < 0.01; Fig. 8). The rate of spontaneous activity was not significantly changed by the thermode placed on the nerve (see below). The spontaneous activity was irregular in most C-fibers (Fig. 3D, insets in Fig. 10). Some C-fibers showed discrete bursts in their spontaneous activity, occurring irregularly (as shown in 3, 10). In 32/169 of the spontaneously active C-fibers ongoing activity was the only ectopic property; in the remaining spontaneously active C-fibers it was combined with ectopic mechano- and/or thermosensitivity.
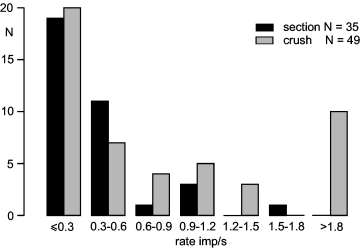
Distribution of rates of spontaneous activity in lesioned unmyelinated nerve fibers after crush (grey columns) or section and resuturing (black columns) of the nerve. The temperature of the nerve during the recording was in the range 22–24 °C.
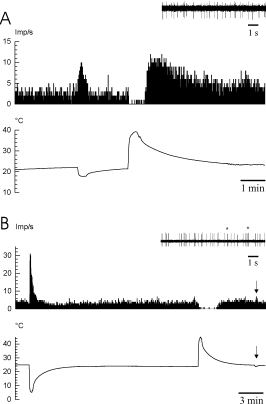
Responses of two spontaneously active C-fibers to cooling or to heating of the nerve. (A) The fiber is activated during cooling, depressed in its activity during heating and exhibited rebound activation after heating. Inset: activity at a temperature of about 22 °C (7 days after nerve crush). (B) The fiber is activated during cooling and depressed during heating without rebound activation after heating. This fiber exhibited a high cold sensitivity (see arrow) and some bursting discharges (see asterisks in inset) (8 days after nerve crush).
The distribution of spontaneous activity of C-fibers exhibited some distinct characteristics (see lower part of Table 1). The proportion of spontaneously active units among cold-sensitive C-fibers was significantly higher than among heat-sensitive C-fibers (27/45 vs. 7/33 fibers, P < 0.005) and significant higher than in mechanosensitive C-fibers (18/33 vs. 10/43 fibers, P < 0.01).
Mechanosensitivity
Mechanosensitivity was observed in 75/169 C-fibers. The proportion of mechanosensitive C-fibers was significantly higher after section and resuturing of the sural nerve than after crush lesion (49/90 vs. 26/79 fibers, P < 0.005; Table 1). The mechanical thresholds to excite the C-fibers varied from 0.45 to 100 mN (18.2 ± 5.2 mN, n = 33). The distribution of the mechanical thresholds of the C-fibers was bimodal, 27/40 C-fibers tested having thresholds of ≤ 20 mN and the remaining C-fibers having mechanical thresholds of ≥ 30 mN (Fig. 4D). No difference was found in mechanical threshold and response profile to mechanical stimulation between both types of nerve lesion. Compared with the A-fibers the mechanical thresholds of the C-fibers were significantly higher (P < 0.001). Low-threshold mechanosensitive C-fibers exhibited preferentially phasic responses and high-threshold ones preferentially tonic responses (Fig. 4C).
The receptive fields of the mechanosensitive C-fibers extended from 9 mm proximal to the lesion site to 15 mm distal to the lesion site depending on the time after nerve lesion and type of nerve injury (Fig. 5B). About 40% of the mechanosensitive C-fibers could only be activated from the lesion site or from sites several millimetres proximal to the lesion site. Most of the remaining mechanosensitive C-fibers could be activated from sites up to 9 mm distal to the nerve lesion (section and resuture) or up to 15 mm distal to the nerve lesion (crush, Fig. 5B). The size of the receptive fields of the mechanosensitive C-fibers varied between 1 and 12 mm along the nerve (inset in Fig. 5B). This is demonstrated for one C-fiber in Fig. 6 (small action potential) in which the mechanosensitivity was present from 6 mm proximal to 2 mm distal to the lesion site.
Thermosensitivity
Thermosensitivity was observed in 94/169 (56%) C-fibers. Forty-five of these thermosensitive C-fibers were activated by cooling, 33 fibers by heating and 16 by both cooling and heating. The proportion of C-fibers that were activated by cooling was higher in nerves with crush lesion than in nerves that were sectioned and resutured (29/79 vs. 16/90 nerve fibers, P < 0.01; Table 1).
Most cold-sensitive C-fibers (42/45, 93%) were activated by cooling steps of < 10 °C starting from a baseline temperature of 22–24 °C, some fibers being very sensitive to small temperature changes (see arrow in Fig. 10B). Seventy-one per cent of these cold-sensitive C-fibers (32/45) responded tonically (Fig. 9). The responses of the remaining cold-sensitive C-fibers were phasic, i.e. these fibers were only activated during lowering of temperature. The peak discharge rates during maximal cooling ranged from 2 to 47 imp/s (8.1 ± 1.5 imp/s, n = 39). Some cold-sensitive C-fibers showed bursting in their discharge during cooling. In 11 of 27 spontaneously active cold-sensitive C-fibers the ongoing activity was depressed during heating of the nerve. Fourteen fibers exhibited a rebound excitation after nerve heating starting at temperatures that were in the range 35–40 °C (Fig. 10A). Ten of these were spontaneously active and activated by cooling; three fibers were silent and heat sensitive; one unit had no other ectopic discharge property. This rebound excitation lasted for 2–6 min. In 16 of the spontaneously active cold-sensitive C-fibers the baseline activity did not change during heating.
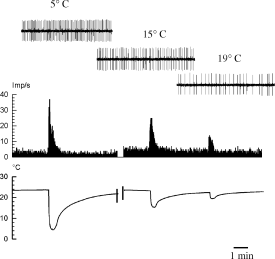
Graded responses of a spontaneously active C-fiber to cooling of the nerve. Insets show original records of 2.5 s duration during the peak temperatures of three cooling stimuli of different intensity (8 days after nerve lesion).
The activation thresholds of heat-sensitive fibers ranged from 32 to 50 °C. Thirty-seven per cent (15/41) of the heat-sensitive fibers were activated by temperatures of 32–37 °C, 26% (11/41) by 38–42 °C and 37% (15/41) responded when the temperature exceeded 42 °C. The peak discharge rates during heating ranged from 2 to 31 imp/s (8 ± 1.3 imp/s, n = 28).
C-fibers that were activated by both cooling and heating exhibited the same response pattern to cooling or heating as those C-fibers that were only activated by one of these stimuli.
Control experiments
Six control experiments were performed in order to test whether nerve fibers can be mechanically activated from the perineurium of the sural nerve. For this purpose the sural nerve was sectioned acutely about 20–25 mm distal to the recording site. Forty-six strands with 108 A-fiber and 322 C-fibers were isolated within the first 5–6 h after sural nerve transection from the proximal end of the nerve. Mechanical stimuli were systematically applied to the sural nerve using a fine-tipped blunt glas rod from sites 5 mm distal to the recording electrodes up to the distal nerve end. In this time period only a few fibers can be activated mechanically from the distal lesion site (Michaelis et al., 1995). Six unmyelinated fibers (2%) could be excited by mechanical stimulation along the nerve. The responses of these fibers were phasic and they had high mechanical thresholds. No C-fiber exhibited spontaneous activity. Myelinated fibers did not respond to mechanical stimulation of the nerve nor did they exhibit spontaneous activity.
Discussion
The aim of this study was to examine the ectopic discharges of cutaneous lesioned myelinated and unmyelinated afferent fibers while regenerating within 4–21 days after crush or section and resuturing of the sural nerve. In the last week of this time period only a few afferent fibers successfully re-innervated the target tissues, most of them being myelinated. These fibers are not included here. This study gives detailed quantitative information, for the lesioned and regenerating A- and C-fibers, about spontaneous activity and responses evoked by mechanical, cooling or heating stimuli applied to the lesioned nerve distal and proximal to the lesion site.
Ectopic properties of lesioned myelinated and unmyelinated afferent fibers
About 22–27% of the C-fibers and 33–40% of the A-fibers exhibited at least one of the tested ectopic discharge properties. These percentages are comparable with those published recently for the ligated and sectioned sural nerve about 20–30 h after nerve lesion (Michaelis et al., 1995, 1999). The ectopic discharges are not generated in perineural nerve fibers. No A-fibers and very few C-fibers were excited from the perineurium of the acutely sectioned sural nerve by mechanical stimulation at high stimulus strength (see also Michaelis et al., 1995). Thermal stimuli applied to the intact sural nerve activated only 4.1% of the C-fibers tested (5/172 fibers by heat stimuli and 2/172 fibers by cold stimuli) and no A-fibers (Blenk et al., 1996). About 15% of the C-fibers in the sural nerve are sympathetic postganglionic (Baron et al., 1988). These fibers are unresponsive to mechanical stimuli at intensities known to excite afferent cutaneous C-fibers, as shown in microneurographic studies on humans (Schmidt et al., 1994) and do not exhibit spontaneous activity generated in the periphery. It is unclear why approximately 60% of the lesioned A- and C-fibers could not be excited by the physiological stimuli and/or did not exhibit spontaneous activity.
Most ectopically active A-fibers were mechanosensitive only; some were spontaneously active or both, most after crush lesion. A few A-fibers were also heat sensitive, most being mechanosensitive and/or spontaneously active (Tables 1 and, Fig. 11). Cold-sensitive A-fibers were virtually absent. Ectopically active afferent C-fibers were either spontaneously active or mechano-, cold or heat sensitive (49% of the C-fibers in the crush lesion model; 64% of the C-fibers in section and resuturing lesion model) or had different combinations of these ectopic discharge properties (Table 1, Fig. 11). Spontaneous activity and cold sensitivity were more prominent after crush lesion; mechanosensitivity was more prominent after section and resuturing; spontaneous activity was more often found in cold-sensitive fibers than in mechanosensitive or heat-sensitive C-fibers (Table 1, Fig. 11). The mechano- and thermosensitivity of the C-fibers were mostly confined distally to the lesion site, but some units could also be excited by stimulation a few millimetres proximal to the lesion site. The proportion of fibers with thermosensitivity and/or spontaneous activity as well as the overlap between the different types of ectopic discharge properties were significantly larger in C-fibers than in A-fibers (Table 1, Fig. 11).
| A-fibers | C-fibers | |||||
|---|---|---|---|---|---|---|
| Crush (n = 77) | Section and resuturing (n = 100) | Neuroma*(n = 100) | Crush (n = 79) | Section and resuturing (n = 90) | Neuroma*(n = 138) | |
| Spontaneous activity | 20 (26%) | 3 (3%) | 6 (6%) | 51 (65%) | 33 (37%) | 52 (38%) |
| Mechanosensitivity | 65 (84%) | 93 (93%) | 98 (98%) | 26 (33%) | 49 (54%) | 60 (43%) |
| Thermosensitivity | 6 (8%) | 8 (8%) | 0 (0%) | 49 (62%) | 45 (50%) | 87 (63%) |
| Cold sensitivity | 0 | 1 (1%) | 0 | 29 (37%) | 16 (18%) | 36 (26%) |
| Heat sensitivity | 6 (8%) | 7 (7%) | 0 | 12 (15%) | 21 (23%) | 35 (25%) |
| Cold and heat sensitivity | 0 | 0 | 0 | 8 (10%) | 8 (9%) | 16 (12%) |
- * Data from Michaelis et al. (1995, 1999).
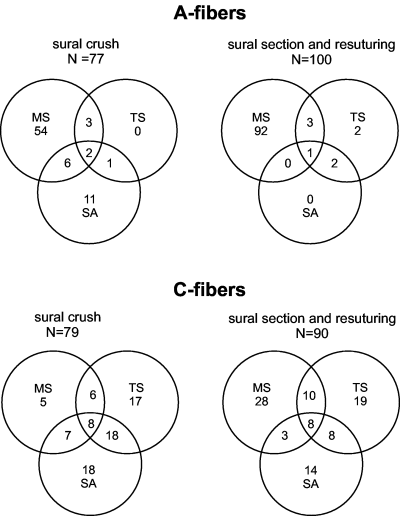
Distribution of spontaneous activity (SA), mechanosensitivity (MS) and thermosensitivity (TS) in A- and C-fibers following crush or section and resuturing of the sural nerve.
The modality of ectopic discharge properties of regenerating nerve fibers are similar in many aspects to those obtained in lesioned cutaneous afferent nerve fibers, which are trapped at the lesion site and prevented from sprouting into the peripheral nerve, 6–30 h after ligation and section of the sural nerve (Michaelis et al., 1995, 1999; Blenk et al., 1996). Table 4 compares the distribution of the ectopic discharge properties in A- and C-fibers between regenerating afferent fibers (following sural nerve crush or section and resuturing) and non-regenerating fibers trapped in the neuroma (Michaelis et al., 1995, 1999). The table shows that ectopic discharge properties of lesioned afferent fibers trapped in the neuroma are quite similar to those developed by regenerating fibers post section and resuturing of the sural nerve. The only exception found was that 8% of regenerating A-fibers develop thermosensitivity but none in the neuroma group. The proportion of fibers with spontaneous activity and the rate of spontaneous activity are significantly higher in both regenerating A- and C-fibers following crush nerve lesion than in the other two nerve lesion models (Table 4).
The receptive fields of individual mechanosensitive A- and C-fibers are either confined to the lesion site or distributed over 1–12 mm along the nerve. Interestingly, no mechanosensitive receptive fields were found outside of the nerve. Regenerating nerve fibers have several sprouts growing at different speeds and this may explain the extent as well as the discontinuity of their receptive fields.
The rate of spontaneous activity in the regenerating C-fibers of our study was significantly higher (0.86 ± 0.20, n = 80) than the rate of spontaneous activity in intact C-fibers in control nerves and in intact C-fibers in nerves in which many axons undergo Wallerian degeneration post lesion of neighbouring nerves (< 0.1 imp/s; Ali et al., 1999; Wu et al., 2001). In intact afferent C-fibers of unlesioned cutaneous nerves spontaneous activity seems only to be present in cold-sensitive C-fibers (Kress et al., 1992; Leem et al., 1993). The rate of spontaneous activity in regenerating nerve fibers was determined at a nerve temperature of 22–24 °C on the surface of the nerve. Therefore, this rate probably is somewhat lower in the cold-sensitive C-fibers at temperatures of 30–33 °C, i.e. at a nerve temperature at which the skin is closed.
Mechanosensitivity, thermosensitivity and spontaneous activity of C-fibers exhibited some overlap (Fig. 11), resulting in several functional types of lesioned C-fibers as defined by the ectopic discharge properties (Table 1). Some of these types of discharge pattern are also present in intact afferent C-fibers innervating hairy or hairless skin of the rat hind limb (Kress et al., 1992; Leem et al., 1993). For example, Leem et al. (1993) report that about 33% of the cutaneous C-fibers recorded in vivo respond to both mechanical and heat stimulation and Kress et al. (1992) that ≥ 50% of the responsive C-fibers are activated by both stimuli. Only about 10% of the lesioned C-fibers in the three types of nerve lesion models (crush, section and resuturing, neuroma) exhibit this combination of evoked impulse activity (Tables 1 and 4; Fig. 10). The reason for this difference could be the high heat thresholds of regenerating C-fibers, which might originally have been polymodal nociceptive in function. These high thresholds to heat stimulation could be related to the down-regulation of the vanilloid-receptor subtype 1 in the axotomized afferent neurons, which is suggested to be responsible for heat-transduction (Michael & Priestley, 1999). In order to prevent damage to the nerve fibers we avoided direct application of temperatures > 50 °C to the nerve. However, the heat thresholds for the C-fibers identified in the in vitro preparation of Kress et al. (1992) were in the range 34–44 °C and these values are in the range of the heat stimuli we have used.
Molecular mechanisms underlying ectopic activity
Mechano- and thermosensitivity of regenerating nerve fibers probably are intrinsic properties of the afferent neurons. These transduction properties are dependent on distinct proteins in the membranes of the cut axons that are synthesized in the cell bodies and continuously carried into the peripheral terminals of the afferent neurons by the axoplasmic transport (Caterina et al., 1997, 2000; Davis et al., 2000; McKemy et al., 2002). The specific transduction characteristics are qualitatively independent of the interaction with the surrounding milieu. However, they are most likely modulated by interactions with the peripheral target tissue. For example, during inflammation the increased sensitivity of nociceptive neurons to heat stimulation is mediated by transcriptional up-regulation of the vanilloid receptor type 1 (VR1), which is triggered by nerve growth factor (NGF), and its trkA receptor in the peptidergic C-neurons and possibly by glia-cell-line-derived neurotrophic factor (GDNF) in the non-peptidergic IB-4-positive C-neurons (Bennett et al., 1998; Nicholas et al., 1999). Inflammatory mediators can acutely sensitize lesioned afferent C- and A-fibers for mechanical stimulation (Michaelis et al., 1998). It is unclear how the VR1-mediated heat transduction, and those transduction mechanisms in afferent neurons that underlie the responses to cold and mechanical stimulation, does change after axotomy of the neurons. However, axotomy is followed by down-regulation of the VR-1 receptor in small-diameter peptidergic and non-peptidergic (IB-4 positive) dorsal root ganglion cells (Bennett et al., 1998; Michael & Priestley, 1999).
The regenerating nerve fibers are reported to interact with Schwann cells, macrophages, fibroblasts and other cells that are activated during Wallerian degeneration distal to the nerve lesion, but also proximal to the nerve lesion, and with various signalling molecules secreted by these cells, e.g. neurotrophic factors such as NGF and brain-derived neurotrophic factor (BDNF) (Heuman et al., 1987; Brown et al., 1991; Meyer et al., 1992; Funakoshi et al., 1993) and cytokines such as tumour necrosis factor α and interleukin 6 (for review see Sommer 2001). The way in which these various molecules modulate the physiological response properties and the spontaneous activity of the regenerating afferent neurons is unknown.
Independent of the change of transduction mechanisms following nerve lesion, sodium channels, potassium channels and voltage-sensitive calcium channels are up- or down-regulated in axotomized sensory neurons. These changes may be responsible for the increased excitability of the lesioned afferent neurons and therefore also for the generation of the spontaneous ectopic activity in them. For example, tetrodotoxin (TTX)-sensitive sodium channels are up-regulated and TTX-resistant channels down-regulated in C-neurons after axotomy (Zhang et al., 1997), involving decreased uptake of NGF or GDNF by the afferent neurons. Both neurotrophines can prevent or attenuate the down-regulation of the TTX-resistant channels in axotomized C-neurons in vivo and in vitro (Dib-Hajj et al., 1998; Cummins et al., 2000; Sleeper et al., 2000). Overall the excitability of the afferent cell bodies in dorsal root ganglia increases in all groups of afferent neurons after axotomy and practically all currents are affected, the largest increase in excitability occurring in afferent C-neurons and the smallest in afferent A-neurons (Abdulla & Smith 2001a,b).
Ectopic activity as a possible trigger of neuropathic pain
It is generally assumed that the changes occurring in the cell bodies of the afferent neurons after peripheral axotomy are representative of what occurs in the peripheral afferent fibers after axotomy in vivo and therefore also explain the generation of ectopic impulse discharges in these terminals. It is questionable whether the ectopic activity generated in the afferent cell bodies of the dorsal root ganglia following peripheral nerve lesion is as important as commonly assumed in triggering neuropathic pain or the equivalent pain behaviour in animal models with nerve lesions. The ectopic activity originating in the dorsal root ganglion seems to occur, first, mainly in afferent neurons with myelinated axons and not in those with unmyelinated axons (Michaelis et al., 1996; X. G. Liu et al., 2000; C. N. Liu et al., 2000) and, secondly, probably only in muscle afferent neurons (Michaelis et al., 2000). Furthermore, the proportion of dorsal root ganglion cells with ectopic activity appears to be low and probably in the range of a few per cent of all afferent cells. Therefore, it is likely that the lesioned peripheral afferent terminal is the main site of origin of ectopic activity that is involved in triggering neuropathic pain and the equivalent neuropathic pain-like animal behaviour. However, it is still puzzling how the distinct changes measured in the dorsal root ganglion cells are related to the ectopic activity generated by the afferent nerve terminals, and how this ectopic activity is related to neuropathic pain or pain-like behaviour in animals. For example, dorsal root ganglion cells of cutaneous afferent neurons with myelinated and unmyelinated fibers do not generate ectopic spontaneous activity after axotomy in vivo (Michaelis et al., 2000), whereas this ectopic activity is readily generated in some of these afferent neurons at their lesion site as shown in this and other studies.
Conclusions
Some lesioned myelinated and unmyelinated cutaneous afferent nerve fibers exhibit ectopically generated spontaneous and evoked discharges while regenerating. A-fibers show predominantly mechanosensitivity, some spontaneous activity and very little heat sensitivity, cold sensitivity virtually being absent. C-fibers show predominantly spontaneous activity, mechanosensitivity and thermosensitivity (activation by cooling and/or heating) with considerable overlap between these ectopically generated discharge characteristics. Comparing these ectopic discharge characteristics with those of the cell bodies of the afferent neurons, it is likely that ectopic discharges originating in the periphery of lesioned afferent neurons are more important in triggering neuropathic pain than those originating in their cell bodies.
Acknowledgements
This work presented here was supported by the Deutsche Forschungsgemeinschaft, by the Bundesministerium für Bildung und Forschung and by AstraZeneca. We thank Hans-Georg Schaible for critical reading of the manuscript and for suggestions.