µ/δ Cooperativity and opposing κ-opioid effects in nucleus accumbens-mediated antinociception in the rat
Abstract
We previously demonstrated that noxious peripheral stimulation (e.g. subdermal capsaicin injection in the hind paw) produces antinociception that is mediated by opioid receptors in nucleus accumbens. The current study used the trigeminal jaw-opening nociceptive reflex responses in the rat to assess the role of intra-accumbens µ-, δ- and κ-opioid receptors in the antinociceptive effect of noxious stimulation and intra-accumbens opioid agonism. Whilst intra-accumbens injection of either the µ-receptor-selective antagonist Cys2,Tyr3,Orn5,Pen7amide (CTOP) or the δ-receptor-selective antagonist naltrindole blocked capsaicin-induced antinociception, neither the selective µ-agonist [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO; 150 or 300 ng) nor the selective δ-agonist D-Pen2,5-enkephalin (DPDPE; 150 or 300 ng) alone induced antinociception. Simultaneous injection of DAMGO and DPDPE (150 ng each), however, produced significant antinociception. Capsaicin-induced antinociception was not blocked by the selective κ-receptor antagonist nor-binaltorphimine, but was blocked by the κ-agonist U69,593. U69,593 also antagonized the antinociceptive effect of the DAMGO/DPDPE combination. Thus, in nucleus accumbens, µ- and δ- but not κ-opioid receptors contributed to capsaicin-induced antinociception; selective activation of individual receptor subtypes was insufficient, but coactivation of µ- and δ-opioid receptors induced antinociception, and κ-receptors appeared to play an antianalgesic role in nucleus accumbens.
Introduction
The importance of nucleus accumbens in pain modulation/antinociception is becoming increasingly apparent. A number of neurotransmitters/receptors have been implicated in accumbens-mediated antinociception; these include NK1 and NK3 tachykinin receptors (Altier & Stewart, 1997), dopamine receptors (Altier & Stewart, 1998; Gear et al., 1999) and nicotinic acetylcholine receptors (Schmidt et al., 2001). Opioid receptor involvement has been demonstrated by intra-accumbens opioid injections that induce antinociception (Dill & Costa, 1977; Yu & Han, 1990) as well as opioid antagonist (i.e. naloxone) injections that attenuate the antinociceptive effect of systemically administered morphine (Dill & Costa, 1977).
The significance of nucleus accumbens in nociceptive modulation was further underscored by the recent observation that it mediates intrinsically activated antinociception, i.e. antinociception evoked by environmental interventions as opposed to pharmacological interventions. Noxious (painful) peripheral stimuli such as paw immersion in water at ≥ 45 °C or subdermal capsaicin administration induce antinociception as potent as that produced by high dose (10 mg/kg) morphine (Gear et al., 1999). Pretreatment by intra-accumbens microinjection of antagonists for either nicotinic, dopaminergic or opioid receptors blocks noxious stimulus-induced antinociception (Gear et al., 1999; Schmidt et al., 2001).
All three opioid receptor subtypes (i.e. µ-, δ- and κ-receptors) are found in nucleus accumbens (Svingos et al., 1997; Svingos et al., 1998; Svingos et al., 1999), and the functions of these receptor subtypes have been shown to differ in various aspects of accumbens physiology. The clearest difference is κ-receptor-mediated antagonism of µ-receptor-mediated effects which occurs in a number of brain systems (reviewed by Pan, 1998; Tsuji et al., 2001). In nucleus accumbens, µ-receptor activation results in dopamine release (Yokoo et al., 1994) as well as reward-related behaviours (Olds, 1982; van der Kooy et al., 1982) whilst κ-receptor activation decreases dopamine release and is aversive (Bals-Kubik et al., 1993; Shippenberg et al., 1993). The difference in the functions of µ-receptors and δ-receptors is less clear; in general, these receptors produce similar effects. Interestingly, selective δ-receptor antagonists can inhibit the action of selective µ-receptor agonists in some situations (Duvauchelle et al., 1996; Yoshida et al., 1999), suggesting a linkage between these two receptors subtypes. On the basis of these findings indicating antagonism between κ-opioid receptors on the one hand and µ- and δ-opioid receptors on the other, in the current study we tested the hypothesis that nucleus accumbens µ- and δ-receptors, but not κ-receptors, mediate both noxious stimulus-induced antinociception and antinociception produced by direct intra-accumbens injection of selective µ- and/or δ-opioid agonists.
Materials and methods
Animals
Experiments were performed on 280–380-g male Sprague-Dawley rats (Bantin and Kingman, Fremont, CA, USA). These animals were housed in groups of two under a 12-h light/dark cycle (lights on at 07.00 h.) in the University of California San Francisco animal care facility. Food and water were available ad libitum. Experimental protocols were approved by the University of California San Francisco Committee on Animal Research and conformed to NIH guidelines for use of animals in research.
Nociceptive assay
Changes in nociception were measured as attenuation (i.e. antinociception) or enhancement (i.e. hyperalgesia) of the trigeminal jaw-opening reflex (JOR) electromyographic (EMG) signal (Gear & Levine, 1995; Gear et al., 1999). The JOR was employed because it is segmentally remote from the hindpaw where the noxious stimulus was applied and thus allows separation of heterosegmental from segmental effects. The validity of the JOR as a nociceptive assay has been reviewed (Mason et al., 1985), and as well we have replicated our major findings using the hind paw withdrawal reflex as a nociceptive assay in awake rats. The antinociceptive effect of capsaicin, injected into the forepaw in these experiments, was blocked by intra-accumbens administration of either an opioid or a dopamine receptor antagonist, similar to what we observed using the JOR (Gear et al., 1999). These results provide further support for the use of the JOR as a valid nociceptive assay.
Anaesthesia
All experiments were performed in rats anaesthetized with an intraperitoneal injection of 0.9 gm/kg urethane and 45 mg/kg α-chloralose (both from Sigma-Aldrich, St. Louis, MO, USA). This method provides a stable JOR EMG signal over the time period required to complete the experiments (Gear & Levine, 1995).
Electrode implantation
To elicit the JOR, a bipolar stimulating electrode, consisting of two insulated copper wires (36 AWG), each with 0.2 mm of insulation removed from the tip, one tip extending 2 mm beyond the other, was inserted into the pulp of a mandibular incisor to a depth of 22 mm from the incisal edge of the tooth to the tip of the longest wire and cemented into place with dental composite resin (Citrix, Golden Gate Dental Supply, Inc, South San Francisco, CA, USA). A bipolar recording electrode, consisting of two wires of the same material as the stimulating electrode with 4 mm of insulation removed, was inserted into the anterior belly of the digastric muscle ipsilateral to the implanted tooth to a depth sufficient to completely submerge the uninsulated end of the wire.
JOR electromyogram
At the beginning of each experiment, stimulation current was set at 3× the threshold for eliciting a JOR. Each data point consisted of the average peak-to-peak amplitude of 12 consecutive jaw-opening reflex EMG signals evoked by stimulating the tooth pulp with 0.2-ms square wave pulses at a frequency of 0.33 Hz. Baseline amplitude was defined as the average of the last three data points, recorded at 5-min intervals, before an experimental intervention. Effects of experimental interventions are expressed as the mean percentage change ± SEM from the baseline for each experimental group; that is, attenuation, as depicted in the figures, represents a negative percentage change in the JOR baseline EMG (see Fig. 1 for example of JOR trace).
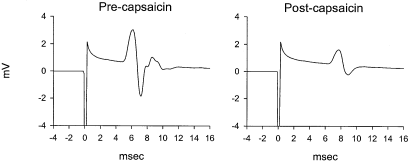
Example of JOR EMG trace (left) before and (right) 30 min after intraplantar capsaicin administration (250 µg). Note that capsaicin attenuated the peak-to-peak amplitude of the EMG. This effect is similar to that observed with morphine (10 mg/kg) administration (see example in Gear et al., 1999).
Cannula placement
For nucleus accumbens injections, 23-gauge stainless steel guide cannulae were stereotactically positioned bilaterally and cemented with orthodontic resin (L.D. Caulk Co., Milford, DE, USA) to allow injections via insertion of a 30-gauge stainless steel injection cannula, which extended beyond the guide cannulae 2 mm, connected to a 2-µL micro syringe (Hamilton, Reno, NV, USA). Injection volumes were 0.5 µL in all experiments and were carried out over a period of 120 s; the injection cannula was left in place an additional 30 s. The stereotaxic coordinates for nucleus accumbens injections were: (from bregma) 1.3 mm rostral, 7.2 mm ventral and ±1.8 mm lateral. Injection sites were verified by histological examination (100-µm sections stained with cresyl violet acetate) and were plotted on coronal sections adapted from the atlas of Paxinos & Watson (1986); (Fig. 2).
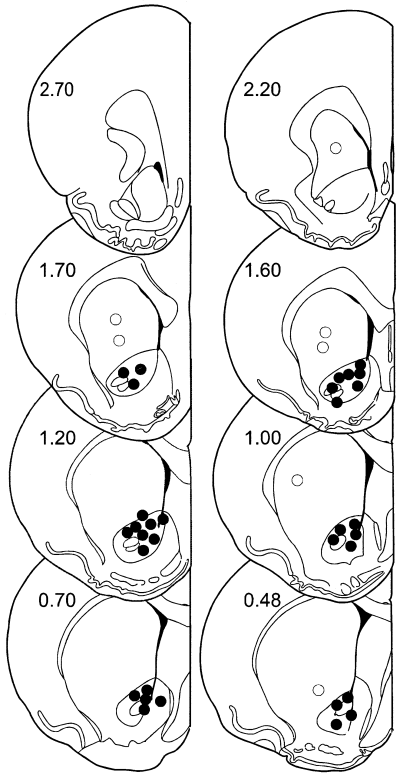
Location of injections. Filled circles are considered to be within the target area, the core region of nucleus accumbens. Open circles depict offsite injections which were deliberately placed in the caudate–putamen 1–2 mm outside the nucleus accumbens. Numbers refer to the distance in mm rostral to bregma (Paxinos & Watson, 1986).
Drugs and doses
Capsaicin was dissolved in Tween 80 (50%) and ethanol (50%) to an initial concentration of 50 µg/µL and then diluted with 0.9% saline to a concentration of 5 µg/µL; subdermal capsaicin injection volume was 50 µL (250 µg) in all experiments. [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO), 150 ng or 300 ng (Johnson et al., 1995; Noel & Gratton, 1995; Zhang & Kelley, 1997), D-Pen2,5 enkephalin D-Pen2,5-enkephalin (DPDPE), 150 ng or 300 ng (Johnson et al., 1995; Meyer & McLaurin, 1995; Zhang & Kelley, 1997) and Cys2,Tyr3,Orn5,Pen7 amide (CTOP) 1 µg (Ableitner & Schulz, 1992; Devine et al., 1993; Badiani et al., 1995) were dissolved in phosphate buffered saline (PBS). U69,593 100 ng (Spanagel & Shoaib, 1994) was dissolved in 45% aqueous 2-hydroxypropyl-β-cyclodextrin. Naltrindole 1 µg (Kelley et al., 1996; Daugéet al., 1999) and nor-binaltorphimine dihydrochloride 1.8 µg (Bodnar et al., 1995; Kelley et al., 1996) were dissolved in distilled water. All drugs and reagents were obtained from Sigma-Aldrich, St. Louis, MO or from Sigma-RBI, Natick, MA, USA.
Because it has been reported that nor-binaltorphimine may not be selective for κ-opioid receptors until several hours after administration, that is, activity at µ-receptors has been reported (Horan et al., 1992; Spanagel & Shoaib, 1994; Wettstein & Grouhel, 1996), intra-accumbens cannulae were placed under pentobarbital anaesthesia (50 mg/kg) and nor-binaltorphimine was administered 1 day prior to the experiment. On the day of the experiment, the rats were anaesthetized with α-chloralose/urethane and the usual experimental protocols were followed.
Data analysis
A two-way repeated measures anova with one between-subjects factor (i.e., treatment) and one within-subjects factor (i.e., time) was used to determine whether there were significant (P ≤ 0.05) differences in antinociceptive responses among the groups. Data were normalized for differences in baseline by calculating the percentage change from baseline for each postintervention data point (Chiang et al., 1990; Chiang et al., 1991; Banks et al., 1992; Gear & Levine, 1995; Takeda et al., 1998; Zhang et al., 1998; Gear et al., 1999; Zhang et al., 1999; Ahn et al., 2001; Belforte et al., 2001; Schmidt et al., 2001). These values were used in the statistical analyses. For each anova the Mauchly criterion was used to determine if the assumption of sphericity for the within-subjects effects was met; if the Mauchly criterion was not satisfied, Greenhouse–Geisser adjusted P-values are presented. If there was a significant between-subjects main effect of treatment group, post hoc contrasts, using the Tukey test, were performed to determine the basis of the significant difference.
Results
Opioid receptor-selective antagonists
To evaluate the contribution of intra-accumbens opioid receptor subtypes to noxious stimulus-induced antinociception, selective antagonists, except nor-binaltorphimine, were administered to nucleus accumbens 10 min prior to the administration of intraplantar capsaicin; nor-binaltorphimine was administered the day preceding the experiment (see Materials and methods). CTOP (µ-receptor antagonist) and naltrindole (δ-receptor antagonist) each blocked capsaicin-induced antinociception, but nor-binaltorphimine (κ-receptor antagonist) did not significantly affect capsaicin-induced antinociception (Fig. 3, Table 1). These findings indicate that µ- and δ-opioid receptors in the nucleus accumbens are necessary for noxious stimuli to induce antinociception and that κ-opioid receptors are not involved.
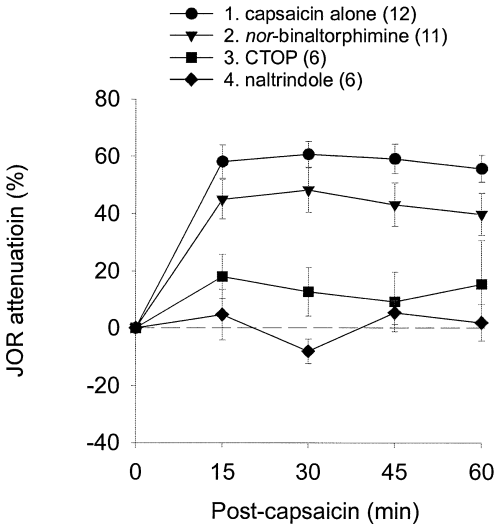
Effect of intra-accumbens administration of selective opioid antagonists on capsaicin-induced antinociception. Note that both CTOP and naltrindole, but not nor-binaltorphimine, blocked attenuation of the JOR by capsaicin. In this and subsequent figures antinociception is plotted as percentage attenuation from baseline of the JOR EMG amplitude on the Y-axis (i.e. greater antinociception is represented as higher positive numbers). The group numbers refer to the Tukey post hoc analyses in Table 1; data are plotted as mean ± SEM and the number of rats in each group is shown in parentheses.
| anovas | Tukey post hoc | |||||
|---|---|---|---|---|---|---|
| Effects | DF | F-value | P-value | Groups | P-value | |
| Fig. 3 | Tx | 3,31 | 16.558 | < 0.001 | 1 vs. 2 | 0.308 |
| Time | 3,93 | 0.353 | 0.674 | 1 vs. 3 | < 0.001 | |
| Time × Tx | 9,93 | 0.797 | 0.561 | 1 vs. 42 vs. 32 vs. 43 vs. 4 | < 0.0010.014< 0.0010.618 | |
| Fig. 4a | Tx | 3,35 | 6.115 | 0.002 | 1 vs. 2 | 0.005 |
| Time | 3,105 | 0.835 | 0.443 | 1 vs. 3 | 0.009 | |
| Time × Tx | 9,105 | 3.114 | 0.008 | 1 vs. 42 vs. 32 vs. 43 vs. 4 | 0.0180.9740.9850.894 | |
| Fig. 4b | Tx | 2,31 | 5.947 | 0.007 | 1 vs. 2 | 0.030 |
| Time | 3,93 | 1.641 | 0.204 | 1 vs. 3 | 0.008 | |
| Time × Tx | 6,93 | 2.541 | 0.053 | 2 vs. 3 | 0.876 | |
| Fig. 5 | Tx | 3,32 | 9.383 | < 0.001 | 1 vs. 2 | < 0.001 |
| Time | 3,96 | 2.227 | 0.107 | 1 vs. 3 | 0.017 | |
| Time × Tx | 9,96 | 1.735 | 0.114 | 1 vs. 42 vs. 32 vs. 43 vs. 4 | 0.0020.3840.6980.921 | |
| Fig. 6 | Tx | 3,28 | 21.087 | < 0.001 | 1 vs. 2 | < 0.001 |
| Time | 3,84 | 5.170 | 0.003 | 1 vs. 3 | < 0.001 | |
| Time × Tx | 9,84 | 2.411 | 0.017 | 1 vs. 42 vs. 32 vs. 43 vs. 4 | 0.5420.2470.023< 0.001 | |
| Fig. 7 | Tx | 3,27 | 10.530 | < 0.001 | 1 vs. 2 | 0.019 |
| Time | 3,81 | 1.762 | 0.161 | 1 vs. 3 | 0.993 | |
| Time × Tx | 9,81 | 0.904 | 0.526 | 1 vs. 42 vs. 32 vs. 43 vs. 4 | < 0.0010.0290.3190.001 | |
| Fig. 7 (U69,593)‡ | Time | 4,24 | 5.217 | 0.003 | n/a† | |
- The discussion and conclusions of this study are based largely on the main effect of Treatment (Tx) and the Tukey post hoc analyses shown in the extreme right column; however, the main effect of Time and the Time–Treatment interaction (Time ×Tx) are shown for completeness. The identity of the groups in the post hoc column is indicated by the numbers which are given in each of the respective figures (or below). †Post hoc analysis was not needed because there were only two groups. ‡One-way repeated-measuresanova for group receiving U69,593 alone (100 ng dose, plot no. 1 in Fig. 7).
Intra-accumbens µ- and δ-receptor-selective agonists
To determine whether activation of opioid receptors in nucleus accumbens is sufficient to produce antinociception, receptor selective agonists were administered into nucleus accumbens either alone or in combination. The combination of DAMGO (µ-agonist, 150 ng) and DPDPE (δ-agonist, 150 ng) induced significantly greater antinociception than similar doses of either agonist alone (Fig. 4a, Table 1). To test for the possibility that a higher dose of either agonist (i.e. equivalent to the total amount of opioid used in the combination) could induce antinociception, DAMGO (300 ng, n = 12) or DPDPE (300 ng, n = 12) was injected alone into nucleus accumbens. The antinociceptive effect of the combination remained significantly greater than that of either agonist alone, even at the higher dose (Fig. 4b, see Table 1 for statistics).
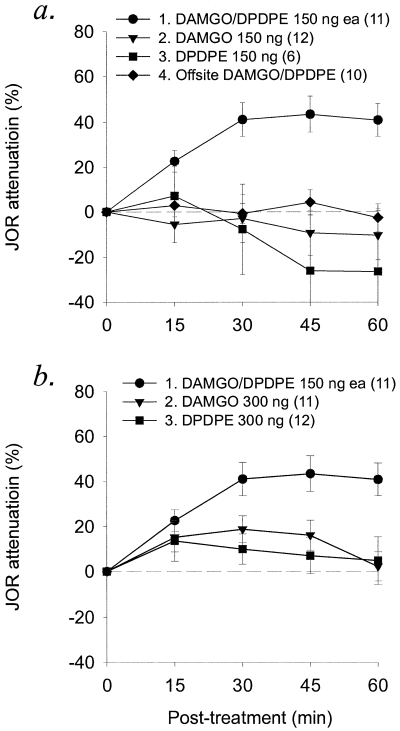
Effect of intra-accumbens administration of selective µ- and δ-opioid agonists alone and in combination. Only the combination of DAMGO (150 ng) plus DPDPE (150 ng) induced significant antinociception compared to either agonist alone, whether administered at (a) the same dose (150 ng each) used in the combination or at (b) twice the dose (300 ng each), equal to the total amount of opioid used in the combination.
To test for the possibility that the DAMGO/DPDPE combination would induce antinociception at a site outside nucleus accumbens, offsite injections were performed using the same doses. Injections within nucleus accumbens resulted in significantly greater antinociception than did offsite injections (Fig. 4a; Table 1).
Intra-accumbens κ-receptor-selective agonists
Intra-accumbens injection of the selective κ-agonist U69,593, 100 or 300 ng alone, failed to attenuate the JOR (Fig. 5), indicating that intra-accumbens κ-receptor activation is not sufficient to produce antinociception. Therefore, to determine the effect of U69,593 (100 ng) on the antinociceptive effect of the DAMGO/DPDPE combination, the three agonists combined were administered to nucleus accumbens. In contrast to the effect of µ/δ-receptor agonist combination, the combination of all three agonists failed to produce antinociception (Fig. 5, Table 1), suggesting that κ-receptor activation inhibits the antinociceptive effect of µ/δ-receptor activation.
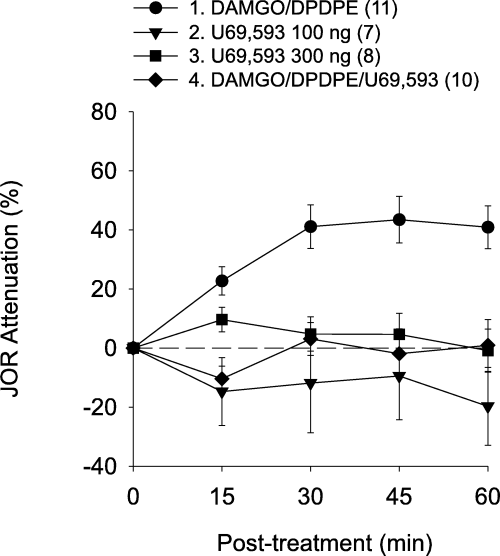
Effect of a κ-agonist on the antinociception produced by intra-accumbens administration of µ- and δ-agonists in combination. Although U69,593 at either dose (i.e. 100 or 300 ng) had no effect by itself, the addition of U69,593 (100 ng) antagonized the antinociceptive effect of the DAMGO/DPDPE opioid combination.
To determine whether κ-receptor activation similarly inhibits noxious stimulus-induced antinociception, U69,593 (100 or 300 ng) was administered into nucleus accumbens 10 min prior to intraplantar capsaicin administration. Intra-accumbens (i.e. onsite) injection of either dose of the selective κ-receptor agonist U69,593, but not extra-accumbens (i.e. offsite) injection, significantly attenuated the antinociceptive effect of capsaicin administration (Fig. 6, Table 1); however, the effect of capsaicin in the presence of the 100-ng dose of U69,593 was significantly different from baseline (Table 1), indicating incomplete blockade. This was not the case for the higher (300 ng) dose of U69,593, which did produce a complete blockade of the antinociceptive effect of capsaicin. These results suggest that activation of κ-receptors in nucleus accumbens can antagonize either noxious stimulus-induced antinociception or µ/δ-opioid-induced antinociception.
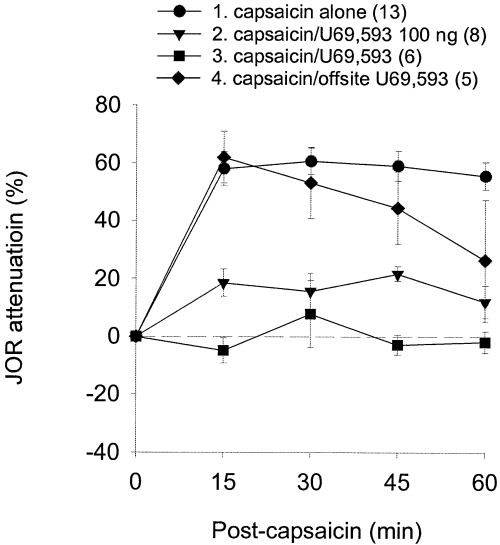
Effect of a κ-agonist on the antinociception produced by capsaicin. Onsite, but not offsite, intra-accumbens U69,593 injection significantly antagonized the antinociceptive effect of intraplantar capsaicin.
To confirm that the effect of U69,593 in the previous experiment was mediated by an action at κ-receptors, intra-accumbens U69,593 (100 ng) was administered 10 min prior to intraplantar capsaicin in rats treated the preceding day with intra-accumbens nor-binaltorphimine (see Materials and methods). U69,593 failed to antagonize the effect of capsaicin in these rats (Fig. 7, Table 1), indicating that the antianalgesic effect of U69,593 was mediated by κ-receptor activation.
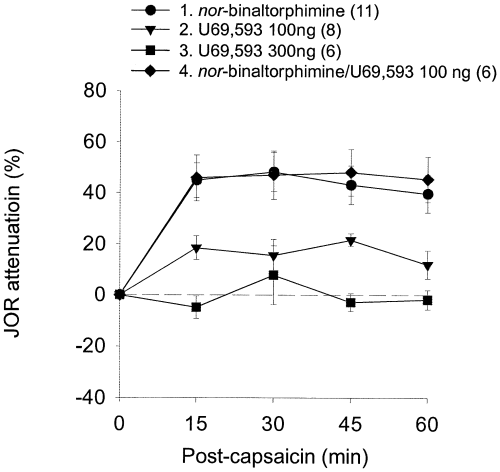
Effect of a κ-antagonist on noxious stimulus-induced antinociception. Whilst pre-treatment with intra-accumbens administration of the κ-antagonist nor-binaltorphimine did not itself affect the JOR, this treatment antagonized the ability of the κ-agonist U69,593 to block the antinociceptive effect of capsaicin.
Discussion
µ/δ-opioid receptor coactivation
In this study we demonstrate that, when injected in the nucleus accumbens, selective antagonists for either µ- or δ-opioid receptors, but not κ-receptors, blocked capsaicin-induced antinociception. Because these results indicated that both µ- and δ-receptors were required for the expression of noxious stimulus-induced antinociception, we performed direct intra-accumbens injection of receptor-selective agonists to determine whether activation of either µ- or δ-receptors alone would be sufficient to induce antinociception. We found that the combination of DAMGO plus DPDPE (µ- and δ-agonists, respectively) was antinociceptive, but that neither alone was effective. The simplest explanation for these findings is that noxious stimulus-induced antinociception depends on coactivation of µ- and δ-opioid receptors.
Antinociception that depends on µ/δ-opioid receptor coactivation has been reported previously (Porreca et al., 1987; Heyman et al., 1989b; Negri et al., 1995). For example, the antinociceptive effect of spinally administered DPDPE is significantly lower in µ-receptor deficient mice than in normal mice (Loh et al., 1998; Matthes et al., 1998). Whilst it is possible this observation could be explained by the nonselective activation of µ-receptors by DPDPE in normal mice, which would appear as a diminished δ-response in µ-knockout mice, our observation that higher doses of the δ-agonist do not mimic the antinociceptive effect of the µ/δ-agonist combination argues against this possibility and suggests instead that µ-receptors participate in δ-agonist-mediated antinociception. Although the mechanism underlying the requirement for µ- and δ-opioid receptor coactivation is unknown, a µ/δ-receptor complex has been proposed (Heyman et al., 1989a; Heyman et al., 1989b; Porreca et al., 1990; Mattia et al., 1991; Cha et al., 1995) and µ/δ-receptor heterodimerization with cross-modulation and synergistic binding of µ and δ agonists was recently demonstrated (Gomes et al., 2000). Furthermore, immunocytochemical studies in nucleus accumbens show that µ- and δ-opioid receptors are colocalized in dendritic spines (Svingos et al., 1997; Svingos et al., 1998). Thus, our findings are compatible with the suggestion that nucleus accumbens opioid-mediated antinociceptive mechanisms are mediated by µ/δ-receptor complexes.
Anti-antinociceptive effects of κ-opioid agonist
Whilst intra-accumbens pretreatment with the selective κ-receptor antagonist nor-binaltorphimine had no effect on capsaicin- or opioid-induced antinociception, administration of the selective κ-agonist U69,593 blocked the antinociceptive effect of both intraplantar capsaicin and intra-accumbens DAMGO/DPDPE. U69,593 had no effect on antinociception when injected alone and also did not block capsaicin- or opioid-mediated antinociception after pretreatment with nor-binaltorphimine, indicating that U69,593 exerted its effect by acting at κ-receptors. Thus, whilst intra-accumbens κ-receptor activation did not itself increase nociceptive responses (i.e. produce hyperalgesia), it did exert a strong antagonism to µ/δ-receptor-mediated antinociception, whether induced directly by injection of opioid agonists or indirectly by noxious stimulation.
It has been suggested that κ-inhibition of µ-effects is a general theme that can be observed in a number of systems (Pan, 1998), including antinociception (Tulunay et al., 1981; Ramarao et al., 1988; Fujimoto & Holmes, 1990; Pan et al., 1997), morphine-induced reward (Bolanos et al., 1996; Kuzmin et al., 1997) and morphine tolerance (Schmauss & Herz, 1987; Takemori et al., 1992; Hooke et al., 1995). Because both κ- and µ-receptors hyperpolarize cells (Pan et al., 1997), κ-antagonism of µ-effects is thought to result from circuit properties such as differential location of these receptor types on pre- and postsynaptic cells (Pan, 1998). In the case of nucleus accumbens, κ-mediated µ/δ antagonism might be explained by opposing actions on dopamine release. Accumbens µ/δ-receptor activation increases dopamine release (Xi et al., 1998; Yoshida et al., 1999); κ-receptor activation decreases dopamine release (Spanagel et al., 1992). We have demonstrated that the antinociceptive effect of capsaicin is dependent on activation of dopamine receptors in nucleus accumbens (Gear et al., 1999) and the importance of dopamine in other accumbens antinociceptive mechanisms has been proposed (Altier & Stewart, 1998). Thus, κ-mediated antagonism of the antinociceptive treatments in this study might result from inhibition of dopamine release in nucleus accumbens. However, it is important to note that the κ-mediated antagonism of capsaicin-induced antinociception in this study resulted from administration of an exogenous agonist; the physiological significance of this effect remains to be determined.
Because of its connections to the mesolimbic dopaminergic reward pathway nucleus accumbens is part of the neurobiological basis of opioid addiction (Wise, 1989). Opioid-induced physical dependence is thought to involve adaptations in the three different opioid receptor subtypes in different brain sites, including nucleus accumbens (Trujillo & Akil, 1991; Noble & Cox, 1996; Pan, 1998), and the aversive symptoms that accompany opioid withdrawal are thought to be secondary to dysfunction of endogenous opioid mechanisms (Trujillo & Akil, 1991). For example, down-regulation of endogenous nucleus accumbens µ-opioid ligands and up-regulation of endogenous κ-opioid ligands are observed during withdrawal (Trujillo & Akil, 1990). It is hypothesized that this withdrawal-induced imbalance in nucleus accumbens underlies κ-mediated dysphoria (Trujillo & Akil, 1991). The molecular findings of decreasing µ-opioid endogenous ligands and increasing κ-opioid endogenous ligands combined with our findings that nucleus accumbens µ- and δ-receptor activation produces antinociception and κ-receptor activation antagonizes that antinociception might define a nociceptive component of withdrawal that forms a component of the motivational process behind compulsive drug use.
In summary, these results demonstrate that both noxious stimulus-induced antinociception and opioid-induced antinociception in nucleus accumbens depend on activation of µ- and δ-opioid receptors. Our observations that administration of neither DAMGO nor DPDPE alone induced antinociception are compatible with the existence of the proposed µ/δ-receptor complex. The ability of a selective κ-agonist to inhibit both forms of antinociceptive treatment may be explained by κ-receptor presynaptic regulation of dopamine release. Whilst nucleus accumbens is well known for its role in mediating the effects of substance abuse (Koob, 1992), the present results contribute to the growing body of evidence that it also plays a key role in pain modulation activated by physiologically relevant stimuli (i.e. pain) as well as by opioid administration. Thus, understanding nucleus accumbens pain modulation mechanisms could potentially shed light on improved strategies for the treatment of pain as well as increase understanding of the neural basis of drug addiction.
Acknowledgements
This work was supported by NIH, U.S. Public Health Service NIDCR K16 DE00386 for B.L.S. and by a postdoctoral fellowship to C.H.T. from CNPq, Brazil.
Abbreviations
-
- CTOP
-
- Cys2,Tyr3, Orn5,Pen7amide
-
- DAMGO
-
- [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
-
- DPDPE
-
- D-Pen2,5-enkephalin
-
- EMG
-
- electromyographic
-
- JOR
-
- jaw-opening reflex
-
- PBS
-
- phosphate buffered saline.




