The speeding of EPSC kinetics during maturation of a central synapse
Abstract
Several factors contribute to the shape of excitatory postsynaptic currents (EPSCs) in CNS neurons, among them the kinetics of presynaptic release, transmitter clearance, and the properties and distribution of postsynaptic receptors. The decays of AMPA receptor-mediated EPSCs at rat cerebellar mossy fibre–granule cell (MF–gc) synapses follow a bi-exponential time-course. The fast component dominates the decay, accounting for 84–94% of the peak amplitude. Here we show that both components of decay, and also the risetimes, became faster during postnatal maturation. At adult, but not immature, synapses, the risetimes and decays of evoked multiquantal EPSCs were similar to those of monoquantal miniature (m)EPSCs. The faster risetimes at mature synapses reflected increased synchrony of multivesicular release, whereas the faster decays appeared to reflect changes in the properties of postsynaptic receptors. Inhibition of glutamate uptake was without effect on evoked EPSCs at both ages. Furthermore, after slowing receptor desensitization with cyclothiazide, the EPSCs at mature synapses decayed as slowly as EPSCs at immature synapses, suggesting that faster glutamate clearance does not account for the developmental speeding of EPSC decay. Our results support previous conclusions that glutamate clearance and receptor deactivation are important determinants of the fast decay component at immature synapses. Desensitization becomes increasingly important during development and plays a major role in shaping EPSC decay at mature synapses.
Introduction
The time-course of synaptic currents depends on a number of factors (for review see: Jonas & Spruston, 1994; Edmonds et al., 1995; Conti & Weinberg, 1999) which include: the composition of the postsynaptic receptors (Raman et al., 1994; Tia et al., 1996), the rate at which they desensitize (Jones & Westbrook, 1996), the affinity of the receptor for the transmitter (Jones et al., 1998), the rate of transmitter clearance from the synaptic cleft (Takahashi et al., 1995; Bergles et al., 1999), the time-course of transmitter release (Diamond & Jahr, 1995) and the occurrence of transmitter overlap between neighbouring release sites (Trussell et al., 1993; Silver et al., 1996b). During development, there are changes in synaptic morphology (Lee & Sheng, 2000) and in the expression of receptors and transporters (Hestrin, 1992; Tia et al., 1996; Furuta et al., 1997; Pouzat & Hestrin, 1997; Petralia et al., 1998; Cathala et al., 2000; Lawrence & Trussell, 2000) which have been associated with changes in several functional properties, such as quantal variance, size and content (Dumas & Foster, 1995; Pouzat & Hestrin, 1997; Bellingham et al., 1998; Wall & Usowicz, 1998). Developmental alterations have also been reported in the relative occurrence of different types of transmission (phasic vs. tonic; Wall & Usowicz, 1997b). Although a number of studies have reported developmental changes in the kinetics of non-NMDA receptor-mediated excitatory postsynaptic currents (EPSCs), except in a few cases (Wu et al., 1996; Chuhma & Ohmori, 1998, 2001; Lawrence & Trussell, 2000), the mechanisms underlying these changes are poorly understood. Indeed, very little is known about the mechanisms that shape EPSCs at mature central synapses.
To determine how the factors shaping excitation at central glutamatergic synapses change during development, we studied evoked EPSCs and spontaneous miniature excitatory postsynaptic current (mEPSCs) at mossy fibre–granule cell (MF–gc) synapses in the cerebellum at two stages of postnatal development. This synapse is well-suited for such investigations. Granule cells are electrically compact and EPSCs are distorted little by electrotonic filtering (Silver et al., 1992; D'Angelo et al., 1995). The analysis of EPSC kinetics is simplified by the ability to evoke release from one (Silver et al., 1996b) or a few of the four or five MFs innervating each granule cell (Fox et al., 1967; Jakab & Hámori, 1988). The immature MF–gc synapse has a simple structure (Hámori & Somogyi, 1983), and it has been concluded that at this stage of development the EPSC decay depends primarily on the rate of receptor deactivation and glutamate clearance (Silver et al., 1996b). However, during the maturation of MF–gc synapses there are developmental increases in morphological complexity (Hámori & Somogyi, 1983) and changes in the expression of glutamate transporters and receptors in the cerebellar granule layer (Mosbacher et al., 1994; Furuta et al., 1997). Here we show that the kinetics of AMPA receptor-mediated EPSCs become faster during development and we investigate the mechanisms underlying this developmental change.
Materials and methods
Cerebellar slices
Parasagittal slices of cerebellar vermis (200–300 µm) were prepared from male Wistar rats, at postnatal days 10–15 (P10-15) and 39–50 (P39-50), with methods based on the preparation of adult guinea pig cerebellar slices (Llinás & Sugimori, 1980). As described previously (Wall & Usowicz, 1997b), male rats were killed by cervical dislocation and decapitated. The cerebellum was rapidly removed and slices were cut on a Vibratome (Pelco, Redding, CA, USA) in cold (2–6 °C) Krebs–Henseleit solution, composed of (in mm): NaCl, 124; KCl, 5; MgSO4, 1.3; CaCl2, 2.4; KH2PO4, 1.2; NaHCO3, 26; d-glucose, 10; (pH 7.4 when bubbled with 95% O2 and 5% CO2, 300 mOsm). Slices were then stored in Krebs–Henseleit solution at room temperature for 1–6 h before recording.
Cerebellar cultures
The cerebella of Sprague-Dawley rat pups (aged 6 days) were removed and minced with a razor blade. The cerebella were further dissociated in culture medium by trituration with a fire-polished Pasteur pipette in the absence of enzymes and passed through a nylon mesh. Aliquots of the resultant cell suspension were plated on acid-washed, 12-mm circular coverslips that had been coated with poly l-lysine (400 µg ml−1 for 1 h) to give final cell densities of 3–4 × 105 cells per coverslip. The cells were maintained at 37 °C in Dulbecco's modified Eagle's medium (DMEM) containing 25 mm KCl and 10% fetal bovine serum for 6–8 days.
Electrophysiological recording
Individual slices were viewed on a Zeiss Axioskop FS microscope with a 63× or a 40× water immersion objective and Nomarski differential interference optics, at a total magnification of 790× or 640× (Carl Zeiss, Oberkochen, Germany). Slices were maintained at 22–24 °C or 30–32 °C with a Peltier-based temperature controller, or by heating the inflow over a block of resistors, and continuously superfused (1–5 mL/min) with Krebs–Henseleit solution, which was bubbled with 95% O2 and 5% CO2. Whole-cell patch-clamp recordings were made from superficially located granule cells, without prior cleaning, using an Axopatch 200-A amplifier (Axon Instruments, Foster City, CA, USA) or an EPC-8 (HEKA, Digitimer, Welwyn Garden City, UK). Patch-pipettes (thick-walled borosilicate glass, Harvard Apparatus, Edenbridge, UK) were coated with Sylgard resin (#184, Dow Corning, MI, USA), fire-polished, and had open-tip resistances of 4–10 MΩ when filled with an intracellular solution containing (in mm): CsCl, 135; HEPES, 10; EGTA, 10; Mg-ATP, 2; (pH 7.2 with TEA-OH, 285 mOsm). The mean whole-cell capacitance of the granule cells studied was 2.9 ± 0.3 pF in P39–50 rats (n = 73) and 3.2 ± 0.1 pF in P10–15 rats (n = 77). The mean series resistance was 22.5 ± 0.4 MΩ (n = 150) before compensation, which was usually set at 80% (with a lag of 10 µs on the Axopatch 200-A amplifier). Low-pass resistance–capacitance filtering of the signals, calculated from the product of the cell capacitance and the residual uncompensated series resistance, was minimal at frequencies below 5 kHz in all the recordings. The effective cutoff frequency of the filter cascade gave filter risetimes of 120 µs in all cases.
Patch-clamp recordings from granule cells in cerebellar cultures were made with an EPC9 amplifier (HEKA, Germany) at room temperature (22–24 °C). The neurons were constantly superfused (1 mL/min) with external solution composed of (in mm): NaCl, 150; KCl, 3; CaCl2, 2; MgCl2, 1; d-glucose, 5; HEPES, 10; (pH adjusted to 7.4 with NaOH). Patch electrodes (thin-walled borosilicate glass, Warner Instruments, Hamden, CT, USA) had open-tip resistances of 5–10 MΩ when filled with an intracellular solution containing (in mm): KF, 120; KOH, 33; MgCl2, 2; CaCl2, 1; spermine, 0.1; and EGTA, 11; (pH adjusted to 7.4 with CsOH). Both whole-cell recordings and recordings in outside-out patches were made but, because solution exchanges were relatively slow in the whole-cell recordings, only the results from patches were analysed in detail.
Synaptic currents
Excitatory postsynaptic currents (EPSCs) were evoked in granule cells in slices by stimulating mossy fibre inputs with square voltage pulses of 200 µs duration (set 1–10 V higher than threshold) at 0.33 Hz. Pulses were delivered by an isolated pulse stimulator (model 2100, A-M Systems, Everett, WA, USA or Digitimer DS 2a, Welwyn Garden City, UK) via a thin-walled patch pipette filled with Krebs–Henseleit solution (2–10 MΩ resistance) which was positioned on the surface of the granule cell layer. EPSCs were recorded at near physiological temperature (30–32 °C). They were also recorded at lower temperatures (22–24 °C), because asynchrony of multivesicular release is more marked at lower temperatures (Katz & Miledi, 1965; Wall & Usowicz, 1997a) and this facilitated investigation of the contribution of asynchrony to the shaping of EPSC time-course. At mature synapses, EPSCs were identified as unitary EPSCs produced by the activation of single inputs from the all-or-none appearance of EPSCs in response to increasing stimulus strength. At immature synapses, although EPSCs also appeared to be generated by a single input (because there was no increase in amplitude upon increasing the stimulus strength) there was variation in the latencies, suggesting the possible activation of more than one input. Nevertheless, there is no doubt that only a few inputs were activated, because each granule cell receives only a maximum of four or five MFs (Fox et al., 1967; Jakab & Hámori, 1988). EPSCs were usually recorded at a holding potential of −70 mV; a few recordings were made at −100 mV. To isolate non-NMDA receptor EPSCs, the NMDA-receptor antagonist d-2-amino-5-phosphonovalerate (D-AP5; 30–50 µm; Tocris Cookson, Bristol, UK) was present in the external solution, and GABAergic transmission was blocked with bicuculline (10–30 µm, Sigma, Poole, UK). For some recordings, the potential contribution of an NMDA receptor-mediated component was further excluded by the addition of 10 µm 7-chlorokynurenic acid (Tocris Cookson). The identity of action potential-dependent non-NMDA receptor-mediated EPSCs was confirmed from their sensitivity to 1 µm tetrodotoxin (TTX) and to 10 µm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; Tocris Cookson). Miniature EPSCs were recorded at −70 mV in the presence of 1 µm TTX, either in the standard concentration of Ca2+ (2.4 mm) or in an elevated Ca2+ concentration (4.8 mm) which approximately doubles the rate of mEPSC occurrence (Wall & Usowicz, 1998).
Drug application to slices
Drugs were initially prepared as 1–10 mm stock solutions. Bicuculline methiodide and TTX were dissolved in deionized water. Cyclothiazide (Tocris Cookson) and CNQX were dissolved in dimethylsulphoxide (DMSO) and 4.65% DMSO in 150 mm NaOH, respectively, at concentrations giving 0.1% DMSO in the final superfusate. Stock solutions of D-AP5 and l-trans-pyrrolidine-2,4-dicarboxylate (PDC; Tocris Cookson) were prepared in 100 mm NaOH. Aliquots of these stock solutions were stored frozen at −20 °C, and were thawed and diluted in filtered (0.22 µm filter) extracellular solution on the day of recording. In some experiments, NMDA receptor activation was enhanced by the omission of Mg2+ and the addition of 10 µm glycine to the external solution. Bicuculline, cyclothiazide, TTX, D-AP5 and PDC were added to the bath solution. CNQX (and in some experiments PDC) was applied locally in the vicinity of the cell being recorded from via a constant-pressure microperfusion system. This system consisted of seven barrels tapering to a single glass tip, 100 µm in diameter (Langton, 1993), positioned ≈ 500 µm from the recorded cell. Superfusion through each barrel was controlled by a solenoid valve (the Lee Company, Westbrook, CT, USA). During some recordings, the correct positioning of the superfusion pipette was verified by recording an inward current in response to application of the GABAA receptor agonist muscimol (Sigma). (The concentration applied (1 µm) was sufficient to overcome the block of GABAA receptors by bicuculline.)
Glutamate-activated currents in cultured neurons
The application systems used to apply glutamate to isolated cells and outside-out patches in culture have been described (Robert et al., 2001). Briefly, one system consisted of several barrels introduced into each compartment of a theta-glass capillary with a tip diameter of ≈ 300 µm. The septum of the capillary was snapped back from the tip so that the flow of solution leaving each side of the theta capillary overlapped. The flow of drug through each barrel was controlled by a solenoid valve (the Lee Company, Westbrook, CT, USA), which was closed or opened by the acquisition software of the EPC9. For experiments where brief (0.5–1 ms) applications were required, glutamate was applied to excised patches using a theta-glass pipette mounted on a piezoelectric bimorph. Patches were positioned near the solution interface and the interface was moved by applying voltage across the bimorph with a constant voltage source (Winston Electronics Co., Millbrae, CA, USA) that was triggered with one of the D–A outputs on the EPC9. The 10–90% risetimes of the open-tip responses obtained with either system were similar (100–300 µs). The glutamate applications were also made in the presence of 100 µm cyclothiazide in the bathing medium. This was initially prepared as a 20-mm stock solution in DMSO and diluted to final concentration in external medium (final DMSO concentration 0.5%). TTX (100 nm, Sigma) and D-AP5 (200 µm, RBI/Sigma) were included in the external solutions to block sodium and NMDA-receptor channels, respectively. Series resistance compensation had no effect on the amplitude or kinetics of the currents in patches and was not routinely employed.
Data acquisition and analysis
In some experiments, EPSCs and mEPSCs were filtered at 2.9–4.5 kHz (−3dB, 10 kHz filter on Axopatch 200-A; 3–5 kHz 8-pole Bessel filter, Frequency Devices, Haverhill, MA, USA) and digitized on-line (50–60 kHz) with a 1401–plus interface from Cambridge Electronic Design (CED, Cambridge, UK), controlled by CED voltage- and patch-clamp software (v. 6.22) or CED Signal Averager software (v. 6.04). In other experiments, EPSCs and mEPSCs were filtered at 5 kHz (7-pole Bessel, EPC8) and digitized on-line (50 kHz) with a Digidata 1200 interface (Axon Instruments) that was controlled by P-clamp software (Clampex v. 8.0). Miniature EPSCs were captured when they crossed a manually set threshold. At many adult synapses, mEPSCs were multiquantal, as demonstrated by the presence of multiple discrete peaks in distributions of their amplitudes (Wall & Usowicz, 1998). To isolate monoquantal mEPSCs at mature synapses, the analysis was restricted to events whose amplitude was consistent with them arising from the release of a single quantum. Amplitude distributions of these mEPSCs were described by a single Gaussian, and the mean amplitudes (10–12 pA at 22–24 °C and 16–19 pA at 30–32 °C) and low coefficient of variation (CV < 20%) of these events indicated they were monoquantal (Wall & Usowicz, 1998). The analysis of EPSC kinetics in P39–50 animals was limited to EPSCs that were multiquantal (> 1.5× the quantal size) and showed a single peak; events with multiple peaks (Wall & Usowicz, 1997a, 1998) were edited from the record. On average, the EPSCs analysed were > 4× the size of the mEPSCs. EPSCs with multiple peaks were also edited from records obtained from P10–15 animals. For each recording, single-peaked, multiquantal EPSCs and monoquantal mEPSCs were aligned on their rising phase and averaged. The decay of the so-obtained mean currents was fitted with functions consisting of two exponential components and a constant steady-state current (maximum likelihood, CED voltage- and patch-clamp software v. 6.22). The constant currents (plateau currents) were small, but present consistently, and their inclusion improved the fits to decays. The zero time point of the fitted exponentials was defined as the time at the peak of the current. Risetimes were measured from mean currents as the time required for the current to rise from 10 to 90% of peak amplitude (after aligning events on the beginning, or in some cases half-amplitude, of their rise). The coefficient of variation of mEPSC amplitudes was calculated after subtracting the background noise [coefficient of variance =√(amplitude variance – noise variance)/mean amplitude].
Glutamate-evoked currents in cultured granule cells and outside-out patches were recorded at −90 mV at room temperature (22–24 °C). The currents were analogue low-pass filtered at 2.9 or 5 kHz (−3dB, 4-pole Bessel, EPC9), digitized on-line at 30 or 100 kHz, and analysed by Igor software (WaveMetrics, Lake Oswego, OR, USA). Ten consecutive responses were averaged. The decay of the mean current evoked in response to 500–700-ms applications of glutamate was fitted with functions consisting of multiple exponentially decaying components and a steady-state plateau current. Zero time was defined as the time of the peak inward current. The decays were well-fitted by bi-exponential functions. Inclusion of a third exponential component did not improve the fits and two of the decay time constants always converged to similar values. The decays of glutamate-evoked currents obtained in the presence of cyclothiazide were described well by the sum of a single exponential component and a steady-state plateau current. The decays of the currents evoked by glutamate applications ≤ 1 ms in duration were adequately fitted by single exponential functions. Inclusion of additional exponential components gave decay time constants that converged to similar values. For the brief applications, zero time was defined as the end of the glutamate pulse, which was evident from the presence of a notch on the current trace.
Results are given as mean ± SEM values. Statistical significance was calculated using a two-tailed paired or unpaired Student's t-test, with significance taken at the P < 0.05 level.
Results
EPSCs at mature synapses were faster than those at immature synapses
To determine whether the factors shaping the kinetics of synaptic currents change during development, we compared multiquantal, single-peaked EPSCs evoked at MF–gc synapses in cerebellar slices from immature (P10-15) and mature (P39-50) rats. Multiquantal EPSCs were identified as synaptic currents with amplitudes > 1.5× the mean quantal size, and currents showing distinct, multiple peaks (Wall & Usowicz, 1997a, 1998) were edited prior to analysis. As illustrated in Fig. 1, such EPSCs at adult synapses had significantly faster rise and decay kinetics than single-peaked EPSCs at immature synapses. These differences were evident at both 22–24 °C and 30–32 °C. Although EPSCs at both immature and mature synapses showed bi-exponential decays (Fig. 1C), the fast component of decay (τfast), and also EPSC risetime, were approximately twice as fast at mature synapses (Table 1). The slow component of decay (τslow) was also faster at mature synapses (Table 1).
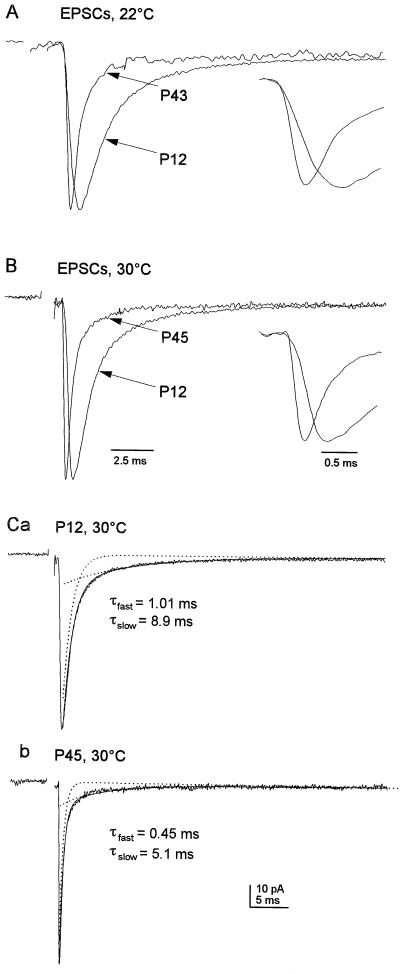
Single-peaked, multiquantal EPSCs were faster at mature than immature synapses. (A) Superimposed normalized averages of 50 evoked EPSCs, recorded in granule cells from a P12 and a P43 rat at 22 °C. These include only single-peaked EPSCs generated by the release of more than one vesicle, as identified by their size (> 1.5 times the mean quantal size). Inset, mean EPSCs from A displayed at an expanded time scale to illustrate the slower rise of the EPSCs recorded from the P12 granule cell. (B) Superimposed normalized averages of 50 evoked EPSCs recorded in granule cells from P12 and P45 rats at 30 °C. Inset, EPSC averages from B displayed at an expanded time scale to illustrate the slower rise of EPSCs from the P12 granule cell. (C) The mean EPSCs in B at each age with the bi-exponential fits to the decays superimposed (solid line). The dotted lines show the individual exponential components. The time constants of the fast and slow decay components are given. The stimulus artefacts, which were wider than those of individual events because events of variable latency were aligned, have been blanked. EPSCs were recorded at −70 mV, low-pass filtered at 3.7 kHz and sampled at 60 kHz.
| Immature synapses (P10–15) | Mature synapses (P39–50) | |||
|---|---|---|---|---|
| 22–24 °C | 30–32 °C | 22–24 °C | 30–32 °C | |
| EPSCs† | (n = 17) | (n = 17) | (n = 18) | (n = 12) |
| Ipeak (pA) | 90.8 ± 10.6 | 123.7 ± 14.3 | 61.7 ± 9.0 | 81.2 ± 12.0 |
| 10–90% risetime (µs) | 415 ± 47 | 263 ± 15 | 237 ± 20 *** | 140 ± 6 *** |
| Decay τfast (µs) | 1350 ± 56 | 940 ± 64 | 699 ± 48 *** | 449 ± 22 *** |
| Afast (%) | 89.0 ± 1.5 | 91.5 ± 1.4 | 85.0 ± 1.2 * | 90.0 ± 1.0 |
| Decay τslow (ms) | 14.1 ± 1.0 | 9.8 ± 0.7 | 11.8 ± 1.0 | 5.8 ± 0.7 *** |
| mEPSCs‡ | (n = 11) | (n = 10) | (n = 9) | (n = 5) |
| Ipeak (pA) | 20.7 ± 2.0 | 25.3 ± 3.0 | 14.2 ± 1.5 | 19.4 ± 1.2 |
| 10–90% risetime (µs) | 167 ± 19 | 154 ± 9 | 164 ± 14 | 136 ± 13 |
| Decay τfast (µs) | 914 ± 46 | 628 ± 38 | 717 ± 42 ** | 461 ± 44 * |
| Afast (%) | 83.5 ± 1.4 | 84.3 ± 2.5 | 87.4 ± 1.6 | 94.1 ± 1.0 * |
| Decay τslow (ms) | 8.9 ± 1.0 | 7.5 ± 0.8 | 6.5 ± 0.8 | 4.4 ± 1.4 * |
- Mean ± SEM values determined for evoked EPSCs and mEPSCs at mature and immature MF–gc synapses at 22–24 °C and 30–32 °C. The number of cells in each group (n) is given. For each cell, 20–100 events were aligned on their rising phase and averaged. The 10–90% risetime of the mean current was measured and the current decay was fitted with a bi-exponential function to give τfast, τslow, and the relative amplitude of the fast component, Afast. The mean values determined for EPSCs and mEPSCs at mature synapses were compared to the corresponding values for immature synapses (at the same temperature). Asterisks indicate the significant differences obtained from these comparisons (* P< 0.05, **P < 0.01, ***P < 0.001; two-tailed Student's t-test). †Multiquantal EPSCs were identified at mature synapses by amplitude. EPSCs at immature synapses may include some monoquantal events as amplitude histograms do not show discrete peaks. ‡Mean mEPSCs at mature synapses included only monoquantal mEPSCs. At immature synapses, all events were included because monoquantal and multiquantal events are not readily discriminated at the ages studied. However, the risetimes and mEPSC amplitudes were not correlated, suggesting that mEPSCs at immature synapses are mainly monoquantal (Wall & Usowicz, 1998).
Factors underlying developmental differences in risetimes
To begin to identify the factors contributing to the developmental speeding of EPSC risetimes, we compared the kinetics of mEPSCs generated by the spontaneous release of a single vesicle at mature and immature MF–gc synapses. Unlike the EPSCs, monoquantal mEPSCs are not affected by differences in the timing of release of multiple vesicles or by overlap of transmitter released from adjacent release sites. However, at the mature synapses, many mEPSCs are multiquantal (Wall & Usowicz, 1998). Therefore, monoquantal mEPSCs were selected for analysis according to size (see Materials and methods). Table 1 shows that the risetimes of monoquantal mEPSCs were similar at immature and mature synapses, and in almost all recordings they were not limited by the effective low-pass filtering imposed on the records (see Materials and methods). The similarity of the mEPSC risetimes makes it unlikely that the developmental speeding of EPSC risetimes arises due to differential electrotonic attenuation of the EPSCs resulting from differences in the sizes of the dendritic tree of granule cells in young and mature rats (Hámori & Somogyi, 1983). The similarity of the mEPSC risetimes also suggests that differences in the rate of receptor activation do not explain the differences in the EPSC risetimes.
Table 1 shows that the mean amplitudes of the mEPSCs were ≈ 25% of the EPSC amplitudes analysed at both immature and mature synapses, indicating that the mean quantal content of the EPSCs was the same. Despite the similar ratio between the mEPSC and EPSC amplitudes at both ages, the relationship between the kinetics of the mEPSCs and EPSCs was markedly different at the two ages. As shown in Fig. 2A and B, the risetimes of the mEPSCs were faster than those of evoked EPSCs at immature synapses, whereas EPSC and mEPSC risetimes were similar at mature synapses (Fig. 2C and D). This developmental difference was evident at both temperature ranges studied. At immature synapses, EPSC risetimes were 2.5-fold slower than mEPSC risetimes at 22–24 °C and they were 1.7-fold slower than mEPSC risetimes at 30–32 °C. At mature synapses, EPSC risetimes were only 1.4-fold greater than the mEPSC risetimes at 22–24 °C, whereas the risetimes of EPSCs and mEPSCs were virtually identical at 30–32 °C (Fig. 2C). At immature synapses, many evoked EPSCs had one or more inflections on their rise and these inflections were more common at lower temperature, as expected if they reflected asynchronous multivesicular release (Katz & Miledi, 1965). The results of these comparisons are summarized in Table 1 and they indicate that increased synchrony of multivesicular transmitter release is largely responsible for the developmental speeding of EPSC risetimes.
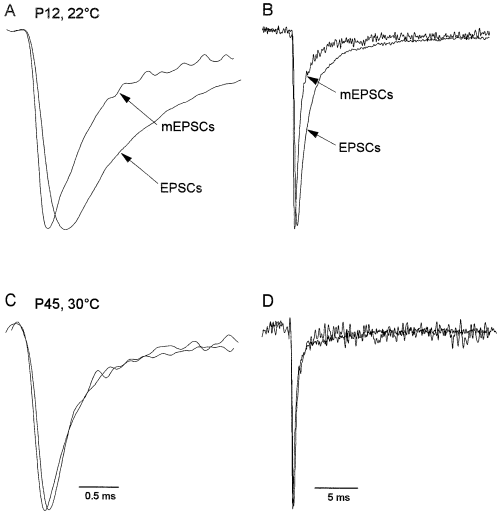
EPSCs were slower than mEPSCs at immature but not mature synapses. (A) Superimposed normalized averages of 50 EPSCs (amplitude 180 pA) and mEPSCs (amplitude 34 pA) recorded in the same granule cell from a P12 rat at 22 °C. The EPSCs were recorded first and then mEPSCs were recorded after application of 1 µm TTX to block Na+-dependent action potentials. Note the slower rise of the EPSC. (B) Same records as in A displayed on a slower time scale to illustrate that EPSC decay was slower than mEPSC decay at immature synapses. (C and D) Superimposed normalized averages of 50 EPSCs (amplitude 137 pA) and mEPSCs (amplitude 19 pA) recorded in the same granule cell from a P45 rat at 30 °C at two different time scales. Note that the mean EPSC and mean mEPSC had similar risetimes (C) and decays (D). EPSCs and mEPSCs were recorded at −70 mV, low-pass filtered at 3.7 kHz and sampled at 60 kHz.
Asynchrony contributed little to developmental differences in EPSC decay
At immature synapses, not only were the risetimes of mEPSCs briefer than those of EPSCs (P < 0.0005), but the fast component of decay was ≈ 50% faster for mEPSCs than for EPSCs (Fig. 2A and B, Table 1, P < 0.002). It was shown previously that lowering release probability at immature MF–gc synapses speeds the decay of EPSCs, and it was concluded that this speeding results from decreased transmitter spillover and faster clearance of the consequently lower concentration of glutamate present in the synaptic cleft (Silver et al., 1996b). In agreement with Silver et al. (1996b), we also found that the decay of EPSCs recorded from immature synapses was speeded when release probability was lowered by altering the extracellular Ca2+/Mg2+ ratio from 2.4/1.3 mm to 0.5/5.0 mm. The resultant decrease in release probability (failures increased from 0.4 ± 0.4% to 50 ± 7% and EPSC amplitude, excluding failures, decreased from 172 ± 1 to 44 ± 13 pA) was accompanied by a 28% reduction in τfast (1120 ± 76 µs vs. 808 ± 50 µs, P < 0.01, n = 5 cells, 30–32 °C) and a 26% reduction in τslow (9.2 ± 1.5 ms vs. 6.8 ± 1.6 ms). However, unlike a previous study (Silver et al., 1996b), we found that lowering release probability also speeded the risetime of EPSCs (from 316 ± 13 to 181 ± 19 µs at 30–32 °C, P < 0.01). This speeding of EPSC risetime is not unexpected given the evidence above for release asynchrony, and it raised the possibility that increased synchrony (resulting from the release of fewer quanta) may also contribute to the speeding of EPSC decay that occurs when release probability is lowered. The speeding of the risetime may have been missed by Silver et al. (1996b) because in that study EPSCs were aligned on the stimulus artefact. Indeed, if we align EPSCs on the stimulus artefact, we observe a slowing of the risetime with a decrease in release probability. This is to be expected because this form of alignment reveals not only temporal differences in the release of multiple vesicles, but also stochastic variations in latency to release from one evoked EPSC to the next (which becomes more evident at a lower release probability).
To determine the effect of release asynchrony on EPSC decay at immature MF–gc synapses, under conditions where similar amounts of glutamate were released, we compared the decay times of EPSCs that had substantially different risetimes but similar peak amplitudes. In each of six cells, evoked EPSCs recorded at 30–32 °C were sorted into two groups, one consisting of events that had risetimes close to those of mEPSCs (‘fast’ EPSCs), and a second group consisting of EPSCs with slower risetimes (‘slow’ EPSCs). The EPSCs in each group were averaged and their amplitude and kinetics compared. By comparing the decays of the fast multiquantal EPSCs with the decays of mEPSCs, this analysis also allowed us to assess whether differences in the amount of glutamate released resulted in different EPSC decays. The larger multiquantal EPSCs would be expected to decay more slowly than the smaller mEPSCs if the speeding of decay that occurs when release probability is lowered is due to faster glutamate clearance or reduced transmitter spillover, as proposed previously (Silver et al., 1996b).
Figure 3A shows three pairs of individual currents recorded from one cell, where EPSCs with similar peak amplitudes, but different risetimes, are superimposed. Note that each slow EPSC has inflections (arrows) on its rising phase. Figure 3B shows mean EPSCs constructed from EPSCs that had fast and slow risetimes, and Fig. 3C shows the same mean currents with their peak amplitudes aligned. It is apparent that the decay of the currents is essentially identical. The mean risetimes of the fast and slow EPSCs differed significantly (190 ± 13 vs. 303 ± 23 µs; P < 0.002), although the amplitudes of the EPSCs in the two groups were similar (145 ± 32 vs. 138 ± 31 pA; −4.2 ± 1.6%). However, despite the marked differences in EPSC risetimes (60 ± 7%), the decays of the fast and slow EPSCs were very similar in each of the six cells studied. Bi-exponential fits to the decay of fast EPSCs gave mean τfast and Afast (relative amplitude of the fast component) values of 897 ± 13 µs and 93.0 ± 1.0% (n = 6), which were virtually identical to the corresponding values for the slow EPSCs recorded from the same cells (901 ± 48 µs and 93.3 ± 1.1%). The τslow values obtained for fast and slow EPSCs were 8.08 ± 1.19 ms and 8.69 ± 1.03 ms, respectively. The average percentage differences for each of the three decay parameters were all below 10% and did not differ significantly from zero. These results indicate that asynchrony of release has little, if any, effect on EPSC decay, provided that EPSCs of similar amplitude are compared.
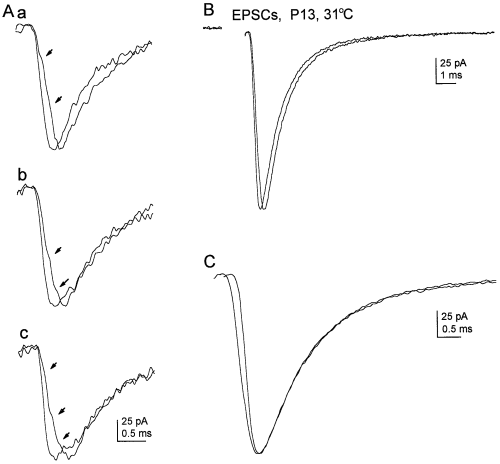
Effect of risetime on EPSC decay. (A) Examples of evoked EPSCs from one granule cell with fast and slow risetimes (P13, holding potential −100 mV; 31 °C). Pairs of EPSCs were chosen that had similar amplitudes. The individual EPSCs in each pair were aligned on the beginning of their rising phases. Arrows point to inflections on the rise of the EPSCs with slow risetimes. Calibrations in Ac apply to each panel (a–c). (B) Mean EPSCs from subsets of events with fast (44 EPSCs) and slow (34 EPSCs) risetimes. The risetimes of the mean fast and slow currents were 165 and 248 µs, respectively. The mean slow EPSC was scaled (multiplied by 1.05) so that its peak amplitude was the same as the fast mean. The stimulus artefact has been blanked. (C) Same mean EPSCs as in B, after aligning their peak amplitudes. The risetimes of the two means are clearly different, but their decays are virtually identical. Bi-exponential fits (not shown) to the decay of the mean fast EPSC gave τfast and τslow values of 832 µs and 13.2 ms (Afast 93.1%). The corresponding τfast and τslow values from the fit to the decay of the mean slow EPSC were 856 and 12.7 ms (Afast 93.1%). EPSCs were low-pass filtered at 3.7 kHz and sampled at 60 kHz.
The analysis above also showed that, at immature synapses, the decay of EPSCs is influenced by the amount of glutamate release. Thus, while the mean risetime of the fast EPSCs (190 ± 13 µs) approached that of mEPSCs at the same developmental stage and temperature (154 ± 9 µs), the decay of the EPSCs, which were ≈ 5–6-fold larger, was slower, particularly for the fast component (mean τfast= 897 ± 13 and 628 ± 38 µs for EPSCs and mEPSCs, respectively; P < 0.001).
An increase in glutamate uptake did not speed EPSC decay at mature synapses
It has been suggested that receptor deactivation and glutamate clearance control EPSC decay at immature MF–gc synapses (Silver et al., 1996a,b). Our finding that large fast EPSCs decay more slowly than small mEPSCs supports a role for glutamate clearance in determining EPSC decay at immature synapses. Therefore, one possible explanation for the speeding of EPSC decay that occurs during maturation of MF–gc synapses might be that glutamate clearance becomes faster because less glutamate is released. This predicts that, at the mature synapses, smaller currents should decay more quickly than larger currents, but we find that the mean decays of mEPSCs and EPSCs at mature synapses are identical. The other possibility that we considered is that glutamate clearance becomes faster as the result of increased glutamate uptake.
Glutamate transporters can influence transmission at immature MF–gc synapses, although at low stimulation frequencies (such as those used here) they do not shape EPSC decay, suggesting that they may be largely extrasynaptic (Sarantis et al., 1993; Overstreet et al., 1999). However, there is an increase in the expression of glutamate transporters in the granule cell layer during cerebellar development (GLT-1 and GLAST; Furuta et al., 1997), which could potentially reflect an increased expression of transporters at synapses. We therefore investigated whether the faster EPSC decay at mature synapses is due, in part, to increased glutamate uptake by recording EPSCs in the absence and presence of the glutamate uptake blocker l-trans pyrrolidine-2,4-dicarboxylate (PDC). The concentration of PDC used for these studies (300 µm) inhibits glutamate transport by several glutamate transporters (GLT-1, GLAST & EAAC1, Arriza et al., 1994), does not directly affect non-NMDA receptors (Sarantis et al., 1993), and has been shown to speed EPSC decay at immature MF–gc synapses under some conditions (Overstreet et al., 1999). Because PDC is itself a substrate, it could increase the extracellular glutamate concentration, not only by inhibiting the uptake of glutamate, but also by release of intracellular glutamate via heteroexchange (Overstreet et al., 1999). Indeed, substrate inhibitors of GABA transporters indirectly evoke currents and affect inhibitory postsynaptic currents in cerebellar granule cells (Wall & Usowicz, 1997a, b; Rossi & Hamann, 1998). Furthermore, PDC has been effective in inhibiting glutamate uptake and prolonging synaptic currents at other synapses (Higgs & Lukasiewicz, 1999; Otis et al., 1999). The effect of PDC was examined at 30–32 °C, because it is predicted that transporter activity is greater at higher temperatures (Tong & Jahr, 1994; Asztely et al., 1997), and hence any role of the transporters in shaping the decay of the EPSCs should be more evident.
We found that PDC did not affect the decay of EPSCs at mature synapses (Fig. 4A). Neither the time constants of the fast and slow components of decay nor their relative amplitudes were significantly different in PDC (mean percentage difference values 20%, P > 0.2, n = 8 cells). It was evident that PDC elevated the extracellular glutamate concentration from the development of small, noisy inward currents (7.5 ± 1.2 pA, n = 5 cells, Fig. 4B) in Mg2+-free saline supplemented with glycine (10 µm). Such currents have been shown to arise from activation of NMDA receptors (Sarantis et al., 1993), which have a higher affinity for glutamate than AMPA receptors. The lack of effect of PDC on the decay of single EPSCs evoked at mature synapses, together with previous results from immature animals (Overstreet et al., 1999), indicates that glutamate uptake does not play a major role in shaping EPSC decay at mature MF–gc synapses.
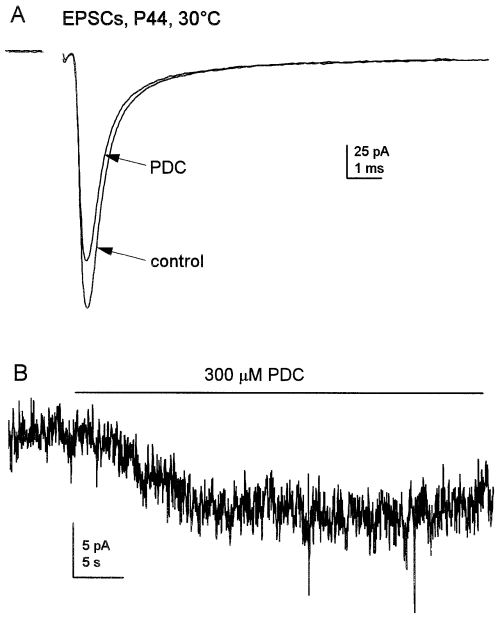
Reducing glutamate uptake at mature synapses had no effect on EPSC kinetics. (A) Superimposed averages of 50 EPSCs, in the presence and absence of 300 µm PDC, that were recorded from a P44 granule cell at 30 °C. The decays of the currents were fitted by the sum of two exponentials (τfast: control 584 µs, PDC 569 µs; τslow: control 10.8 ms, PDC 10.3 ms; fits not shown). The relative amplitude of the fast component was 86% in control and 89% in PDC. The EPSCs were low-pass filtered at 3.7 kHz and sampled at 60 kHz. The stimulus artefact has been blanked. (B) PDC (300 µm) evoked a small noisy inward current in a P40 granule cell at 22 °C. The recording was made in nominally Mg2+-free extracellular solution that was supplemented with 10 µm glycine to enhance NMDA receptor activation. The current was low-pass filtered at 3.4 kHz and sampled at 5 kHz.
The effect of cyclothiazide on receptor desensitization, deactivation and ESPC decay
Our results indicate that increased glutamate clearance does not contribute to the speeding of EPSC decay that accompanies maturation of MF–gc synapses. In contrast, the possibility that alterations in postsynaptic receptor properties contribute to the developmental speeding of decay is supported by comparison of the decays of mEPSCs at the two developmental ages. Both τfast and τslow for mEPSCs were reduced at mature synapses (1.3–1.4-fold and 1.4–1.7-fold, respectively) relative to the corresponding values at immature synapses (Table 1). These significant (P < 0.05) differences in synaptic current decay, under conditions of minimal glutamate release, suggest developmental changes in postsynaptic receptor properties. Such changes could reflect either faster receptor deactivation or faster desensitization.
Previous results from studies of AMPA receptors in cultured granule cells suggested that receptor desensitization is too slow to contribute to the fast component of EPSC decay (Silver et al., 1996a). However, Overstreet et al. (1999) have shown that cyclothiazide, a compound that markedly slows AMPA receptor desensitization (Partin et al., 1993; Trussell et al., 1993; Yamada & Tang, 1993), substantially slows the decay of EPSCs at immature MF–gc synapses. These results suggest that desensitization does contribute to EPSC decay, even in young animals. It is also known that the expression of GluR4, and flop splice variants thereof, increases during cerebellar development at times roughly coincident with synaptogenesis, and the increased expression of GluR4-flop subunits would be expected to speed receptor desensitization (Lomeli et al., 1994; Mosbacher et al., 1994; Ripellino et al., 1998). However, although many results with recombinant channels suggest that developmental increases in GluR4-flop expression should speed receptor desensitization, previous work has failed to find evidence for the expression of such channels in cultured granule cells (Silver et al., 1996a). This might reflect differences in flip/flop expression under the culture conditions employed, or alternatively that the properties of native AMPA-type channels in granule cells do not parallel those of channels expressed in heterologous systems. Although in some systems cyclothiazide was reported to have little or no effect on AMPA receptor deactivation (Trussell et al., 1993; Yamada & Tang, 1993; Partin et al., 1996; Atassi & Glavinovic, 1999), there are also reports that it slows deactivation substantially (Patneau et al., 1993; Raman & Trussell, 1995). If cyclothiazide markedly slows the deactivation of granule cell AMPA receptors, this would complicate the interpretation of cyclothiazide's slowing of granule cell EPSCs (Overstreet et al., 1999).
Given these somewhat conflicting data, we sought to obtain further insight into the relative contribution of receptor deactivation and desensitization to shaping EPSC decay at the two developmental times. We first determined the effects of cyclothiazide on receptor deactivation and desensitization, and then compared these results with the effect of cyclothiazide on the kinetics of mEPSCs and EPSCs. In postmigratory granule cells, AMPA receptors are largely segregated to synaptic regions (Silver et al., 1996a; Smith et al., 2000), and it is therefore not feasible to record AMPA receptor-mediated currents in patches pulled from these cells in slices. The effects of cyclothiazide on receptor deactivation and desensitization were therefore determined using outside-out patches from the soma of granule cells in primary cultures of cerebellum. Granule cells were maintained in vitro for 6–8 days and currents were evoked by the rapid application of 2 or 5 mm glutamate. Receptor desensitization was induced by a 500- or 700-ms application of 2 or 5 mm glutamate. This evoked whole-cell currents that decayed with a single time constant of ≈ 4 ms (not shown), similar to the time constant determined previously by Silver et al. (1996a) in granule cells cultured for 3–5 days. However, as shown in Fig. 5A, we routinely detected more rapidly desensitizing currents in outside-out patches from these same cells (mean glutamate-evoked current at −90 mV, 177 ± 10 pA; mean 10–90% risetime, 304 ± 25 µs; n = 6 patches). In each patch, the decay of these currents contained two exponential components. Bi-exponential fits to the decays gave mean time constants (τfast and τslow) of 0.95 ± 0.05 and 4.51 ± 0.28 ms (22–24 °C). The bi-exponential fit to one of the currents is shown in Fig. 5A. The relative amplitude of the fast component was, on average, 64 ± 4% of the peak inward current (n = 6).
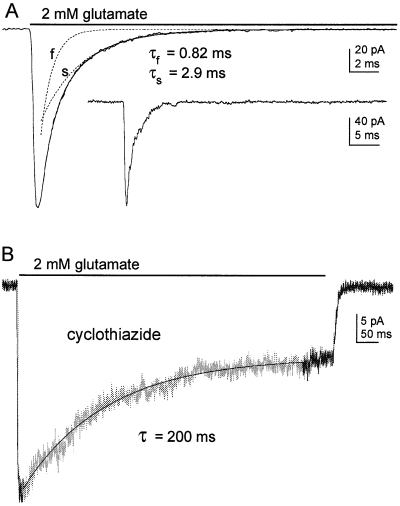
AMPA receptor desensitization in cultured granule cells. (A) Mean current evoked by 2 mm glutamate at −90 mV in an outside-out patch from a cultured granule cell maintained in vitro for 7 days. The record is the average of 10 consecutive responses obtained at 8-s intervals. The bi-exponential fit (solid line) is superimposed on the data. The values for τfast and τslow and the corresponding individual exponential components (dotted lines) are indicated. The inset shows one of the individual responses on a slower time scale. (B) Typical response to the application of glutamate (2 mm) in another granule cell patch in the continuous presence of cyclothiazide (100 µm). The current is described by a single exponential decay and a sustained steady-state current. The single exponential fit to the desensitizing component (τ = 200 ms) is superimposed (solid line) on the current trace.
AMPA receptor desensitization was profoundly slowed by cyclothiazide (100 µm), as shown in Fig. 5B. In the continuous presence of cyclothiazide, the rapid application of glutamate evoked slowly and incompletely desensitizing currents in outside-out patches (mean amplitude at −90 mV, 119 ± 18 pA, n = 5 patches). On average, 62 ± 4% of the current decayed with a time constant of 288 ± 31 ms, whereas the remainder of the current did not desensitize significantly on the time scale studied here. This represents a > 300-fold slowing of desensitization. Furthermore, given the known differential effect of cyclothiazide on recombinant channels formed from flop and flip splice variants (Partin et al., 1996), the slowly decaying component and the plateau current almost certainly arise from channels displaying flop and flip phenotypes, respectively.
The effect of cyclothiazide (100 µm) on AMPA receptor deactivation was investigated using short (1 ms) pulses of glutamate. As illustrated in Fig. 6, AMPA receptors deactivated quickly in the absence of cyclothiazide. Single exponential fits to the decays of the currents gave a mean deactivation time constant (τdeact) of 721 ± 30 µs (n = 5 patches, 22–24 °C). In the presence of cyclothiazide, τdeact was increased to 1090 ± 36 µs (n = 4 patches). This represents only a 50% slowing of deactivation. In addition to slowing the decay of the currents evoked by brief applications of 5 mm glutamate, cyclothiazide also slowed the rise of the currents. This is apparent in Fig. 6C, where the responses in panels A and B have been superimposed on a faster time scale. The 10–90% risetimes were 232 ± 21 and 382 ± 15 µs in the absence and presence of cyclothiazide (n = 5 and 4 patches, respectively). The cause of this slowing is unclear, but it was consistent and was more pronounced at lower glutamate concentrations.
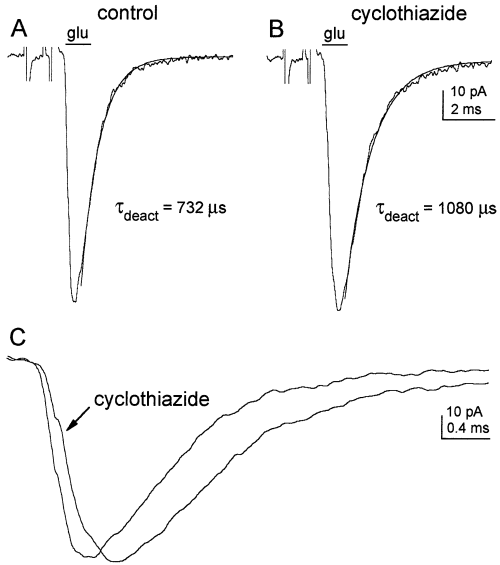
AMPA receptor deactivation in cultured granule cells. (A) Current evoked by a 1-ms application of 5 mm glutamate in an outside-out patch from a granule cell maintained in vitro for 7 days. The decay of the current has been fitted with a single exponential function (solid line) with a time constant of 732 µs. (B) Current evoked in the same patch by a 1-ms application of 5 mm glutamate after equilibrating the patch in 100 µm cyclothiazide. The single exponential fit to the decay gave a τdeact of 1080 µs. The traces in panels A and B are the means of 10 consecutive responses obtained at 2-s intervals. The artifacts associated with the voltage pulse applied across the piezoelectric bimorph have been truncated and the application bars were aligned with the onset of the glutamate-evoked currents. (C) The currents in A and B aligned and superimposed on a faster time scale to illustrate the slower rise of the current in cyclothiazide.
Having determined the relative effects of cyclothiazide on receptor deactivation and desensitization, we then looked at its effects on monoquantal mEPSCs and EPSCs. We found that cyclothiazide slowed the decay of the mEPSCs at both developmental ages. When examined at the same temperature as the effects of cyclothiazide on receptor deactivation and desensitization (22–24 °C), the τfast at immature and mature synapses was significantly greater (P < 0.01; 3.1 ± 0.4- and 3.3 ± 0.5-fold, respectively) when measured in the presence of cyclothiazide. The relative proportion of the fast component was reduced from 80.4 ± 4 to 57.9 ± 7.0% (P < 0.05) at immature synapses and from 84.2 ± 3.8 to 67.5 ± 3.3% (P < 0.01) at mature synapses. Cyclothiazide also increased τslow, but to a smaller extent: τslow was 1.8 ± 0.3- and 1.8 ± 0.4-fold greater than the control values at immature and mature synapses, respectively. Similar effects on mEPSCs were observed at 30–32 °C, as illustrated in Fig. 7A and B and summarized in Table 2. On average, τfast was 3.4-fold larger (P < 0.05) at immature synapses and 2.6-fold greater at mature synapses (P < 0.01). This slowing of the fast component in the decay of mEPSCs was accompanied by a reduction in its relative amplitude (Table 2, P < 0.01). The τslow values in cyclothiazide were 2.1- and 1.4-fold the corresponding control values at immature and mature synapses, respectively (immature, P < 0.05; mature, P = 0.23). Thus, at both developmental ages, cyclothiazide slowed the fast component of decay for monoquantal mEPSCs at 22–24 °C more than 200%, or four times as much as it slowed the apparent rate of receptor deactivation. The slowing of the slow component of decay was roughly 1.5× greater than the slowing of deactivation.
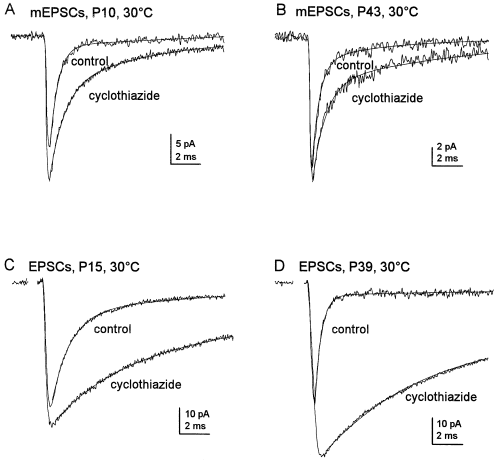
Effect of cyclothiazide on the decay of mEPSCs and EPSCs at immature and mature synapses. Mean currents before and during the application of 100 µm cyclothiazide (30 °C) were formed by aligning individual currents on the half-amplitude of their rising phases. The decays of these mean currents were fitted by the sum of two exponentials (smooth solid lines) defined by the time constants τfast and τslow and by the relative proportion of the fast component Afast. (A) Mean mEPSCs at an immature synapse (P10). τfast and τslow were 530 µs and 4.57 ms in control (average of 40), and 1300 µs and 9.89 ms in cyclothiazide (average of 40). Afast was reduced from 87% in control to 72% in cyclothiazide. (B) Mean monoquantal mEPSCs at a mature synapse (P43). Values for τfast and τslow were 429 µs and 5.08 ms in control (average of 30) and 990 µs and 7.38 ms in cyclothiazide (average of 45). Afast was 93% in control and 83% in cyclothiazide. (C) Mean evoked EPSCs at an immature synapse (P15). τfast and τslow were 1360 µs and 10.3 ms in control (average of 25) and 4680 µs and 21.28 ms in cyclothiazide (average of 25). Afast was reduced from 83% in control to 45% in cyclothiazide. (D) Mean evoked EPSCs at a mature synapse (P39). τfast and τslow were 440 µs and 16.9 ms in control (average of 25) and 6380 µs and 34.3 ms in cyclothiazide (average of 25). Afast was 88% in control and 52% in cyclothiazide. Note that cyclothiazide prolongs the decay more, and produces a larger increase in the plateau current, for evoked EPSCs than for mEPSCs. The EPSCs and mEPSCs were recorded at −70 mV, low-pass filtered at 5 kHz and sampled at 50 kHz. Stimulus artifacts in C and D were blanked.
| Ipeak (pA) | τfast (µs) | Afast (%) | τslow (ms) | Ιplat (pA) | |
|---|---|---|---|---|---|
| mEPSCs | |||||
| Immature (n = 3) | |||||
| Control | 24.4 ± 5.4 | 520 ± 6 | 83.9 ± 3.4 | 6.4 ± 1.5 | 0.21 ± 0.1 |
| Cyclothiazide | 23.1 ± 1.5 | 1760 ± 289 | 71.0 ± 1.2 | 13.5 ± 2.5 | 0.16 ± 0.1 |
| Mature (n = 5) | |||||
| Control | 16.9 ± 1.3 | 413 ± 18 | 89.8 ± 1.7 | 5.9 ± 1.1 | 0.15 ± 0.1 |
| Cyclothiazide | 18.7 ± 0.3 | 1058 ± 106 | 71.5 ± 3.0 | 8.4 ± 0.8 | 0.43 ± 0.2 |
| EPSCs | |||||
| Immature (n = 5) | |||||
| Control | 208 ± 81 | 960 ± 113 | 86.5 ± 1.4 | 11.3 ± 1.1 | 2.8 ± 0.8 |
| Cyclothiazide | 231 ± 66 | 4286 ± 563 | 64.5 ± 6.0 | 20.7 ± 2.1 | 7.5 ± 2.2 |
| Mature (n = 4) | |||||
| Control | 51 ± 4 | 595 ± 55 | 86.4 ± 2.0 | 13.5 ± 1.4 | 0.9 ± 0.3 |
| Cyclothiazide | 60 ± 6 | 5015 ± 1302 | 62.0 ± 3.4 | 27.2 ± 4.9 | 3.8 ± 1.8 |
- Mean ±SEM values for the peak inward current at −70 mV (Ipeak), the time constant and relative amplitude of the fast component of decay (τfast and Afast), the time constant of the slow component of decay (τslow), and the amplitude of the steady-state inward plateau current (Iplat). For evoked EPSCs and monoquantal mEPSCs at both ages, all measurements were made before and after cyclothiazide in the same cells at 30–32 °C. The number of cells in each group (n) is given in parentheses. (The control values are not included in the values given in Table 1.) The mean ‘x-fold’ changes in each parameter, and their significance, are given in the text.
In agreement with previous results (Overstreet et al., 1999), cyclothiazide produced a marked slowing of EPSC decay at immature MF–gc synapses (Fig. 7C, Table 2). On average, 100 µm cyclothiazide slowed τfast nearly 400% (values are 4.7 ± 0.9 fold those in control, 30–32 °C, P < 0.01) and significantly decreased its relative amplitude (Table 2). Cyclothiazide also markedly slowed the decay of multivesicular EPSCs at mature synapses, but the slowing was 2× that at immature synapses, as illustrated in Fig. 7C and D and summarized in Table 2. The τfast values in cyclothiazide were 8.1 ± 1.8-fold larger than the control values measured in the same cells (P < 0.01) and the amplitude of the fast component was reduced from 86.4 ± 2.0 to 62.0 ± 3.4% (30–32 °C). At both developmental stages, cyclothiazide caused significant increases in τslow and the amplitude of the plateau current (P < 0.05). However, unlike the changes in τfast, which were more marked than the changes in τfast for mEPSCs, at both developmental stages the changes in τslow were similar in size to the corresponding increases seen for mEPSCs.
At the mature synapses investigated with cyclothiazide, the EPSCs were, on average, only ≈ 25% of the amplitude of the immature EPSCs, both in the absence and presence of cyclothiazide. However, the decays of the currents in cyclothiazide were slightly slower for the mature synapses (Table 2). This result further supports the idea that, under control conditions, the faster decays of EPSCs at mature synapses do not simply reflect faster transmitter clearance secondary to the release of smaller amounts of glutamate.
In addition to its effects on EPSC decay, cyclothiazide also caused increases in peak EPSC amplitude at both developmental times. The average increases were 25 and 18% at immature and mature synapses, respectively (Table 2). These increases are consistent with the action of cyclothiazide to slow entry into desensitzation (Partin et al., 1996) and the notion that desensitization and activation proceed in a parallel fashion from common closed states (Vyklicky et al., 1991; Raman & Trussell, 1995; Partin et al., 1996; Robert et al., 2001). We cannot exclude the possibility that, at the immature synapses, a cyclothiazide-induced increase in transmitter release (Diamond & Jahr, 1995; Ishikawa & Takahashi, 2001) and subsequent slower glutamate clearance and spillover also contribute to the slower decay in cyclothiazide. However, alterations in transmitter release cannot explain the cyclothiazide-induced slowing of EPSC decay at mature synapses, because we have shown above that the rate of decay of synaptic currents at mature synapses is independent of current amplitude. In total, the results support the conclusion that the developmental speeding of EPSC decay is not related to faster glutamate clearance but rather to a developmental increase in the speed of desensitization.
Cyclothiazide also consistently slowed EPSC risetimes (from 220 ± 12 to 338 ± 328 µs at mature synapses (P < 0.01) and from 258 ± 16 to 367 ± 26 µs at immature synapses, 30–32 °C, P < 0.01). Previous work demonstrated that cyclothiazide prolongs the period during which release probability remains high and thereby increases multivesicular release asynchrony (Diamond & Jahr, 1995). Whilst this action may contribute to the slowing of EPSC risetimes, it is noteworthy that the risetimes of monoquantal mEPSCs were also slowed by cyclothiazide, and at mature synapses this slowing was statistically significant (162 ± 4 vs. 238 ± 12 µs, n = 5, P < 0.01). This result, together with cyclothiazide's effect of slowing the rise of glutamate responses evoked in patches (Fig. 5C), indicates that cyclothiazide also has a direct effect on channel kinetics that slows the apparent rate of channel activation.
Discussion
Developmental alterations in EPSC risetimes
The two-fold developmental speeding of the risetime of single-peaked, multiquantal EPSCs appears to result exclusively from an increase in the synchrony of multivesicular transmitter release that occurs on a timescale of < 200 µs. The risetimes of monoquantal mEPSCs did not differ at the two ages. Developmental reductions in transmitter release asynchrony have only been reported at a small number of central synapses (Chuhma & Ohmori, 1998; Rohrbough & Spitzer, 1999). At the calyx of Held, developmental increases in presynaptic Ca2+ currents, the Ca2+ sensitivity of the release machinery, and the capacity for Ca2+ clearance, all reduce asynchrony and phase-lock transmitter release to the action potential (Chuhma & Ohmori, 1998, 2001). Whether similar mechanisms operate at the MF–gc synapse is presently unclear.
The determinants of the fast component of EPSC decay
In a previous study it was concluded that granule cell AMPA receptors desensitize too slowly for desensitization to be a major determinant of EPSC decay at MF–gc synapses (Silver et al., 1996a). We show here, however, that AMPA receptors in cultured granule cells display two components of desensitization with time constants of ≈ 1 and ≈ 4 ms (at 22–24 °C). The slower time constant is similar to that determined by Silver et al. (1996a) at similar temperature. The fast (1 ms) and slow (4 ms) components we have measured are likely to arise, respectively, from channels containing the flop and flip versions of GluR4 (or GluR4c; Gallo et al., 1992). Because expression of the flop versions of these subunits increases during postnatal development (Gallo et al., 1992; Mosbacher et al., 1994), the difference between our results and those of Silver et al. (1996a) probably reflects the more mature phenotype of the cells we studied, which were maintained in vitro for longer times.
At immature MF–gc synapses, large multiquantal EPSCs decayed more slowly than monoquantal mEPSCs. The decay of synaptic currents at immature MF–gc synapses is therefore partially determined by the amount of glutamate released. These results support previous work indicating that the rate of glutamate clearance is a determinant of the fast decay of synaptic currents (Silver et al., 1996a, 1996b), as does our observation that the fast component of EPSC decay is slower than the fast component of desensitization. In addition, however, cyclothiazide slowed the fast decay component of both mEPSCs and EPSCs substantially more than it slowed deactivation of currents in outside-out patches. One interpretation of these results is that desensitization also contributes to the decay of synaptic currents at immature synapses. It is also possible, however, that the slowing reflects greater sensitivity of the channels to glutamate. It is known that cyclothiazide produces leftward shifts in the EC50 value for agonist activation of AMPA receptors (Patneau et al., 1993; Yamada & Tang, 1993). The modest effect we observed of cyclothiazide on deactivation demonstrates that any direct effect of cyclothiazide on the rate of agonist dissociation is minor. However, cyclothiazide-induced increases in apparent affinity may support channel activation at synaptic glutamate concentrations that would normally be insufficient to open the channels and such an effect would prolong current decay. Thus the contribution of desensitization to the decay of EPSCs at immature synapses remains uncertain.
The decay of EPSCs was faster at mature MF–gc synapses, and in the adult the decay of EPSCs was as fast as the decay of monoquantal mEPSCs, indicating that the rate of decay is independent of the amount of glutamate released. As at immature synapses, cyclothiazide slowed both mEPSC and EPSC decay substantially more than it slowed AMPA receptor deactivation, and it slowed EPSCs more than mEPSCs. These results suggest that the decay of synaptic currents at mature synapses is primarily determined by the rate of desensitization. Although the fast component of decay was faster than the fast component of desensitization determined in patches, this may reflect the more mature phenotype of the cells in situ.
One caveat to the conclusion that desensitization determines the fast component of decay at mature synapses is the possibility that cyclothiazide slows the deactivation of synaptic AMPA receptors more than it does the deactivation of AMPA receptors present on the soma of granule cells in culture. Indeed, AMPA receptors are largely absent from the soma of granule cells in situ once they receive synaptic input (Silver et al., 1996a; Smith et al., 2000). However, deactivation of synaptic receptors would have to be slowed four times more than that of receptors in patches to account for the effect on mEPSC decay. Two observations also indicate that such slowed deactivation alone cannot explain the results. First, it is difficult to reconcile the greater effect of cyclothiazide on multivesicular EPSCs than on monoquantal mEPSCs if the effect of cyclothiazide is primarily to slow deactivation (for example, by increasing mean open time or slowing the rate at which glutamate dissociates). Second, in addition to slowing the decay of the fast component, cyclothiazide also significantly reduced its relative amplitude. Neither result would be expected if, in cyclothiazide, the decay of the currents simply follows the glutamate transient with a greater lag.
For evoked EPSCs, cyclothiazide might also alter the shape of the glutamate transient, because it is known to increase and prolong presynaptic release at glutamatergic synapses (Diamond & Jahr, 1995; Ishikawa & Takahashi, 2001). If the decay of the synaptic currents followed the glutamate concentration profile in the synapse, then alterations in release might cause alterations in the relative amplitude of the fast and slow components of decay. However, such an effect cannot account for the reduced relative amplitude of the fast component in the decay of monoquantal mEPSCs.
The simplest explanation for our results is that desensitization becomes faster during development and becomes the dominant determinant of the fast component of EPSC decay. This occurs in parallel with developmental changes in alternative splicing and the increasing expression of GluR4 and GluR4c subunits (Gallo et al., 1992; Mosbacher et al., 1994; Ripellino et al., 1998). A developmental increase in the rate of receptor desensitization has been recently described in the avian nucleus magnocellularis, where receptor desensitization shapes EPSC decay and where the developmental speeding likewise correlates with a change in the relative expression of flip and flop splice variants (Otis et al., 1996; Lawrence & Trussell, 2000).
The slow component of EPSC decay
Cyclothiazide increased the duration of the slow component of EPSC decay less than it did the fast component, at both mature and immature synapses. The time constant of the slow component was greater than the time constant of the slow component of desensitization determined in outside-out patches. Therefore, it is possible that spillover may contribute to the slower component, not only at the immature synapses, as previously suggested (Silver et al., 1996a,b), but also at the mature synapses. In addition, the effect of cyclothiazide on the slow component was similar to its effect on the apparent rate of deactivation. The slow component of decay may also reflect the activity of channels that have escaped from desensitization and which can re-open in the presence of maintained levels of extracellular glutamate. Some AMPA-type channels, especially GluR4flip channels, recover from desensitization very quickly (τrecov 6 ms; Lomeli et al., 1994), and the decay of the synaptic currents in cyclothiazide suggests that glutamate remains elevated for tens of milliseconds at MF–gc synapses. The greatly prolonged currents in cyclothiazide are perhaps not surprising because active glutamate uptake does not appear to shape the decay of single EPSCs at either immature or mature MF–gc synapses. Although Monte Carlo simulations suggest that the passive clearance of glutamate from synapses is extremely rapid, with the majority of diffusion-limited clearance complete in < 200 µs (Clements, 1996), our results raise the possibility that this is not the case at the synapses studied here.
Functional significance
At adult MF–gc synapses, the fast component of EPSC and mEPSC decay (τfast≈ 450 µs, 30 °C) is similar to EPSC decay in various neurons of the auditory system (τdecay 200–300 µs, 33 °C; Raman et al., 1994), as well as hippocampal basket cells (τfast≈ 370 µs, 34 °C; Geiger et al., 1997). The receptors present at each of these synapses contain the GluR4 subunit (Mosbacher et al., 1994; Geiger et al., 1995; Rubio & Wenthold, 1997), which appears to be a prerequisite for extremely rapid AMPA-receptor signalling (Mosbacher et al., 1994).
In the auditory system, the rapid time course of EPSCs is important for coincidence detection and permits sound localization on the basis of interaural time differences (Reyes et al., 1996). In the cerebellum, simultaneous activity in two or more mossy fibres is required to fire action potentials in granule cells (D'Angelo et al., 1995), and many granule cells (≈ 50) must fire simultaneously to activate their postsynaptic targets, the Purkinje cells (Barbour, 1993). The developmental shortening of granule cell EPSCs may make coincidence detection more stringent and shift the optimum frequency range over which this crucial cerebellar circuit functions. Unlike some central synapses where rapid glutamate uptake is important in curtailing the EPSC (Bergles & Jahr, 1997; Bergles et al., 1999), at mature MF–gc synapses it appears that it is rapid desensitization that ensures discrete AMPA-receptor signalling. However, this property may also limit the ability of AMPA receptors to follow sustained high frequency input. Indeed, at high transmission frequencies, AMPA-receptor EPSPs drop out (presumably due to the accumulation of desensitization) and the temporal summation of individual events is sustained solely by NMDA receptors (D'Angelo et al., 1995).
Acknowledgements
This work was supported by The Wellcome Trust (M.M.U.), a Beit Memorial Fellowship (M.J.W.), Leverhulme Trust Fellowships (J.R.H., M.M.U.), and NS37904 (J.R.H.).
Abbreviations
-
- Afast
-
- relative amplitude of the fast component of the decay of fast EPSCs
-
- CNQX
-
- 6-cyano-7-nitroquinoxaline-2,3-dione
-
- D-AP5
-
- d-2-amino-5-phosphonovalerate
-
- DMSO
-
- dimethylsulphoxide
-
- EPSC
-
- excitatory postsynaptic current
-
- mEPSC
-
- miniature excitatory postsynaptic current
-
- MF–gc
-
- Mossy fibre–granule cell
-
- P
-
- postnatal day
-
- PDC
-
- l-trans-pyrrolidine-2,4-dicarboxylate
-
- τfast
-
- fast component of decay
-
- τslow
-
- slow component of decay
-
- TTX
-
- tetrodotoxin.




