Glycine triggers an intracellular calcium influx in oligodendrocyte progenitor cells which is mediated by the activation of both the ionotropic glycine receptor and Na+-dependent transporters
Abstract
Using fluo-3 calcium imaging, we demonstrate that glycine induces an increase in intracellular calcium concentration ([Ca2+]i) in cortical oligodendrocyte progenitor (OP) cells. This effect results from a calcium entry through voltage-gated calcium channels (VGCC), as it is observed only in OP cells expressing such channels, and it is abolished either by removal of calcium from the extracellular medium or by application of an l-type VGCC blocker. Glycine-triggered Ca2+ influx in OP cells actually results from an initial depolarization that is the consequence of the activation of both the ionotropic glycine receptor (GlyR) and Na+-dependent transporters, most probably the glycine transporters 1 (GLYT1) and/or 2 (GLYT2) which are colocalized in these cells. Through this GlyR- and transporter-mediated effect on OP intrcellular calcium concentration [Ca2+]i, glycine released by neurons may, as well as other neurotransmitters, serve as a signal between neurons and OP during development.
Introduction
During recent years, evidence has emerged that oligodendroglial cells express a complex array of functional receptors to a variety of neuroligands previously known to affect mostly neurons. These include not only neurotransmitter but also neuropeptide and neurohormone receptors (Belachew et al. 1998a; Verkhratsky et al. 1998) which, via several pathways activate molecular cascades regulating intracellular calcium ([Ca2+]i). Actually, [Ca2+]i increase is already known to be induced in oligodendroglial cells by stimulation of AMPA/kainate-3 type glutamate (Holtzclaw et al. 1995), ATP (P2y and/or P2u; Kirischuk et al. 1995a,b), adrenergic (Cohen & Almazan 1993) and muscarinic (Cohen & Almazan 1994) receptors. Interestingly, the expression by oligodendrocytes of carbachol and ATP receptors is controlled by oligodendrocyte–neuronal contacts (He et al. 1996). Taken together, these data suggest that oligodendroglial calcium signalling could be a target on which converge various signals involved in the neuronal regulation of the oligodendroglial growth and fate.
Regarding these signals, it is now established that glycine receptors (GlyR) are expressed in oligodendroglial lineage cells (Kirchhoff et al. 1996; Belachew et al. 1998a,b). The peak of glycine-induced chloride current is observed at the progenitor stage (OP), thus suggesting a maximum expression of the GlyR during a transient developmental period which takes place just before the O4-positive stage (Belachew et al. 1998b). The pharmacological properties and presumably the molecular structure of the oligodendroglial GlyR are specific (Belachew et al. 1998b) but the functional consequences of GlyR activation in OP cells still remain to be elucidated.
Spinal cord oligodendrocytes are known to possess a furosemide-sensitive Na+/K+/2Cl- uptake system that maintains an unusually high intracellular chloride concentration (Hoppe & Kettenmann 1989). As a consequence of this chloride uptake, the calculated Nernst chloride equilibrium potential (ECl = −35 mV) is more positive in oligodendrocytes than neurons and therefore, as already demonstrated for GABAA (Gilbert et al. 1984), GlyR activation-induced increase of chloride conductance should result in a depolarization of oligodendrocytes. Moreover, in cultures derived from cortex, oligodendrocytes express both low-voltage- (T-type) and high-voltage (presumably L-type)-gated calcium channels (Von Blankenfeld et al. 1992). In cultured murine precursor cells of oligodendrocyte lineage, GABA-induced depolarization of the cells exceeds the opening threshold for voltage-gated calcium channels (VGCC) and thereby produces Ca2+ influx and a measurable [Ca2+]i increase which exclusively result from a Ca2+ entry via VGCC as removal of extracellular calcium ([Ca2+]o) inhibits this [Ca2+]i rise (Kirchhoff & Kettenmann 1992). Here, we demonstrate that the activation of GlyR, the other major ligand-gated anion channel, also induces calcium influx in cultured OP cells derived from newborn rat cerebral cortex as is the case for developing cortical neurons (Flint et al. 1998). Furthermore, we also provide evidence that this depolarization-induced calcium increase could be in part the consequence of the activation of a Na+-dependent electrogenic uptake, most probably the 2Na+/Cl-/glycine uptake system (Aragon et al. 1987) through the glycine transporters 1 (GLYT1) and/or 2 (GLYT2) which are both expressed by cultured OP cells. To date, two different glycine transporter genes have been cloned: (i) three isoforms of GLYT1 (a, b and c) with different amino terminals can be produced by alternative splicing and/or alternative promoter usage and are thought to be exclusively present in astroglial processes surrounding N-methyl-d-aspartate and glycinergic synapses (Guastella et al. 1992; Liu et al. 1992; Smith et al. 1992; Borowsky et al. 1993; Kim et al. 1994; Adams et al. 1995; Zafra et al. 1995a); and (ii), GLYT2, expressed mainly in presynaptic nerve terminals but also in glial elements selectively in the brainstem and spinal cord where it could specifically modulate glycinergic neurotransmission (Liu et al. 1993; Zafra et al. 1995b; Morrow et al. 1998).
The demonstration of glycine transporter expression in OP cells allows us to hypothesize that GLYT1 and GLYT2 also regulate, together with GlyR, glycine-mediated neurono–oligodendroglial interactions.
Materials and methods
Cell culture
Preparation of B104 conditioned medium (B104-CM)
The B104 rat CNS neuroblastoma cell line was maintained in the logarithmic phase of growth in Dulbecco's modified Minimum Essential Medium (DMEM; GIBCO, Belgium) supplemented with 10% fetal calf serum and 2 mm glutamine. For the production of conditioned medium, confluent B104 cultures were washed twice with phosphate buffered saline (PBS) and incubated in serum-free DMEM containing 2 mm glutamine and the N1 supplement (insulin 5 μg/mL, transferrin 5 μg/mL, progesterone 20 nm, putrescine 100 μm and selenium 30 nm). After 3 days, the medium was collected, filtered (0.22 μm) and stored at −20 °C until use. The same conditioning procedure was used with MEM instead of DMEM for the preparation of the MEM-B104-CM.
Primary oligodendrocyte progenitor cell cultures
Primary oligodendrocyte cultures were prepared using a slight modification of the isolation procedure described by Avellana-Adalid et al. (1996). The cerebral cortices of 1–3-day-old rat pups were dissected and collected in PBS supplemented with glucose at 4.5 g/L, carefully freed of meninges and vessels, and dissociated by sieving successively through a 225 μm and a 25 μm nylon mesh. Cells were collected in PBS containing 25 mm HEPES. The cell suspension was layered on top of a precentrifuged (30 min at 26 000 g) Percoll density gradient (1.04 g/mL; Pharmacia, Sweden) and centrifuged for 15 min at 26 000 g. Cell debris which remained in the top aqueous phase were discarded and the interphase below the debris and just above the red blood cells was resuspended in PBS–HEPES. The suspension was centrifuged three times (10 min at 400 g) in PBS–HEPES to eliminate Percoll. The final pellet was resuspended in DMEM supplemented with N1, biotin (10 ng/mL) and 30% (v/v) of B104-CM. Five millilitres of the cell suspension were then seeded on an uncoated 25 cm2 tissue culture flask (Falcon; Becton–Dickinson, USA) at a concentration of 4 × 106 cells/mL. After 24 and 48 h, the flask was smoothly shaken and the suspension transferred into a new flask, thus eliminating adherent cells. After these preplatings, the progenitor cells are present as spheroid aggregates termed ‘oligospheres’ (150–300 μm diameter). To obtain an oligodendrocyte commitment, oligospheres were switched to DMEM-N1 medium and seeded on polyornithine-coated (0.1 mg/mL) glass coverslips in the centre of 35 mm plastic Petri dishes (NUNC, Denmark) at a density of 10–25 oligospheres per coverslip.
Calcium imaging
Cells were loaded with the calcium indicator dye fluo-3 AM (6 μm; Molecular Probes, USA) by bath application for 45 min at 37 °C. Fluo-3 AM is a nonratiometric indicator dye that increases cellular fluorescence intensity with increased intracellular calcium concentration. Loaded cells were then washed three times with Locke solution containing (in mm): NaCl, 154; KCl, 5.6; glucose, 5.6; HEPES, 10; CaCl2.2H2O, 2.3. The responses of the cells to experimental conditions were recorded as digitized images from a Bio-Rad MRC 1000 laser scanning confocal system coupled to Zeiss Axiovert 135 microscope with a plan-NEOFLUAR objective (40 ×, 1.3 n.a., oil immersion). The TCSM program (Bio-Rad, USA) was used to control the confocal microscope and obtain a series of images at intervals from 2–5 s. The different reagents diluted in Locke solution were applied by a fast microperfusion system (SPS-8, List-Medical, Germany). Strychnine, nifedipine and bicuculline were obtained from Sigma (USA), glycine from UCB (Belgium) and EGTA from ACROS (New Jersey, USA). In the sodium-free Locke solution, NaCl was substituted with an equimolar concentration of choline chloride. The series of digitized fluorescence images were analysed by a program which determined the average level of fluorescence above background level of each cell and for every time point sampled. The locations of cells were delimited by placing rectangular boxes around every cell in a field. A ‘background’ box was also defined in a noncellular area of the scanned image. The average intensity of the pixels within a boxed cellular region was calculated and the average intensity of the pixels within the ‘background’ box defined for the image was subtracted from this value. In order to compensate for variable dye loading between cells, these background-corrected values were normalized by conversion to percentage changes relative to a baseline measurement for each boxed cellular region at the start of a time series (Ft/F0). Statistical analysis was performed using GraphPAD Prism and GraphPAD InStat software (USA).
Immunocytochemistry
The coverslip cultures were fixed in 4% (v/v) paraformaldehyde for 10 min at room temperature. Nonspecific binding was blocked by a 60-min treatment in a PBS solution containing non-fat dry milk (15 mg/mL). This was followed by an overnight incubation at 4 °C with goat polyclonal anti-GLYT1 or sheep polyclonal anti-GLYT2 antibodies (Chemicon Int., Temecula, USA) both at 1 : 5000 dilution. As secondary antibodies, a TRITC-conjugated antigoat IgG (Sigma, USA) at 1 : 400 dilution (60 min incubation at 37 °C) or a TRITC-conjugated antisheep IgG (Jackson ImmunoResearch, USA) at 1 : 250 dilution were used, respectively. For double stainings, the coverslips were then postfixed for another 10 min incubation in 4% (v/v) paraformaldehyde and the A2B5 and O4 stainings were performed as described by Belachew et al. (1998a) using primary antibodies obtained from Boehringer–Mannheim. Triple PBS rinses were performed between each step. For cell counting, all the stains were analysed in triplicate by counting at least 10 fields per coverslip. The control for antibody specificity omitted the primary antibody in the staining protocol. The immunofluorescence images were obtained by Z-series with a Bio-Rad MRC-1000 confocal microscope.
Results
Glycine induces an increase of intracellular calcium concentration in OP cells through the opening of voltage-gated calcium channels
The cultured cells were studied in the outgrowth zone of expanded oligospheres 3 days after switching to DMEM-N1. We have previously shown that, in this growth factor-free medium, the cultures contain mainly A2B5-positive cells with many of them already coexpressing a galactocerebroside immunophenotype (Belachew et al. 1998a). We also demonstrated that there was virtually no neuronal or glial fibrillary acidic protein-positive cells (< 1%) in such cultures (Belachew et al. 1998a).
To investigate whether GlyR activation could affect intracellular oligodendroglial calcium concentration, we used 3-day-old cultures because the peak density of GlyR expression occurs at this intermediate stage of differentiation (Belachew et al. 1998b).
OP cells were imaged using confocal microscopy and the calcium indicator dye fluo-3 in Locke standard extracellular solution. All the recordings were made in the presence of bicuculline (100 μm) to avoid any nonspecific increase of intracellular calcium that would result from a glycine-induced cross-activation of GABAA receptors. In such conditions, the application of glycine (0.5 mm) results in a prolonged rise of [Ca2+]i in around 50% of the cells (30 out of 61 tested cells). A glycine-evoked change in [Ca2+]i was not observed in these OP cells (16 out of 61 tested cells) which do not exhibit an intracellular calcium response to depolarization induced by high extracellular K+ concentration (50 mm). The glycine-elicited [Ca2+]i increase in OP cells (n = 7; Fig. 1A) is completely abolished in a calcium-free extracellular solution supplemented with EGTA (2 mm). Moreover, nifedipine (10 μm) an l-type calcium channel blocker, reversibly inhibits the glycine-induced [Ca2+]i rise in all the tested OP cells (n = 41; Fig. 1B). These data thus suggest that, in OP cells, glycine triggers a depolarization-induced calcium entry through VGCC.
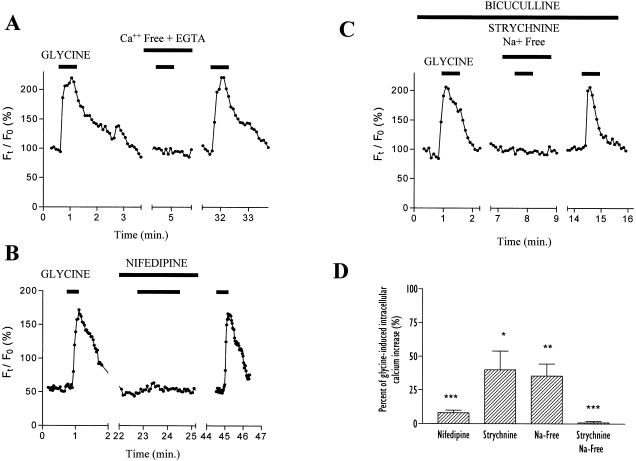
Fluo-3 measurements of glycine-induced intracellular calcium increase in oligodendrocyte progenitor cells. (A) Time-course of glycine-evoked [Ca2+]i rise in the presence of bicuculline (100 μm). The calcium response is abolished in calcium-free extracellular solution supplemented with EGTA (2 mm). Glycine was applied at 0.5 mm for 40 s. (B) Glycine-induced calcium increase is completely inhibited by simultaneous application of nifedipine (10 μm) in Locke solution. (C) Glycine-induced calcium increase is completely inhibited by simultaneous application of strychnine (30 μm) in a sodium-free Locke solution. (D) Mean amplitudes (± SEM) of relative glycine-evoked [Ca2+]i rises elicited in the presence of nifedipine (n = 41), strychnine (n = 8), Na+-free medium (n = 7) or in both conditions simultaneously (n = 11). Results are expressed as percentage of glycine-induced [Ca2+]i responses and statistical data (using Student's t-test) are derived from comparison with these control responses. *P < 0.05, **P < 0.01, ***P < 0.0001.
Glycine-induced calcium influx in OP cells is mediated by both GlyR and Na+-dependent transporters, most probably GLYTs
Strychnine (30 μm), a competitive antagonist of GlyR, produces a reversible but variable inhibition of glycine-induced calcium responses (Fig. 1D). In three of eight OP cells, the strychnine block of [Ca2+]i increase was complete, whereas the inhibition was only partial in the other tested OP cells, despite a virtually full blockade of OP GlyR at such strychnine concentrations (Belachew et al. 1998b). This suggested that the glycine-induced depolarization in OP cells and its subsequent calcium entry is only partly due to GlyR activation. As it was recently demonstrated that the glial glycine uptake transport systems mediated by GLYT1b and GLYT2 induce inward depolarizing uptake currents in HEK 293 cells transfected with these transporters (López-Corcuera et al. 1998), we suspected the presence of such transporters in oligodendroglial cells. Immunocytochemical stainings (Fig. 2) indeed show that all the A2B5-positive (98 ± 2%, mean ± SEM) and the majority of O4-positive OP cells (76 ± 5%, mean ± SEM) express GLYT1 in vitro. We also observed the expression of GLYT2 in most of the A2B5-positive OP cells (90 ± 3%, mean ± SEM). These two transporters are thus colocalized with GlyR because, as we previously shown, GlyR is expressed in more than 90% of the cells at this stage after 3 days of differentiation in DMEM-N1 (Belachew et al. 1998b).
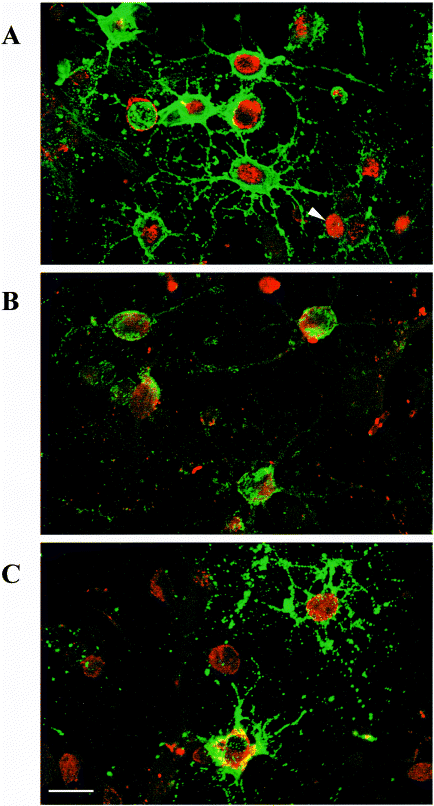
Confocal microscopy images of GLYT1 and GLYT2 immunofluorescence stainings in oligodendroglial cells after 3 days of differentiation in vitro. (A) Double stained OP cells with A2B5 (green) and anti-GLYT1 (red) antibodies. The GLYT1 staining is only located on the cell body and not on oligodendroglial processes. GLYT1-positive A2B5 cells are probably already at the O4 stage (arrow). (B) A2B5-positive OP cells (green) are also stained by anti-GLYT2 (red) antibodies. (C) The GLYT1 staining (red) remains in O4-positive cells (green). Many GLYT1-positive O4-negative cells are probably still A2B5-positive OP cells. Scale bar, 10 μm
In order to investigate the role of the GLYTs and/or less specific electrogenic transporters in glycine-induced [Ca2+]i increase in OP cells, we blocked these Na+-dependent transporters by using Na+-free Locke solution. In such conditions, a partial inhibition of glycine-evoked calcium responses was observed (n = 7; Fig. 1D). Finally, if strychnine (30 μm) and external Na+ removal are combined, a full inhibition of the glycine response is obtained (n = 11; 1, 3), thus demonstrating that a glycine-triggered [Ca2+]i increase in OP cells is actually due to the activation of not only GlyRs but also Na+-dependent transporters, most probably GLYTs.

Pseudocolored fluorescence images of OP cells loaded with fluo-3. Illustrated example of progenitor cells fluorescence that reflects [Ca2+]i variations in response to external application of successively control medium (A and C), glycine (0.5 mm) in Locke solution (B), or glycine with strychnine in a Na+-free Locke solution (D). Images are pseudocolored according to the scale, which represents a linear increase in fluo-3 fluorescence. This example was selected to underline that glycine-induced [Ca2+]i increase was delayed in one of these OP (arrow) which suggests an intercellular calcium wave propagation through gap junctional contacts which are known to be present in OP cells (Takeda et al. 1995).
It is noteworthy that the inhibition in Na+-free solution is greater (65%) than that expected based on strychnine-resistant inhibition (40%). This discrepancy could arise from inhibition of Na+-dependent chloride transporters affecting the chloride gradient and thus influencing GlyR signalling.
Discussion
Glial cells, obviously non-excitable according to the classical view of excitability (i.e. the ability to generate action potentials), express voltage-gated Ca2+ channels that constitute an important pathway for calcium entry in oligodendroglial cells (Kirischuk et al. 1995b). Such calcium influx through VGCCs and the resulting increase of [Ca2+]i are likely to regulate various intracellular events in OP cells (including metabolic reactions, gene expression or ion transport systems) that could ultimately be linked to the myelinating function. In this study, we report that exogenously applied glycine induces an important elevation of [Ca2+]i in cortical OP cells. We show that this effect is observed only in cells which express functional VGCCs, as demonstrated by [Ca2+]i increase in response to depolarization. Glycine-evoked [Ca2+]i rise results from a depolarization-induced calcium influx as it is completely suppressed by VGCC blockade or removal of extracellular calcium. However, we cannot exclude that plasmalemnal calcium entry could secondarily trigger a calcium release from intracellular stores but, so far, such a Ca2+-induced Ca2+-release mechanism has not been demonstrated in oligodendroglial cells (Kirischuk et al. 1995b).
We consider that glycine-induced depolarization and calcium influx in OP cells are mediated by the simultaneous activation of the ionotropic GlyR and the Na+-dependent transporters, the best candidates being glycine transporters, since: (i) we demonstrate by immunostaining the presence of GLYT1 and GLYT2 in the majority of OP cells, and these transporters (2Na+/Cl–/glycine) are known to be electrogenic, and thus their activation produces an inward depolarizing uptake current (López-Corcuera et al. 1998); (ii) we have previously shown the presence of GlyR in OP cells (Belachew et al. 1998b), the activation of which should induce a depolarization as is the case after GABAA receptor activation; (iii) glycine-evoked [Ca2+]i increase is significantly, but only partly, inhibited either by application of saturating concentrations of strychnine or by choline substitution of extracellular Na+, which are conditions that block the GlyR and Na+-dependent transporters, respectively; and, (iv) glycine-evoked [Ca2+]i increase is totally abolished by the simultaneous blockade of oligodendroglial GlyR and Na+-dependent transporters, therefore demonstrating that both types of membrane proteins transduce the effect of glycine leading to an increased OP [Ca2+]i. Due to the lack of availability of pharmacological agents that would specifically block both GLYT1 and GLYT2, we cannot provide conclusive, but only circumstantial, evidence demonstrating that GLYTs are the sole Na+-dependent transporters implied.
The coexpression of glycine receptors and transporters and the consequences of their stimulation on calcium homeostasis has not been reported in oligodendroglial cells. This oligodendroglial colocalization of GLYT1 and GlyR is, however, not surprising considering the in situ hybridization data that show a temporal and spatial coincidence of GLYT1 and GlyR β-subunit mRNA expression (Zafra et al. 1995b). Contrary to GLYT1, GLYT2 expression is prominently neuronal and restricted to the brainstem, the spinal cord and the cerebellum, where it colocalizes with GlyR and glycine immunoreactivity (Jursky & Nelson 1995; Luque et al. 1995; Poyatos et al. 1997). Such correlating distributions of GLYTs and GlyR suggest that GLYTs reuptake activity could regulate the glycinergic neurotransmission by modulating glycine concentrations locally bathing presynaptic elements of glycinergic synapses in vivo. It is also known that in cultured astroglial cells, the initiation and the maintenance of GLYT1 expression require the presence of neurons whereas we show that it is not the case for oligodendroglial GLYTs (Zafra et al. 1997). Consequently, we consider that glycine, via its receptors and transporters, could be involved in a bidirectional neuron–oligodendrocyte dialogue that could regulate oligodendroglial development through glycine-induced [Ca2+]i increase. There is currently good evidence that neurons influence oligodendrogliogenesis through the release of growth factors (Calver et al. 1998) and electrical activity (Demerens et al. 1996). Neurotransmitters may also play a role in this neuronal regulation of oligodendrocyte behaviour. However, as oligodendroglial calcium signalling can be triggered by numerous membrane receptor systems (Verkhratsky et al. 1998), the question of the specificity of such effects arises. In other words do oligodendrocytes distinguish, for instance through spatiotemporal cell-specific pattern of [Ca2+]i rise, between different stimulations that all increase [Ca2+]i? At present, the functional responses of oligodendroglial cells that are triggered by an elevation of [Ca2+]i has still to be investigated. In cortical OP cells, calcium influx can induce the phosphorylation of the cAMP response element binding protein (CREB, Pende et al. 1997) that is known to be a crucial factor for Ca2+-dependent gene expression. Very recent data support the idea that CREB phosphorylation, which is increased when [Ca2+]i rises, could mediate neuronal signals that, coupled to specific transduction cascades, may play different regulatory roles at specific stages of oligodendrocyte differentiation (Sato-Bigbee et al. 1999). Interestingly, OP cells also express a G-protein-coupled [Ca2+]o-sensing receptor (CaR, Chattopadhyay et al. 1998). The stimulation of CaR by [Ca2+]o elevation increases OP proliferation by triggering a [Ca2+]i rise which is likely to activate an outward K+ channel (Chattopadhyay et al. 1998) whose role is essential for the regulation of OP cell cycle (Knutson et al. 1997). Considering this emerging evidence for a central role of [Ca2+]i in oligodendroglial development, we would expect that glycine-induced [Ca2+]i elevation in OP cells could be one of several neuronal signals modulating proliferation, migration or differentiation of OP cells, and therefore ultimately myelination and possibly remyelination, thus opening therapeutic prospects.
Acknowledgements
We thank Dr Frank Kirchhoff (Max Delbrueck Center for Molecular Medicine, Berlin, Germany) for his helpful comments on the manuscript. This work was supported by the Fonds National de la Recherche Scientifique (FNRS), the Concerted Action of the Government of the French Community of Belgium, the Fondation Médicale Reine Elisabeth (FMRE) and the Ligue Belge de la Sclérose en Plaques. We thank P. Ernst-Gengoux for her technical support and expertise. B. Rogister and S. Belachew are, respectively, Research Associate and Research Assistant of the FNRS.
Abbreviations
-
- AMPA
-
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
-
- B104-CM
-
- B104 cell line conditioned medium
-
- [Ca2+]i
-
- intracellular calcium concentration
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- EGTA
-
- ethylene glycol-bis(β-aminoethyl ether)-N-tetraacetic acid
-
- GABA
-
- γ-aminobutyric acid
-
- GlyR
-
- glycine receptor
-
- GLYT
-
- glycine transporter
-
- HEK
-
- human embryonic kidney (cell line)
-
- HEPES
-
- N-(2-hydroxyethyl)piperazine-N′-2-ethanesulphonic acid
-
- MEM
-
- minimum essential medium
-
- OP
-
- oligodendrocyte progenitor
-
- PBS
-
- phosphate-buffered saline
-
- VGCC
-
- voltage-gated calcium channels




