Death-signalling cascade in mouse cerebellar granule neurons
Abstract
Molecular mechanisms of neuronal cell death are still largely unknown. In the present study, the signal transduction pathway of cell death in cerebellar granule neurons was examined by employing various death-preventative agents. When death was induced by the depletion of serum and a depolarizing level of potassium, transient increase in active c-Jun, mitochondrial membrane potential (Δψ) loss, activation of caspase-3 (-like) proteases, and nuclear condensation and fragmentation were observed. The protein synthesis inhibitor cycloheximide blocked all these phenomena, whereas RNA synthesis inhibitor actinomycin-D, survival factor such as insulin-like growth factor-1, brain-derived neurotrophic factor, high K+ (25 mm) and overproduced antiapoptotic protein Bcl-2, prevented Δψ loss, caspase activation, and nuclear change, but not an increase in active c-Jun. The caspase inhibitor z-Asp-CH2-DCB (carbobenzoxy-l-aspartyl-α-[(2,6-dichlorobenzoyl) oxy]methane) only inhibited activation of caspases and nuclear change. These results suggest that the death signal in cerebellar granule neurons is sequentially transduced in the order of c-Jun activation, de novo RNA synthesis, mitochondrial Δψ loss, activation of caspase-3 (-like) proteases and nuclear change.
Introduction
Cell death is involved in a variety of biological events including development and maintenance of tissue homeostasis. Dis-regulation of cell death leads to various diseases in multicellular organisms (Vaux 1993;Rudin & Thompson 1997). In the mammalian nervous system, naturally occurring programmed cell death seems to be required to form and refine the neuronal circuit (Raff et al. 1993). Following the emergence of functional neural networks, sustenance for neurons is required in order to maintain the nervous system because most mature neurons cannot proliferate. Inappropriate neuronal cell death results in disruption of the nervous system, as observed in neurodegenerative diseases (White 1996) or traumas including ischaemia (Choi & Rothman 1990). To understand the mechanisms underlying the emergence and maintenance of functional neural networks, the investigation of the molecular mechanisms regulating neuronal cell death and survival is crucial.
At least a part of molecular machinery regulating cell death is phylogenically conserved (Vaux 1993). Genetic studies of programmed cell death in the nematode Caenorhabditis elegans have pointed to 11 genes involved in completing this process (Ellis et al. 1991). Three of 11 genes control the onset of the death: two ced-3 and ced-4 genes are required to execute death, and the third gene ced-9 plays a part of guardian to death (Hengartner & Horvitz 1994). Mammalian homologues of ced-3 and ced-9 are the genes for the caspase family and the Bcl-2 family, respectively, both of which have been shown to be involved in the regulation of mammalian cell death (Horvitz et al. 1994;Kumar & Harvey 1995;Kroemer 1997).
The caspases are the cysteine proteases which cleave their substrates at the C-terminal site of specific aspartic acid (Martin & Green 1995). The caspase family, now consisting of 11 members, is divided into three subgroups from on the basis of their primary structures: caspase-1 (-like), caspase-2 (-like) and caspase-3 (-like) protease subfamilies (Alnemri et al. 1996). Caspases are translated in inactive precursor forms and are thought to be activated by specific proteolytic cleavage, and their activation is likely one of the critical steps in the death-signalling transduction pathway (Kumar & Harvey 1995). In particular, caspase-3 (-like) protease activation is observed in all kinds of apoptotic cell death thus far analysed and the cell death is prevented by caspase-3 (-like) protease inhibitors, suggesting that caspase-3 (-like) proteases are involved in cell death (Kuida et al. 1996;Faleiro et al. 1997).
The Bcl-2 family has among its constituents anticell death proteins such as Bcl-2 and Bcl-xL and pro-cell death proteins like Bax and Bak (Merry & Korsmeyer 1997). Inasmuch as Bcl-2 suppresses cell death induced by a variety of stimuli in multiple cell lineage (Tsujimoto 1996), it seems negatively to regulate a key event in the common cell death machinery on which multiple death-signalling pathways converge. We and others have recently reported that the loss of mitochondrial membrane potential (Δψ) is one of the substantial steps in death-signalling, and that Bcl-2 prevents Δψ loss (Zamzami et al. 1995;Marchetti et al. 1996;Shimizu et al. 1996), suggesting that Bcl-2 functions to maintain Δψ in preventing cell death. As Bcl-2 blocks the activation of caspases as well as Δψ loss whereas caspase-3 (-like) protease inhibitor cannot prevent disruption of Δψ (Shimizu et al. 1996), the Δψ loss seems to be upstream of the activation of caspase-3 (-like) proteases, although certain caspases were activated upstream of Δψ loss in some systems (Susin et al. 1997).
Cell death of cerebellar granule neurons induced by the depletion of serum and a depolarizing level of extracellular potassium (Thangnipon et al. 1983) shows apoptotic features such as fragmentation of nuclei and DNA (D'Mello et al. 1993;Ankarcrona et al. 1995). This death requires de novo RNA and protein synthesis (Yan et al 1994). The transcription factor c-Jun has been shown to transiently increase upon granule neuron death (Millar & Johnson 1996) and is likely to be one of the key molecules regulating neuronal cell death (Ham et al. 1995). Newly transcribed genes through c-Jun activation might commit to pursuit the death process. Previous studies demonstrated that granule neuron death is inhibited by high concentrations of potassium (Gallo et al. 1987), insulin-like growth factor-1 (IGF-1) (D'Mello et al. 1993), brain-derived neurotrophic factor (BDNF) (Kubo et al. 1995), or overproduced Bcl-2 protein (Tanabe et al. 1997). In addition, several groups have recently reported that caspases are involved in the death of cerebellar granule neurons induced by depletion of serum and a depolarizing level of potassium (Schulz et al. 1996;Armstrong et al. 1997;Ni et al. 1997). Prevention by Bcl-2 and involvement of caspases in the death of cerebellar granule neurons implies the existence of a common process of intracellular cascade of death signalling in neurons as well as cells of other lineages.
Despite accumulating information about individual events which occur during neuronal cell death, the sequential order of these events has not yet been fully elucidated. As a model of neuronal cell death, we have characterized cell death in cerebellar granule neurons depleted of serum and a depolarizing level of potassium, utilizing several anticell death agents.
Materials and methods
Mice
Mice overexpressing the human bcl-2 gene under the control of neuron-specific enolase promoter (line 57) on a C57BL/6J background have been described previously (Martinou et al. 1994). The C57BL/6J mice purchased from CLEA (Tokyo, Japan) were also used in this study. The care of all mice used in this study was in compliance with the Osaka University Medical School Guideline for the Care and Use of Laboratory Animals.
Purification of cerebellar granule neurons
Cultures enriched in granule neurons were obtained from dissociated cerebella of 7-day-old bcl-2 transgenic mice and their littermates, or C57BL/6J mice, as described previously (Tanabe et al. 1997). Removed cerebella were treated with 1 mg/mL trypsin and 12.5 μg/mL DNase I, triturated in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) (Bio-Whitakker, Walkersville, MD, USA), and recovered cells plated at a density of 2.5 × 105 cells/cm2 on a 35 mm dish, in which 18 × 18 mm cover glasses coated with poly-d-lysine were placed where indicated. The culture medium was replaced with DMEM containing 10% FBS supplemented with 20 mm KCl (final KCl concentration is 25 mm) 5 h after seeding the cells. To reduce non-neuronal cells, cytosine arabinofranoside (10 μm) was added to the culture medium 24 h after plating. In using bcl-2 transgenic mice and their littermates, each removed cerebellum was treated separately because genotypes were not known at the time of dissection. The genotype of each cerebellum was determined as described previously (Tanabe et al. 1997). Cells from C57BL/6J mice were prepared from several cerebella together. Granule neurons were matured and differentiated for 7 days in vitro (Gallo et al. (1987);Tanabe et al. 1997), and then used in the experiments.
Treatment of cultures and quantification of cell death
Cell death was induced by exchanging DMEM containing 10% FBS with high K+ (25 mm KCl) to serum-free DMEM with low K+ (5 mm) on day in vitro 7 or 8. Actinomycin-D (Act-D) (Wako, Osaka, Japan), cycloheximide (CHX) (Wako, Osaka, Japan), z-AspCH2-DCB (carbobenzoxy-l-aspartyl-α-[(2,6-dichlorobenzoyl)oxy]methane) (z-D) (Peptide Institute, Mino, Osaka, Japan), IGF-1 (Boehringer Mannheim, Germany) and BDNF (kindly provided by Sumitomo Pharmaceutical Co., Osaka, Japan) were added directly to serum-free DMEM with low K+. Serum-free DMEM with high K+ (high K+) was prepared by supplementing DMEM with 20 mm KCl (final concentration of KCl is 25 mm). In using z-D, cells were preincubated with the inhibitor for 2 h before death induction. To quantify cell death, live and dead neurons were counted under a phase-contrast microscope. Live and dead neurons were distinguished by round cell bodies with complex neurites and bright pyknotic small cell bodies with fragmented or disintegrated neurites, respectively. Morphological nuclear changes, such as condensed and fragmented nuclei, were also used to quantify the viability of cells under a fluorescence microscope as described below.
Western blotting
Cerebellar granule neurons seeded on to a 35-mm dish, were washed with phosphate-buffered saline (PBS) twice and lysed with extraction buffer (50 mm Tris–HCl, pH 7.4, 1% sodium dodecyl sulphate, 100 mm NaCl, 20 mm EDTA). After boiling the lysates for 5 min, 20 μg of total protein were separated by 12.5% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and analysed by Western blotting using anti-c-Jun monoclonal antibody which reacts with only c-Jun phosphorylated at Ser63 (Santa Cruz, CA, USA).
Fluorescence microscopy
Mitochondrial membrane potential (Δψ) and morphological changes of nuclei were examined by fluorescence microscopy. Cerebellar granule neurons seeded on 18 × 18 mm cover glasses were stained at the indicated times with Rhodamine 123 (10 μm) (Molecular Probes, Eugene, OR, USA) and Hoechst 33342 (10 μm) (Calbiochem, La Jolla, CA, USA) for 15 min, then analysed for Δψ by fluorescence microscope (Leica LSM410) with excitation at 488 nm and emission at 525 nm, and for nuclear morphology by fluorescence microscope (Olympus BHS-RFL-LSM) with excitation at 360 nm and emission at 420 nm, respectively. Δψ loss after death induction was quantified as follows. The control culture without death induction was exposed to serum-free DMEM with Rhodamine 123 for 15 min, and the fluorescent intensities of five randomly chosen fields (136 mm2 for each field) were averaged and regarded as a standard (= 1). Cells were then incubated under death-inducing conditions in the presence or absence of anticell death agents; five fields were chosen at each time point, and ratios of averaged intensity of Rhodamine 123 in the fields to the standard were calculated.
Measurement of caspase-3 (-like) protease activity
Cerebellar granule neurons were washed three times with PBS and collected in the assay buffer (50 mm Tris-HCl pH 7.4, 10 mm EGTA, 1 mm EDTA), then incubated with 1 μm digitonin for 10 min at 37 °C to recover cytosolic proteins. Cytosolic proteins (10 μg) was incubated with 100 nmol Ac-DEVD-MCA (Peptide Institute, Mino, Osaka, Japan) in 500 μL of assay buffer at 37 °C for 60 min. The fluorescent 7-amino-4 methylcoumarin (AMC) product was measured with excitation at 380 nm and emission at 460 nm by fluorescence spectrophotometer (Hitachi F-4500). One unit was defined as the amount of enzyme required to release 1 pmol AMC/min at 37 °C.
Statistical analysis
The non-parametric Kolmogorov–Smirnov test were used to compare differences between two assays of cell death. In the experiments of mitochondrial Δψ, a two-way factorial analysis of variance was done to test for association between treatments and time after cell death induction. Scheffe's post hoc multiple comparisons test was done for a more detailed analysis.
Results
Induction of cerebellar granule neuron death by low K+ and serum depletion
To investigate the intracellular events occurring during neuronal cell death, cerebellar granule neurons were used as a model system, in which neuronal cell death was induced by depletion of a depolarizing level of potassium and serum because it is considered to mimic physiological death of those neurons (Gallo et al. 1987). Cerebellar granule neurons were prepared from bcl-2 transgenic mice and their littermates or C57BL/6J mice, as described in the Materials and methods. Neurons were cultured in the presence of a depolarizing level of K+ (25 mm) included in DMEM with 10% FBS for 7 days. Under these conditions, the neurons differentiated and matured into round soma with complex networks of neurites (Gallo et al. 1987). The bcl-2 transgenic mice-derived neurons matured and differentiated in vitro similarly to those derived from their littermates and C57BL/6J mice as reported previously (Tanabe et al. 1997). Differentiated cerebellar granule neurons in vitro underwent cell death upon reducing extracellular potassium concentration from high (25 mm) to low (5 mm) (Gallo et al. 1987). Because changing to a fresh medium containing 10% FBS was toxic to matured cerebellar granule neurons regardless of the potassium concentrations (Schramm et al. 1990), FBS was omitted from the fresh medium when lowering the potassium concentration throughout this study.
Effects of death-preventative agents on death of granule neurons
As the previous studies have shown that pyknotic cells and condensed/fragmented nuclei were observed when cerebellar granule neurons were treated by the depletion of serum and a depolarizing level of potassium (Ankarcrona et al. 1995;Tanabe et al. 1997), the viability of cerebellar granule neurons was assayed by counting living and dead cells under a phase-contrast microscope and also by counting normal and apoptotic (condensed and fragmented) nuclei staining with Hoechst 33342 under a fluorescence microscope. More than 80% of neurons incubated for 24 h in serum-free DMEM with low K+ showed bright pyknotic soma with disintegrated neurites under a phase-contrast microscope, and about 90% of these neurons exhibited condensed or fragmented nuclei as stained with Hoechst 33342, which are typical features of apoptosis (Fig. 1A,B). There was no statistically significant difference between the two assays (P = 0.0649, not significant), although nuclear changes appeared to be slightly faster than the destruction of neuronal soma and neurites. We then examined the effects of death-inhibitors on cellular and nuclear morphological changes (Fig. 1B). A transcriptional inhibitor Act-D (1 μg/mL) and a protein synthesis inhibitor CHX (10 μg/mL) rescued about 90% of neurons from death, IGF-1 (25 ng/mL) and BDNF (50 ng/mL) rescued about 55%, high K+ (25 mm KCl) about 85%, and overproduced Bcl-2 about 65%, assayed by both the appearance of neurons under a phase-contrast microscope and nuclear morphology under a fluorescence microscope (Fig. 1A,B). There were no significant differences between the two viability assays. These results suggest that Act-D, CHX, IGF-1, BDNF, high K+ and Bcl-2 function as cell death inhibitors upstream of the apoptotic nuclear and cytoplasmic changes in the death-signalling cascade. In contrast, neurons treated with z-D (100 μm), a general inhibitor against caspases, showed almost the same appearance and viability (less than 10%) as control neurons 24 h after death induction under a phase-contrast microscope, whereas more than 90% of nuclei exhibited normal features, but some were slightly smaller (Fig. 1A,B), suggesting that z-D functions upstream of nuclear changes in the death-signalling cascade, and cytoplasmic changes require other signal(s) which is not blocked by z-D. zVAD-fmk, another general caspase inhibitor, showed very similar effects to z-D (data not shown).
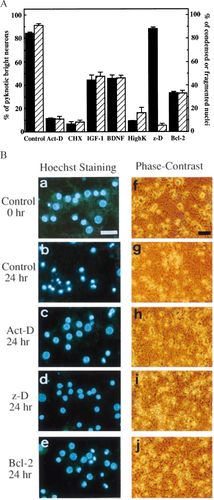
, BDNF, high k+ or z-D as described in Materials and methods. The bcl-2 transgenic mouse-derived neurons were used to examine the effect of Bcl-2. (A) Percentage of apoptotic cells was determined at 24 h by phase-contrast microscopy (filled column) and by fluorescence microscopy with Hoechst staining (hatched column). Results are expressed as mean ± SD. (B) Appearances of nuclei (a–e) and neurons (f–j) examined by a fluorescence microscope after staining with Hoechst 33342 and by a phase-contrast microscope respectively. (a,f) Normal (control) neurons at 0 h. (b,g) Control neurons at 24 h. (c,h) Act-D treated neurons at 24 h. (d,i) z-D treated neurons at 24 h. (e,j) bcl-2 transgenic mouse-derived neurons at 24 h. Scale bars: white bar, 25 μm (a–e); black bar, 50 μm (f–j).
Cycloheximide blocks transient increase of phosphorylated c-Jun during the early phase of the death-signalling cascade in cerebellar granule neurons
To analyse the intracellular death-signalling pathway preceding morphological changes, we initially examined the level of c-Jun expression in cerebellar granule neurons incubated under death-inducing low K+ and serum-free conditions in the presence or absence of various death-preventative agents (Fig. 2). The monoclonal antibody used here recognizes only c-Jun phosphorylated at Ser63 which is an active form of c-Jun protein. In control neurons, the amount of phosphorylated c-Jun protein increased during the 3 h incubation after death induction and then gradually decreased to an undetectable level in 24 h. We also examined cyclin D1 and CDK4 by Western blot analysis, but no significant change was observed (data not shown), consistent with their mRNA analysis in a previous report (Millar & Johnson 1996). When neurons were treated with CHX, no significant increase in phosphorylated c-Jun was observed at any indicated times. Act-D did not inhibit, but slightly retarded, the increase in phosphorylated c-Jun protein. Because only a small amount of c-Jun protein was detected in untreated cerebellar granule neurons by Western blot analysis using an antibody which recognizes both inactive and active c-Jun protein (data not shown) and CHX but not Act-D inhibits the increase of active c-Jun, the increase in the amount of active c-Jun is probably due to translation from pre-existing mRNA rather than the phosphorylation of pre-existing inactive c-Jun. IGF-1, BDNF and high K+ treated neurons and neurons overexpressing Bcl-2 also did not inhibit the increase of active c-Jun, although some differences in their activation/inactivation profiles were reproducibly observed (Fig. 2). The expression pattern of c-Jun in z-D treated neurons was almost same as that of control neurons (Fig. 2). These results suggest that CHX functions upstream of the c-Jun activation, while the action sites of Act-D, IGF-1, BDNF, high K+, Bcl-2 and z-D were downstream of the c-Jun activation.
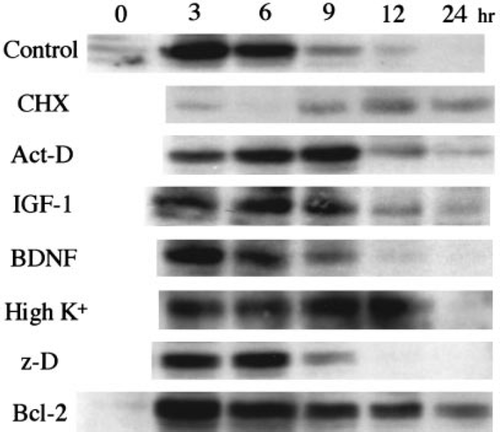
4 h after death induction, then analysed by Western blotting using anti-c-Jun phosphorylated at Ser63 monoclonal antibody, as described in Materials and methods.
Cycloheximide, actinomycin-D, insulin-like growth factor-1, brain-derived neurotrophic factor, high K+ and Bcl-2 prevent loss of mitochondrial membrane potential (Δψ)
The loss of Δψ has been reported to be associated with apoptosis and to be prevented by Bcl-2 (Zamzami et al. 1995;Marchetti et al. 1996;Shimizu et al. 1996). However, mitochondrial Δψ loss during the death process has not been well studied for cerebellar granule neurons. We next examined Δψ in cerebellar granule neurons undergoing death by measuring Rhodamine 123 intensity (Fig. 3). Rhodamine 123 is a lipophilic cation taken up to mitochondria in proportion to mitochondrial Δψ (Johnson et al. 1981). Control neurons reduced mitochondrial Δψ between 3 and 6 h after death induction. Neurons treated with Act-D or CHX maintained Δψ in both soma and neurites until 24 h after death induction. High K+, Bcl-2, IGF-1 and BDNF partially prevented the reduction of Δψ, and the extent of prevention appeared to be proportional to their efficiency in inhibiting cell death; this suggested that high K+, Bcl-2, IGF-1 and BDNF maintained Δψ in preventing cell death. There was no statistically significant difference between z-D treated neurons and control neurons in the reduction of Δψ at any time points. The Δψ measured using TPP+ (Brand 1995) and JC-1 (Reers et al. 1995), both of which are also lipophilic cations taken up to mitochondria in proportion to mitochondrial Δψ, gave results consistent with those using Rhodamine 123 (data not shown). These results suggest that the functional sites of all death-preventative agents used in the study, except for z-D, are upstream of Δψ loss in the death-signalling pathway.
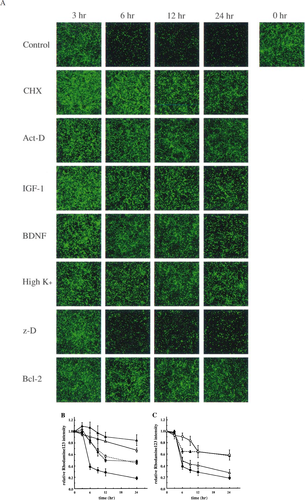
, P < 0.0001). Scheffe's post hoc multiple comparisons test to determine where treatment differences actually occurred showed that differences between control and z-D were not significant but others are significantly different from the control. Symbols in panel B: filled circle, control; open triangle, Act-D; filled triangle, CHX; open square, IGF-1; filled square, BDNF. Panel C: filled circle, control; filled triangle, high K+; open triangle, z-D; open circle, Bcl-2.
Cycloheximide, actinomycin-D, insulin-like growth factor-1, brain-derived neurotrophic factor, high K+, Bcl-2 and z-D inhibit activation of caspase-3 (-like) protease activity
Recently, Armstrong et al. 1997) have demonstrated that caspase-3 was activated in cerebellar granule neurons undergoing apoptosis induced by the depletion of serum and a depolarizing level of potassium. To examine whether death-preventative agents function upstream or downstream of caspase-3 (-like) protease activation, we analysed the preventative effects of these agents on the caspase activation after low K+ and serum withdrawal. We measured the caspase-1 (-like) and −3 (-like) activities using fluorogenic tetrapeptide substrates, Ac-YVAD-MCA and Ac-DEVD-MCA, respectively. In control neurons, the Ac-DEVD-MCA cleaving activity was observed from 2 h after death induction, increased by 6–9 h, and then decreased by 24 h whereas no Ac-YVAD-MCA cleaving activity was observed (Fig. 4A and data not shown). The time course of caspase-3 (-like) protease activation was coincident with the previous report by Armstrong et al. 1997). On the other hand, all death-preventative agents used here suppressed, in varying degrees, the increase in Ac-DEVD-MCA cleaving activity. The CHX and z-D almost completely inhibited activation of caspase-3 (-like) proteases. Effective prevention was observed in Act-D, high K+ treated neurons as well as neurons overproducing Bcl-2, whereas the preventative effects of IGF-1 and BDNF were partial such that about 30% of activity in control neurons was detected at 3, 6, 9 h in IGF-1 or BDNF treated neurons (Fig. 4A,B). The extents of suppression for caspase-3 (-like) protease activation by these agents, except for z-D, correspond to their preventative efficacy on both the nuclear changes and cell death. These results suggest that all the agents used here act upstream of or at the activation step of caspase-3 (-like) proteases.
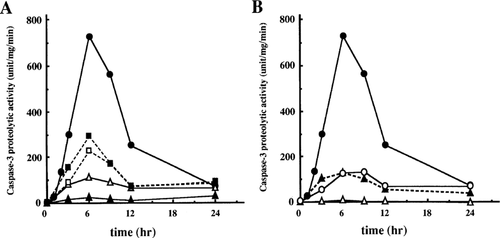
Effects of death-preventative agents on the induction of caspase-3 (-like) protease activity in death-induced neurons. Cerebellar granule neurons were incubated in serum-free Dulbecco's modified Eagle's medium with low K+ in the presence or absence of various death-preventative agents. Cell lysates were prepared at the indicated times after the induction of cell death, and caspase-3 (-like) protease activity was measured using Ac-DEVD-MCA as described in Materials and methods. Symbols in panel A: filled circle, control; open triangle, Act-D; filled triangle, CHX; open square, IGF-1; filled square, BDNF. Panel B: filled circle, control; filled triangle, high K+; open triangle, z-D; open circle, Bcl-2. Representative data in at least three independent experiments are shown.
Discussion
Recent studies have demonstrated that cell death induced by the depletion of serum and a depolarizing level of potassium in cerebellar granule neurons requires: (i) de novo RNA and protein synthesis (Kubo et al. 1995); (ii) increase in c-jun mRNA (Millar & Johnson 1996); and (iii) activation of caspase-3 (-like) protease(s) (Ni et al. 1997). In addition, we and others have previously reported that granule neuron death induced as above was prevented by Bcl-2 (Tanabe et al. 1997), IGF-1 (D'Mello et al. 1993), BDNF (Kubo et al. 1995) or a depolarizing level of potassium (Galli et al. 1995). In the present study, we confirmed the previous observations and also showed that cerebellar granule neurons undergoing cell death by low K+ and serum depletion are accompanied by a transient increase in phosphorylated c-Jun and loss of Δψ. To determine the sequential order of these events, we used a variety of neuronal death-preventative agents such as RNA synthesis inhibitor Act-D, protein synthesis inhibitor CHX, depolarizing level of potassium (25 mm KCl) (high K+), caspase family inhibitor z-D, and survival factors as IGF-1, BDNF and Bcl-2. Assuming that reactions downstream of the functional step of each inhibitor do not proceed, it is plausible to determine the order of the events comparing the effects of each inhibitor on the death-associated events described above together with the information from time course analysis.
Previous reports showed that mRNA level of transcription factor c-Jun increases upon neuronal cell death (Millar & Johnson 1996), c-Jun protein is essential for pursuing death in neurons (Ham et al. 1995) and it triggers apoptosis in fibroblast (Bossy-Wetzel et al. 1997). In control cerebellar granule neurons, the active c-Jun protein increased. CHX suppressed the increment whereas other treatments did not, and some of those delayed subsequent decrease in phosphorylated c-Jun, suggesting that c-Jun activation requires protein synthesis, and all death-preventative agents except for CHX function downstream of c-Jun activation in the death-signalling transduction pathway. As c-Jun is a transcription factor and Act-D does not prevent the activation of c-Jun, de novo gene expression activated by c-Jun, which is inhibited by Act-D, appears to be involved in the neuronal cell death machinery. Although c-Jun was shown to be used as a survival signal transducer in some cases (Herdegen et al. 1997), the c-Jun protein is detected slightly in neurons under culture conditions with a depolarizing level of potassium (DMEM plus 10% FBS with 25 mm KCl), so that the survival signal from high K+ treatment is not likely to use c-Jun as a survival signal itself. High K+ treatment also does not seem to interfere with the increase in phosphorylated c-Jun by death-signalling, indicating the action site of high K+-derived signal is downstream of c-Jun activation. In the presence of IGF-1 or BDNF, activation of c-Jun was also observed, suggesting that IGF-1 and BDNF-derived signals function downstream of c-Jun activation as in the case of high K+ treatment. However, it is also possible that the increased c-Jun was derived from dying cells which escaped the death-preventative effect of IGF-1 and BDNF because of their partial protection from the death of cerebellar granule neurons. In this case, neurons living by the action of IGF-1 or BDNF might not activate c-Jun. As it was reported that c-Jun is also up-regulated by IGF-1 (Monnier et al. 1994) or BDNF (Gaiddon et al. 1996;Courtney et al. 1997) as well as in regenerating neurons (Herdegen et al. 1997), the possibility was not excluded that IGF-1 and BDNF use c-Jun as a survival signal.
It has been reported that the disruption of Δψ is a crucial step in death cascade in other systems (Zamzami et al. 1995;Shimizu et al. 1996). We found that mitochondrial Δψ was also reduced in cerebellar granule neurons undergoing death. CHX and Act-D maintained Δψ in both soma and neurites until 24 h, while the time course of Δψ reduction in z-D treated neurons was similar to that of control neurons. High K+, Bcl-2, IGF-1 and BDNF partially prevented Δψ loss, and their efficiency was correlated with the death-preventative competency. These results suggested that all death-preventative agents, except for z-D, act upstream of Δψ loss, whereas z-D acts downstream.
One of the phylogenically conserved steps in death cascade is the activation of caspases (Martin & Green 1995). Control neurons showed activation of caspase-3 (-like) proteases while all death-preventative agents inhibited their activation. As the preventative efficiencies for the activation were well correlated with those for cell death assessed by phase-contrast microscopic analysis in all death-preventative agents except for z-D and corresponded to those for apoptotic nuclear changes in all those agents, this suggested that all the agents act upstream of at the activation of caspases in the death-signalling pathway.
Morphological change of nuclei is one of the features of apoptosis. We previously reported that cerebellar granule neurons in a serum-free medium with low K+ for 24 h revealed condensed and fragmented nuclei (Tanabe et al. 1997). The percentage of condensed and fragmented nuclei per total nuclei agreed well with that of pyknotic cells in neurons treated with all death-preventative agents, except for z-D treated neurons at 24 h. The broad caspase inhibitor z-D blocked nuclear condensation almost completely (< 10% apoptotic nuclei in total), whereas it did not protect the degeneration of soma and neurites (> 90% pyknotic neurons in total). These results indicate that CHX, Act-D, IGF-1, BDNF, high K+ and Bcl-2 prevent apoptotic changes of both nuclei and cytoplasm, whereas z-D inhibits only nuclear condensation and fragmentation but not cytoplasmic destruction. One possible explanation is that z-D does not inhibit the activation of all caspase family proteases, some of which are involved in cytoplasmic destruction. Ac-DEVD-CHO, a caspase-3 (-like) protease specific inhibitor, protected neither nuclear nor cytoplasmic changes in spite of their partial preventative effect on caspase-3 (-like) protease activity in vitro (results not shown); this suggested that caspases other than caspase-3 (-like) proteases or more than one caspase might be involved in nuclear destruction in this cell death, although it is possible that Ac-DEVD-CHO was not effective due to less membrane permeability than that of z-D. Alternatively, another signal derived downstream of Δψ loss may entail the destruction of cytoplasm and cell membrane. This is consistent with the recent report that the inhibition of caspases blocked nuclear change but not cytoplasmic change and cell death in fibroblasts (McCarthy et al. 1997).
From the results shown above, we conjectured the signal transduction pathway of death in cerebellar granule neurons could be as follows: (i) transient activation of c-Jun; (ii) de novo RNA synthesis; (iii) reduction of Δψ; (iv) activation of caspase-3 (-like) proteases; and (v) nuclear condensation and fragmentation (Fig. 5). The neuronal cell death signalling cascade described above indicates that a part of the cascade (Δψ loss to nuclear change) is shared with other cell death systems demonstrated previously (Zamzami et al. 1995;Marchetti et al. 1996;Shimizu et al. 1996). Furthermore, the death signalling is transduced from cell-lineage-/death-stimuli-peculiar events (i.e. transient c-Jun activation and de novo RNA/protein synthesis) to cell death-general events (i.e. Δψ loss, caspase activation and nuclear change), implying that cell lineage- or death-stimuli-specific signals converge into the common pathway. The signal for cytoplasmic change seems to begin with mitochondrial Δψ loss or activation of caspases which are not blocked by caspase inhibitor z-D.
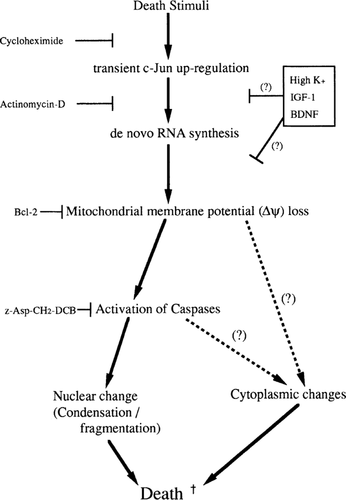
Model of death-signalling cascade in neurons. Scheme was established from the integrated results of the present study. Predicted functional site(s) of death-preventative agents are also shown. Possible signalling pathways involved in cytoplasmic change are discussed in the text.
In other cell death systems, several molecules are supposed to link the events described above. AIF (apoptotic inducing factor), released from mitochondria upon Δψ loss, induces apoptotic changes of nuclei (Susin et al. 1996, Susin et al. 1997) and activation of caspases (Susin et al. 1997). Cytochrome c is released by death stimuli from mitochondria to cytoplasm, and activates caspase-3 (-like) proteases in the presence of cytoplasmic factors and dATP (Kluck et al. 1997;Yang et al. 1997). DFF (DNA fragmentation factor) is cleaved by caspase-3, then translocates to nuclei to trigger DNA ladder formation (Liu et al. 1997). To understand the precise molecular mechanism of neuronal cell death, analyses on the molecules and/or reactions which link each event described here in neurons, including the three molecules mentioned above, are essential.
The scheme of neuronal death signalling cascade which we have proposed in the present study is an important step toward not only understanding the precise death-signalling cascade in neurons but also for developing a strategy to treat neurodegenerative diseases or brain injuries such as ischaemia.
Acknowledgements
We are indebted to Drs C. Nakayama and T. Takino (Sumitomo Pharmaceutical Co.) for recombinant human BDNF protein. We also thank Dr D.W. Rackham for the proof-reading in English. This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science and Culture of Japan.




