‘Heaven’ for serpents? A mark–recapture study of tiger snakes (Notechis scutatus) on Carnac Island, Western Australia
Abstract
Abstract Animals resident on small islands provide excellent opportunities to carry out detailed mark–recapture studies. Populations are closed and ecosystems are often simpler than those of mainland sites. These factors enable the study of cryptic species that have otherwise been neglected. Snakes are notable for their secretive nature and, as a result, few natural populations have been accurately described through long-term mark–recapture monitoring. A population of tiger snakes (Notechis scutatus) was studied on Carnac Island, a small limestone island (16 ha) off the coast of Western Australia. Population estimates show that snake density is very high, with more than 20 adult snakes per ha. This equates to a biomass of more than 100 kg of a top predator concentrated in a very small area. Such a high predator density can be explained because adult snakes feed mainly on chicks of nesting birds that breed in large colonies on Carnac but forage elsewhere. Substantial annual growth rates in body size in most individuals suggest that food availability is high on Carnac. Growth rates decreased more sharply in adult females than in males, whereas annual changes in body mass were similar in both sexes, probably because of the high energetic costs of reproduction experienced by females. Surprisingly, the sex ratio was highly biased, with males largely outnumbering females.
Introduction
The complex structure of most biological populations can make accurate general studies on population dynamics difficult to achieve, even at the species level. However, these types of studies provide much of the fundamental basis for investigating hypotheses relating to physiology and evolutionary biology, as well as for conservation. Because many animal species are very secretive, precise data on population size and population structure, although essential, are often not available.
To cope with the complexities of population biology, investigators can select simple biological systems where population parameters are relatively easy to identify and quantify. The information gathered can then serve as a basis for understanding more complex situations. Simple systems provide the opportunity to understand better the interaction between the studied species and other taxa within the habitat. Nonetheless, even in favourable systems, collection of data sets on vertebrate populations may take several years. It is largely for this reason that studies of population structure and population dynamics have concentrated on a few vertebrate taxa that generally exhibit gregarious behaviour during reproduction, such as colonial birds, and some mammal, lizard and amphibian species. Conversely, taxonomic bias exists against some groups that are notoriously difficult to study, such as snakes (Seigel & Collins 1993; Shine & Bonnet 2000). Most population ecology studies to date have focused on a few Northern American and European snake species (Fitch 1999; Madsen et al. 2000) but not exclusively (for example see Shine 1991; Sun et al. 2000). However, most extant snake taxa are found in tropical countries and yet the population dynamics of these species have received little attention. South America, Africa, South-East Asia and Australia contain most of the taxa of snakes; however, published data based on mark–recapture projects are still very scarce for these regions.
Within Australia specifically, with the exception of very few species (two pythons, two elapids and one acrochordid; Schwaner 1985; Shine 1991; Shine & Madsen 1997; Webb & Shine 1998; Madsen & Shine 2000a,b), detailed investigations on the structure of snake populations are not available in the literature. This is despite the fact that the continent supports approximately 170 species. In fact, there is a virtual absence of published population data for snakes occurring in the largest state, Western Australia. There are several reasons for this, foremost of which is likely to be the apparent low densities of many species, the cryptic habits of many others and the elusive nature of snakes in many environments (Seigel & Collins 1993). Other reasons include the difficulty of conducting suitable trapping regimes, such as those typically employed for population studies on most mammals and birds; or the low ‘academic payoff’ when studying ectothermic vertebrates (Bonnet et al. 2002). Reptiles such as snakes, however, can be useful model organisms for this type of research because of their pivotal roles in many ecosystems (for example, Pough 1980; Shine & Madsen 1997). Furthermore, their generally sedentary nature removes many of the factors that confound most population studies (i.e. emigration or immigration often confuse issues such as relationships between reproductive success and population density).
The purpose of this paper is to report on the results of a 3-year mark–recapture study to describe the population size and structure of the Western tiger snake, Notechis scutatus occidentalis, on Carnac Island, 8 km south-west of the port of Fremantle in Western Australia. Carnac Island provides a useful environment for investigations into population structure and dynamics of this large venomous elapid because on Carnac, tiger snakes are abundant, easy to capture and docile.
METHODS
Study area and species
Carnac Island is one of the many continental islands forming a dissected chain parallel to the west coast of Western Australia, from Penguin Island in the south to the Houtman Abrolhos in the north. It is one of only two islands in Western Australia that is known to support a population of tiger snakes, the other being Garden Island. Carnac is a small island (16 ha) consisting of a low limestone plateau up to 20 m a.s.l. largely covered by white sands. It has relatively homogenous low vegetation and little relief (Rippey & Rowland 1995).
The faunal assemblages of Carnac have been dynamic and influenced by human activities. Of the five colonial nesting seabirds that utilize the island, the Silver Gull (Larus novaehollandiae) is the most abundant, with an estimated 3000–4000 breeding pairs in 1981 (Lane 1979; Dunlop & Storr 1981). Gull numbers are increasing around Perth in response to the availability of food at domestic rubbish disposal sites (Dunlop & Storr 1981) and thus constitute a significant portion of the island's biomass. Terrestrial vertebrate fauna on Carnac include the King skink, Egernia kingii, the gecko Christinus marmoratus, the small skink Morethia obscura, and the introduced house mouse, Mus domesticus. Frogs are the staple dietary item of tiger snakes on the mainland (Shine 1987; Cogger 1992; Bush et al. 1995; X. Bonnet, pers. obs.) but there are no frogs on Carnac, because of the lack of permanent or intermittent water bodies.
The origin of tiger snakes on Carnac Island remains unclear. It is possible that the population may be derived, at least in part, from individuals marooned by the rising sea level 5000–7000 years ago when Carnac was separated from the mainland. Alternatively, given that tiger snakes are proficient swimmers, founder individuals may have reached the island on their own. Another origin for the population was suggested by Cann (1986), who stated that a founder population of some 80 individuals was released on the island approximately 70 years ago. Department of Conservation and Land Management files indicate that this introduction occurred in approximately 1929.
The tiger snake is a large and stout venomous elapid (Cogger 1992). Dorsal body colouration is variable but, in western populations, is typically black to dark brown, sometimes with distinct transverse dorsal yellow bands. Body size varies among broadly disjunct populations.
Adult mean total body length is approximately 80–110 cm, and body mass ranges from 200 to 850 g (Schwaner 1990; Cogger 1992). The tiger snake is viviparous, producing litters ranging in size from 10 to 40 young on average (total length = 15–24 cm, body mass = 3.3–9.5 g; Bush et al. 1995). The taxonomic status of the genus is unresolved (Cogger 1992; Scott et al. 2001), but for the purposes of this paper, Western Australian populations are referred to as Notechis scutatus occidentalis (Storr et al. 1986).
Measurements
During 31 trips to Carnac, data were gathered on 313 individual snakes, many caught repeatedly over a 3-year period during spring in the years 1997–1999. The following information was recorded:
- 1
Snout–vent length (SVL: from the tip of the snout to the subcloacal scale) to the nearest 0.5 cm.
- 2
Sex by eversion of the hemipenes.
- 3
Presence and type of prey ingested (palpation and/or regurgitation).
- 4
Reproductive status (enlarged follicles are detectable by palpation).
- 5
Presence of body or head injuries (see Bonnet et al. 1999).
- 6
Body mass to the nearest 1 g in adults and to the nearest 0.1 g in small snakes (less than 100 g).
If prey was determined to be recently ingested, or when enlarged follicles were detected, body mass was not included in the analyses. As a means of permanent identification, each snake was individually scale clipped and a silver number was painted on the back of adult snakes for short-term identification to avoid frequent recapture (i.e. within the same day). Each snake was then released at its capture site as soon as practicable (1–3 h).
Population size estimates and analyses
The island was thoroughly investigated during each searching session. Snakes were captured by hand and kept in calico bags until measured. A small number of animals (<5%) were observed but eluded capture. Between two and six people searched for varying periods of time per day (2–4 h), depending upon weather conditions (i.e. high ambient temperatures shortened the searching period because snakes could not be located or were too difficult to catch, whereas cool, cloudy days increased available daily searching time). The mark–recapture regime did not unduly disturb the snakes because released individuals were frequently observed to be basking at, or near, their capture site within 30 min of liberation. We did not observe any long-distance or rapid dispersal of snakes, which might suggest that they were trying to escape observers.
Over the course of the study, handling time for data collection decreased, with recaptured snakes processed in less than 10 min, whereas newly caught snakes required 30 min to collect basic information, such as ventral scale counts. Hence, we were able to progressively increase the area searched, from 20% of the island surveyed in 1997, to 60% in 1998 and 80% in 1999 (approximately 9% of the island is covered with very thick bushes and this area was not searched). Such increases in the study area were factored into estimates of the total population size of the island and subsequent calculations of density. Habitat was very similar in the different search areas and our analyses revealed no significant differences in the number of snakes, or snake size, among them. Because almost the entire island was surveyed in 1999 and not in other years, only population size estimates performed using 1999 data could be considered accurate. However, it was still possible to estimate the total population size on Carnac by taking into account the area searched in 1997 and 1998.
Population size was estimated using the capture program (Otis et al. 1978; 1991 release). The capture procedure assumes a closed population (i.e. no births, deaths or migration between sampling periods), and is generally used for experiments covering short periods of time (Otis et al. 1978). Our study conforms relatively well to these conditions. The Carnac tiger snake population is well isolated from any other. Population size was estimated separately for 1997, 1998 and 1999, and births did not influence our analysis because our survey periods were concentrated within 1–2 months of each year (neonates require more than 1 year to become adult). Mortality may have occurred during a survey period. However, given an annual survival rate of at least 0.7 (estimated without taking into account interannual recapture probabilities), survivorship for a much more limited period would very probably have been greater than 0.9, which is satisfactory to run capture. Sometimes, the island was surveyed during 2 (or more) consecutive days. We pooled several capture sessions to limit the inflation of the mark–recapture matrix, giving a total of nine sessions spread out through the survey period. Finally, capture provides the opportunity to test different models including heterogeneity of capture probabilities in populations (Mh), time-specific variation in probabilities of recapture (Mt), behavioural response after initial capture (Mb) and different combinations of these models. In all of the 3 years, capture detected strong effects of time and interindividual heterogeneity in the probabilities of captures (as expected for ectothermic animals). We systematically selected the model that estimated population under time variation and individual heterogeneity in capture probabilities (Mth) of Chao et al. 1992). The goodness-of-fit test module among the three with the maximal value always proposed this model. In practice, the different models proposed (Mh, Mt, Mth…) provided consistent population estimates.
Except on few occasions, the distribution of the data was normal, and the assumption of homogeneity of the variance was verified in all regressions (all P > 0.10 in all Levene's tests). Although the F-test is robust to the violation of the normality and homogeneity assumptions (Statistica 1995), we also used non-parametric tests (Mann–Whitney U-test and Kruskal–Wallis anova) when appropriate. No result was altered using non-parametric tests rather than parametric tests. Statistical analyses were performed using Statistica 5.1 and 6.0. Means are expressed with the standard deviation, except for population size estimates, which are given with their standard error.
Results
Effect of sex and body size on catchability
We captured 313 tiger snakes, of which 312 were sexed. Adults represented most of the captures (93%), whereas juveniles and neonates were rarely found (Table 1; χ2 = 479.0, d.f. = 2, P < 0.001, comparing the number of individuals of the three age-classes). The number of identified males (n = 221) was significantly greater than the number of females (n = 91; χ2 = 28.3, d.f. = 1, P < 0.001, comparing the number of individuals for each sex; and χ2 = 13.3, d.f. = 2, P = 0.001 comparing the number of males and females among the three age-classes). Such a highly biased sex ratio may occur in our data set due to lower catchability of females compared with males. Similarly, adult snakes are generally more conspicuous than any other age class, and hence may be over-represented in our data set. We tested these possibilities by comparing the catchability of snakes grouped by size and sex following the methodology of Marti (1990). We classified individuals into three discrete size categories: small (mean SVL < 58.5 cm [range 20–69.5 cm, n = 70], presumably young snakes), medium (mean SVL 82.7 cm [range 70–89.5 cm, n = 131]) and large (mean SVL > 97.9 cm [range 90–110 cm, n = 112] presumably old snakes). Because our data set incorporates mostly adult snakes, our classification did not separate the neonates and the juveniles from the relatively small adult snakes. That is, our small-class snakes (n = 70) include the small number of neonates and juveniles (n = 20) we caught and the small adults (n = 50).
| Age category | Males | Females |
|---|---|---|
| Adults | 214 | 78 |
| Juveniles | 4 | 8 |
| Neonates | 3 | 5 |
Large snakes were more readily captured than those in the medium class, which in turn were more catchable than the small ones (anova with size category and sex as the factors and the total number of captures in the year of first capture of each individual as the dependent variable, F2,306 = 8.71, P < 0.001; Fig. 1). In contrast, sex had no influence on catchability (F1,306 = 0.85, P = 0.36, interaction between size class and sex F2,306 = 0.65, P = 0.52; Fig. 1). Thus, the strong under-representation of juveniles, neonates and small adults was probably due to their more cryptic habits, but the highly biased sex ratio was probably not affected by any problem of differential catchability between the sexes.
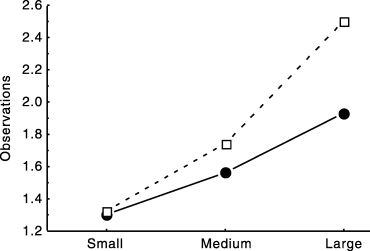
Influence of size and sex on catchability in tiger snakes. (), Males; (□), females.
Because blind and partially blind snakes occur on Carnac Island due to pecking by gulls (Bonnet et al. 1999), we also tested the possibility that snakes with a partial or total loss of vision would display more conspicuous behaviour and so be captured more frequently than other snakes. We classified the snakes into three categories: normal snakes (both eyes intact, n = 270), half blind (one eye destroyed or both eyes partly destroyed, n = 21), and fully blind (both eyes destroyed, n = 22). To take into account the effect of size on catchability, we performed an ancova with ‘eye’ category as the factor, total number of captures in the first year of capture as the dependent variable, and SVL as the covariable (F2,309 = 5.41; P < 0.005). Blind snakes were more often captured (adjusted mean number of captures per year was 2.59 ± 1.16) than partly blind snakes (adjusted mean number of captures per year was 1.92 ± 1.16), which in turn were more often captured than normal snakes (adjusted mean number of captures per year was 1.72 ± 1.33). Thus, loss of vision had an effect on the probability of capture for tiger snakes. We note, however, that the limited sample size for fully blind and partly blind snakes weakens the power of this analysis (1 − β was less than 0.4).
Population size estimates
Population size estimates were carried out separately for each year of the study (Table 2). As one might expect, the population size estimate increased with the area of the island searched (Table 2). But the area-corrected population size and hence the estimated density remained stable (Fig. 2). Overall, we estimate that approximately 300–400 adult snakes live on Carnac. The estimated snake density was 22 adult snakes per ha (range 18–29). We did not attempt to estimate the number of neonates and juveniles.
| Year | Marked individuals | Total no. captures | Population size estimate | 95% confidence interval | Area-corrected population size |
|---|---|---|---|---|---|
| 1997 | 99 | 194 | 178 ± 24 | 143–241 | 322 |
| 1998 | 153 | 277 | 281 ± 33 | 231–362 | 387 |
| 1999 | 172 | 242 | 304 ± 35 | 251–392 | 362 |
- Marked individuals provides the number of snakes newly caught within a given year (hence discounting year-to-year recaptures); the total number of captures includes captures and recaptures between the nine capture sessions per year (see text for details). Population size (±SD) was estimated using the capture program under time variation and individual heterogeneity in capture probabilities. The last two columns provide the 95% confidence interval in the population size estimate and the area-corrected population size (see text for details).
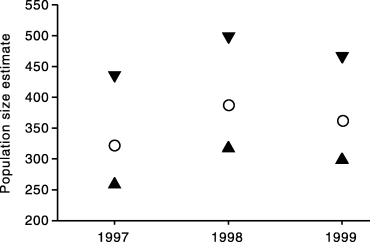
Population size estimates in tiger snakes during 3 years on Carnac Island. Estimates are given with their 95% confidence interval. Estimates are presented as ‘area-corrected’ to take into account the surface of the island searched each year (see text). (), Population estimate; (▴,▾), 95% confidence intervals.
Characteristics of the snakes
The morphological characteristics of the snakes are shown in Table 3. Males attained larger body sizes than females (anova with sex as the factor and SVL as the dependent variable; F1,309 = 46.0, P < 0.001). On average, relative to their size, males were in similar body condition to conspecific females (ancova with sex as the factor, log body mass as the dependent variable [excluding snakes with prey in the stomach and gravid females] and log SVL as the covariate; F1,162 = 0.20, P = 0.66 for the slopes; F1,163 = 2.18, P = 0.14 for the intercepts).
| Age category | SVL (cm) | Total length (cm) | Body mass (g) |
|---|---|---|---|
| Adults (n = 293) | 86.0 ± 12.2 | 100.6 ± 13.6 | 441.2 ± 168.9 |
| Juveniles (n = 12) | 45.4 ± 3.2 | 53.5 ± 3.5 | 57.7 ± 13.0 |
| Neonates (n = 8) | 28.3 ± 2.9 | 33.7 ± 3.2 | 15.8 ± 5.8 |
- SVL, snout–vent length.
Growth rate
We recaptured 83 individuals in different years for which it was possible to calculate annual growth rate. Among them, 16 were recaptured in 3 consecutive years. Most (n = 55, 66% of the total) of the snakes exhibited significant growth, from 2.0 cm to 29.0 cm per year. On average, the tiger snakes gained 5.1 ± 6.1 cm in length per year. We found a strong negative relationship between annual growth rate and SVL (r = −0.61, r2 = 0.38, F1,81 = 49.2, P < 0.00001; Fig. 3). We found a significant sexual difference in annual growth rates, with a marked decrease in annual gain in SVL in adult females (ancova with sex as the factor, annual gain (cm) as the dependent variable and SVL as the covariate; F1,790 < 0.001, P = 0.99 [slopes], F1,80 = 6.65, P < 0.013 [intercepts]). In this specific analysis, to meet the assumptions of normality and of the homogeneity in the variance, we omitted the extreme values in annual growth rate (i.e. greater than 20 cm per year) and performed this analysis again; the results remained unchanged. Deleting the 16 ‘duplicate’ snakes from analyses did not change any result.
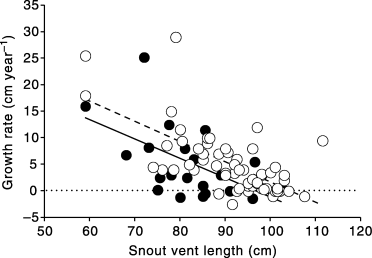
Relationship between snout–vent length and annual growth rate of Carnac Island tiger snakes. (), Females; (), Males.
Annual changes in body mass were highly and positively correlated with annual growth rate in body length (r = 0.70, r2 = 0.49, F1,61 = 58.1, P < 0.001; Fig. 4). We also found a strong negative relationship between initial SVL (and initial BM) and future changes in body mass (r = −0.55, r2 = 0.30, F1,61 = 26.5, P < 0.001). In contrast to what was observed with growth rate, females did not gain less in mass than conspecific males when initial body size was taken into account (ancova with sex as the factor, annual gain mass (g) as the dependent variable and SVL as the covariate; F1,59 = 0.32, P = 0.58 [slopes], F1,60 = 0.15, P < 0.70 [intercepts]).
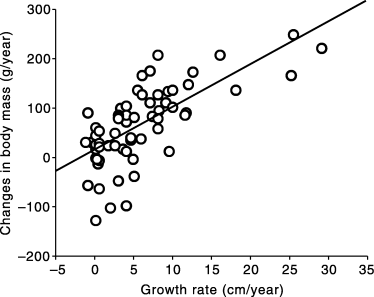
Relationship between annual growth rate and annual changes in body mass of Carnac Island tiger snakes.
Food habits
The main prey consumed by tiger snakes on Carnac have already been reported: sea gull chicks represent 83% of the prey, mice 15% and lizards 2%, respectively (Bonnet et al. 1999). Here, we focus on the influence of sex and body size on feeding frequency. For that we palpated the stomach, and/or examined the faeces of 275 different snakes (we did not include intra-annual or interannual recaptures in this analysis). Among them, 77 (28%) showed evidence of a recent meal (partially digested prey and/or fluid, brownish faeces with feathers, hairs or scales). The size of the snake did not statistically influence the probability of detecting a recent meal (logistic regression with presence or absence of prey as the dependent variable and SVL as the independent variable: χ2 = 2.42, d.f. = 1, P = 0.12), nor did we find any sex difference in this probability (53 males among 190 vs. 24 females among 84 recently fed; χ2 = 0.01, d.f. = 1, P = 0.91).
Discussion
The data presented in this paper provide strong confirmation that islands, such as Carnac, offer excellent opportunities for gathering information on animals that are otherwise notoriously difficult to study (Shine 1987; Schwaner 1989, 1991; Sun et al. 2000). In addition, small islands may well constitute primordial refuges for snakes, lizards, tortoises and other vertebrates that have been, and still are, destroyed in very large numbers on mainland areas (Main 1961; Schwaner 1985; Stanner & Mendelssohn 1987; Schwaner & Sarre 1988; Sun et al. 2000).
Our data show that Carnac Island is an unusually productive ‘heaven’ for tiger snakes, at least during the periods (spring to early summer) over which we have investigated the island (recent work, however, suggests that animals may suffer during the long and dry summer; X. Bonnet, D. Pearson, M. Ladyman, D. Bradshaw, unpubl. data). Although very small, this island supports a stable population of snakes, and the density we recorded is one of the highest reported for sedentary vertebrates (19–25 adult snakes per ha). In comparison, Schwaner and Sarre (1988) estimated densities of 4–13 snakes per ha in other island populations of tiger snakes (other ‘serpent heavens’) in South Australia. What is important about both our estimates of population size and biomass is that the tiger snakes of Carnac Island are resident animals. Therefore, the very high population density cannot be directly compared to the transitional concentrations of other animal species (i.e. African ungulates, Garter snakes) during migrations or at emergence from hibernation sites.
In terms of biomass of top predators (more than 100 kg for the 16 ha of the study area using the minimalist estimate of 250 adults weighing, on average, 400 g each), the very high density is even more surprising. It would be quite unimaginable to find a tiny island of 16 ha safely accommodating approximately 10 carnivorous mammals weighing 10 kg each. However, the very low energy requirements of ectotherms relative to endotherms render such a situation viable for snakes (Pough 1980). Notably, ectothermy allows animals to store large body reserves when food is abundant (during the peak seabird breeding season for example; Schwaner 1985), and to starve over very long periods of time if and when food becomes scarce. Hence, ectotherms have the physiological ability to exhibit a strong time uncoupling between the phases of energy gathering and the processes of energy allocation for maintenance, growth and reproduction; something usually impossible for endotherms (Bonnet et al. 1998; Schneider et al. 2000). The silver gulls that seek refuge on the island forage almost exclusively on the mainland, so this partitioning of habitat use provides important supplementary energy to the trophic system of Carnac Island (Smith & Carlile 1993a,b). A long-term study carried out on water pythons at Fogg Dam (in the vicinity of Darwin, Northern Territory) provides an example of a comparable system (Madsen & Shine 1996). Madsen and Shine (1996) reported that the relative biomass of the pythons was one of the highest for terrestrial vertebrates. Water pythons forage almost exclusively on dusky rats; again their particular physiology enables the pythons to feast during rodent proliferation and to fast over long time periods when food is scarce.
Because of their relative abundance and their large size, tiger snakes may play a key role in the Carnac Island ecosystem. In the absence of observations covering an entire yearly cycle it would be very speculative to estimate the total number of prey consumed by tiger snakes. However, they probably capture several thousand silver gull chicks per year, as well as a substantial number of lizards and mice. In support of this, many snakes were caught with one to four prey items in the stomach and most of them exhibited a substantial annual growth indicating that they feed regularly. The impact of the tiger snakes on the community of resident vertebrates of Carnac is likely to be very strong. Tiger snakes may well be opportunists, taking whatever prey is abundant; being frog-eaters on mainland wet areas and eating seabird chicks on islands.
We have already reported elsewhere the surprising phenomenon of tigers snakes blinded by seagull pecking that survive and breed ‘normally’ (Bonnet et al. 1999). In the present paper we show that the loss of vision apparently translates into differential catchability among blind, partly blind and normal snakes, supporting the premise that the better the vision, the lower the catchability. Although still requiring confirmation, such a result has not previously been documented, to our knowledge, for any animal species. Perhaps vision is used to select more suitable microhabitats, enabling the snakes to bask without exposure to potential predators? The present study has also reinforced an already documented fact; large individuals are much easier to see and to catch (Bonnet & Naulleau 1996).
In our population, female body size positively influenced fecundity (unpubl. data), thus any reduction in female growth rate would presumably decrease future reproductive success. The lower growth rate of females relative to males suggests that the energy costs of reproduction influence growth in this sex, resulting in the observed sexual size dimorphism, characterized by males attaining larger sizes (Shine 1987; Schwaner & Sarre 1988). Such sex divergence in growth rate could not be explained by sexual divergence in foraging efficiency, because the proportion of females found with prey in the stomach equalled that of males. Annual changes in body mass were not different between the sexes. Thus, the most parsimonious explanation is that females allocate a large proportion of their resources to vitellogenesis.
Other results are more puzzling. We observed a highly biased sex ratio. We do not know whether this bias is due to differential mortality of the sexes or if it is a result of clutch bias. Interestingly, Schwaner (1985) and Schwaner and Sarre (1988) reported complex and fluctuating patterns of sex ratio among different island populations of tiger snakes in South Australia and Bass Strait. However, they interpreted these fluctuating patterns as the effects of differential catchability over the seasons (i.e. gravid females bask more often) and concluded that the sex ratios were probably not skewed. Further investigations are certainly needed to determine to what extent the highly biased sex ratio we observed on Carnac Island is unique or representative of other tiger snake populations (Shine & Bull 1977).
Our data of population size and population structure for tiger snakes in Western Australia, can serve as a comparative data set for long-term monitoring of the populations of this species. Carnac snakes are larger than other tiger snakes we have measured in Western Australia. Despite the observation of Abbott (1978) describing the Carnac tiger snakes as dwarf individuals, we recorded a mean SVL of 76.1 cm (n = 32) in adult snakes from Perth lakes versus 86.0 cm (n = 293) on Carnac (P < 0.0001). They have a very peculiar diet (Bonnet et al. 1999), they exhibit a very specific defensive behaviour when harassed by humans (unpubl. data) and the island population includes numerous long-lived blind individuals (Bonnet et al. 1999). Although we cannot yet tease apart the effects of phenotypic plasticity on diet and morphology from genetic divergence among populations, the unusual characteristics of the Carnac tiger snakes justifies close, long-term scrutiny. A broad survey of the literature (see Shine 1987; Schwaner 1989, 1990, 1991; Schwaner & Sarre 1990; and references therein) suggests that each area (i.e. each island) studied in the past supports a unique population of tiger snakes in terms of body size, population density, sex ratio, diet, catchability and probably many other features despite the virtual absence of genetic variation throughout the whole range of populations from eastern to western Australia (S. Keogh, pers. com.). Such a situation highlights the potential for comparison between all life history traits of mainland snakes. Most notable is the potential to study the effects of phenotypic plasticity on life history traits over a very wide range of ecological situations.
Acknowledgements
Long-term population studies are simply not possible without the help of volunteers. Thus we would like to thank W. Gibb for taming the wild seas, R. Cambag and D. Philippe for technical support. Other volunteers and data scribes include S. Ford, E. Stead-Richardson, S. Sharpe, T. Finston, R. Perry, C. and J. Chatton, M. Stuckey, R. Shine, Z. Ebra and P. Byrne. A special thanks to the French Embassy of Canberra (A. Moulet and A. Littardi), Australian Research Council-International Researcher Exchange Scheme and Programme International de Collaboration Scientifique (number 659), which provided funds to carry out fieldwork and to Conservation and land Management for the issuing of licences. The University of Western Australia's Animal Ethics Committee approved all procedures. C. Bonnet and three anonymous referees provided useful comments on the manuscript.




