Multiple effects of shrubs on annual plant communities in arid lands of South Australia
Abstract
Abstract The presence of shrubs in arid lands creates spatial heterogeneity that affects the distribution and performance of annual plants; several possible mechanisms have been implicated. A preliminary survey in a chenopod shrubland in South Australia showed differences in the distribution of annual plants under canopies of Atriplex vesicaria and Maireana sedifolia (the two dominant shrub species) and open spaces. A series of experiments were conducted to test the potential contribution to these patterns of nutrient enrichment under shrubs, differential seed accumulation, stress reduction by the canopy, competition by shrub roots, and protection against grazing. The germinable soil seed-bank under A. vesicaria and M. sedifolia was different from that in open spaces, but these differences can only explain a fraction of the differences observed in the growing annual plant community in different microsites. The soil under A. vesicaria had higher total nitrogen content than soil in open spaces, whereas soil under M. sedifolia had lower available phosphorus than open spaces. Although annual plant densities under A. vesicaria were higher than in open spaces, experimental removal of shrubs increased their density, suggesting that shrub canopies inhibit annual plants in this system. Surprisingly, trenching of open areas close to shrubs (severing lateral shrub roots) decreased annual plant density. We suggest that water moves laterally through shrub roots, in a process akin to a hydraulic lift, increasing water availability for the annual plants. Exclusion of vertebrate grazers had a stronger effect on annual plant biomass in open spaces than under M. sedifolia, suggesting that this shrub provides shelter against herbivory. Overall our results show that shrubs can have simultaneously facilitative and inhibitory effects on the annual plant community through different mechanisms, but more importantly that different shrub species have different effects. This is a potential mechanism allowing for species coexistence of annual plants.
Introduction
Understanding the causes and consequences of spatial heterogeneity has became one of the central toils of modern ecology (Levin 1992). Although spatial heterogeneity has been important in theoretical ecology for some time (O'Neil et al. 1986; Kotliar & Wiens 1990) field ecologists in the past have been mostly interested in landscape-scale heterogeneity, and considered small-scale heterogeneity to be merely noise obscuring the properties of the system (Chesson 1986). Only recently the importance of small-scale heterogeneity has gained broad attention (Tilman & Kareiva 1997; Dieckmann et al. 2000). Small-scale heterogeneity may be particularly important in determining community structure because the strength of different biotic interactions can be modulated by environmental conditions (Reynolds et al. 1997).
Small-scale heterogeneity may result from a combination of biotic and abiotic factors. Soil heterogeneity is present at a variety of scales, affecting the distribution of plant species (Reynolds et al. 1997). In addition, in systems where perennial plants form a discontinuous cover, such as savannas and shrublands, canopy presence alters the environment in ways that influence the performance of smaller organisms. Scattered shrubs in arid lands seem to play an important role in structuring the annual plant community. This spatial heterogeneity can result in a patchwork of microenvironments with different balances between facilitation and interference (Aguiar et al. 1992; Aguiar & Sala 1994).
It is a common observation that in arid and semi-arid systems, annual plants are more abundant beneath the canopies of shrubs than in the open spaces (Went 1942; Halvorson & Patten 1974; Cody 1986; Emmerson & Facelli 1996). There may be several factors that contribute to this pattern. Seeds may accumulate preferentially under shrubs (Reichman 1984; Chambers 1994), which in itself can produce higher plant densities. Establishment conditions can be also more favourable under shrub canopies because the accumulation of fine wind-blown material and organic matter under shrubs enhances nutrient availability (Turner et al. 1966; Garner & Steinberg 1989; Callaway et al. 1991; Belsky & Canham 1994; Belsky 1994) and soil water retention (Jordan & Nobel 1979). Hydraulic lift can also increase water availability around shrubs (Dawson 1993; Burgess et al. 1998), whereas the reduced radiation under the canopy can reduce temperature (Hunter & Aarsen 1988; Valiente-Banuet & Ezcurra 1991) and hence alleviate water stress (Belsky 1994). These effects can be conditioned by the environment (Callaway et al. 1996) and are probably species-specific (Callaway 1998). At the same time, however, the shrub can have negative effects on herbaceous plants, by reducing resource availability (Aguiar & Sala 1994). Shrubs may also provide partial protection from herbivores to annual plants by reducing access to plants living underneath their canopies (Atsatt & O'Dowd 1976; Hjälten & Price 1997; Rousset & Lepart 1999). On the other hand, shrubs may also attract herbivores, either because they are a source of food in themselves or because they provide shade and protection against predators (Whal & Hay 1995).
In spite of a recent increase in interest in facilitative interactions in arid lands, we have little understanding of how all of these possible interactions between shrubs and the herbaceous components balance each other. It is widely accepted that the strength of the different interactions should vary with time, depending on seasonal and yearly variations (Callaway & Walker 1997). To what extent different species of shrubs produce different effects is not quite clear. The possible combination of species-specific positive and negative effects may constitute complex forces structuring communities (Callaway 1998).
The aim of the present study was to investigate the effects of shrubs on the structure of the annual plant community in the arid shrublands of South Australia. We used a combination of field surveys and experiments to analyse annual plant community structure in open areas and immediately beneath the dominant chenopod shrubs in the system. Specifically we asked:
- 1
What is the distribution of the seed-bank and the growing annual plant community in these microsites?
- 2
Are there differences in the soil associated with the presence of these shrubs and, if so, do these differences affect the performance of the dominant annual species?
- 3
What are the effects of the presence of a shrub canopy and water availability on the community structure of annual plants?
- 4
Do shrubs provide any protection to annual plants from vertebrate grazers?
Methods
Study site
This study was conducted at Middleback Field Centre (137°24′E, 32°57′S), 21 km north-west of Whyalla, South Australia. The climate is arid (average annual rainfall of 209 mm) with a slight concentration in the winter months. The temperatures range from average 28.9 °C maximum and 18.7 °C minimum in January, to 16.8 °C maximum and 7.3 °C minimum in July. Soils are predominantly brown calcareous earths with clay-loam texture, and calcium carbonate has accumulated at variable depths, usually within the first 50 cm. The pH is slightly alkaline, and nutrient availability is low (Crocker 1946). The vegetation in these sites consists largely of an Acacia papyrocarpa low open woodland with a chenopod steppe understorey, dominated by Atriplex vesicaria (bladder saltbush) and Maireana sedifolia (pearl bluebush). Nomenclature follows Jessop and Toelken (1986). These shrubs have contrasting life cycles. Whereas A. vesicaria is a relatively fast grower with a life span of approximately 30 years (Crisp 1978), M. sedifolia grows more slowly, reproduces infrequently, lives more than 150 years (Crisp 1978), and is less palatable to vertebrate grazers than A. vesicaria.
The annual plant community is relatively diverse. Most species grow in winter–spring, and only a few species grow during summers with good rainfall. The annual plant community has been impacted by stock and rabbit grazing (Meissner & Facelli 1999) and is presently dominated by Carrichtera annua, an introduced forb. For the sake of the present study we included in the annual plant guild some species that are facultative, short-lived perennials but behave as annuals in most years, namely Danthonia caespitosa, Stipa nitida and Sclerolaena spp.
Two sites (located approximately 5 km apart) were used. One site was a portion of a grazed paddock, and was used for the surveys (which included destructive sampling) and the grazing exclusion experiment. The other was a permanent exclosure that had not been grazed for 20 years, and was used for the experimental manipulation of the effects of shrubs on the annual plant community.
Soil seed-banks in open spaces and beneath shrub canopies
We assessed the germinable soil seed-bank (i.e. the seeds that readily germinate under favourable moisture conditions) under shrubs of each species and in open spaces. This measure provides estimates of the potential plant community (Bertiller 1996), but it is not a complete assessment of the seed populations, because seeds of some species may not germinate, or the germination fractions of the various species may be different (Simpson et al. 1989). Soil samples were collected from the grazed site in April 1996, before any winter germination occurred, in an area of intermediate grazing located between 400 and 600 m from a water point. We located six random points, and at each point chose the two A. vesicaria and M. sedifolia plants closest to the tape. A random point between the two individuals of each species was also located to represent open spaces between shrubs. From beneath each shrub and in the open spaces, we collected the soil from three regularly spaced quadrats (20 cm × 20 cm × 2 cm) deep. This depth was selected because it contains the majority of the seeds (Kemp 1989). The three sub-samples were pooled and thoroughly mixed. Three days after the samples were collected, 150 g of soil from each sampled site was placed into aluminium trays in the glasshouse. The trays were watered at least three times a week, or as needed, to keep the soil moist without water logging it. Seedlings were identified and counted weekly until emergence ceased (late August). Once a seedling was identified it was removed to minimize possible density-dependent effects on further germination. We restricted the analyses to species that were present in four or more trays from at least one environment. All other species, together with a few unidentified individuals, were pooled in the ‘Other’ category. A large proportion of grass seedlings died before identification was possible, and therefore we pooled all grasses in a single group.
Annual plant communities in open spaces and beneath shrub canopies
In early September 1996 we sampled the annual plant community at the same sites we had sampled the soil seed-bank, to confirm our observations that there were floristic differences between sites under shrubs and open spaces. Ten shrubs of each species and 10 open spaces were sampled. We placed three quadrats (20 cm × 20 cm) beneath each shrub and around random points in open spaces. All annual plants in the quadrats were identified and counted, and the data of the three sub-samples were pooled. All the forbs that were not present in at least four out of the 10 sites for each environment, and a few unidentified individuals were included in the ‘Other’ category for further analyses. Grasses were placed into a single category as positive identification was often not possible.
Soils properties in open spaces and beneath shrub canopies
We collected soil from under 10 mature A. vesicaria, 10 mature M. sedifolia, and in 10 associated open spaces at the grazed site. The sampling units were randomly located along a transect, using the nearest suitable site from a random point. Each sample was 20 cm × 20 cm × 7 cm deep. Soil was collected to this depth because nutrients are concentrated in the topsoil (Charley & Cowling 1968). Soil was analysed for total nitrogen using the modified Kjeldahl method (Bradstreet 1965), and available phosphorus using the bicarbonate-extractable method (Watanabe & Olsen 1965). We measured pH using a Hanna Instruments HI 8424 pH meter using 8 g soil in 40 mL deionized water at 20°C.
We also conducted a glasshouse experiment to assess the growth of Carrichtera annua (the dominant annual species) when grown in the different soils. We used 10 pots for each soil type, filled with 150 g air dried, sieved soil. In each pot two seeds of C. annua were planted. The pots were watered with 50 mL water three times per week to ensure that water was not limiting. The few seedlings that failed to establish were replaced with transplants. The experiment started on 1 July 1996 and the plants were harvested on 15 August and were oven dried at 80°C for 48 h and weighed.
Direct effects of shrubs on the annual plant community
This experiment was conducted on A. vesicaria in the permanent exclosure, thus eliminating the effects of grazing by sheep and rabbits, but not kangaroos. Five sites, each having at least four shrubs of similar size and canopy structure, were located. Within each site (blocks), two randomly selected shrubs were removed by sawing at the base of the plant. Because A. vesicaria dies after substantial defoliation, this was adequate to eliminate the shrub activity, avoiding the disturbance that uprooting produces. We removed the shrubs in early April 1996.
Within each site four open areas 1.5 m away from the nearest shrub were selected. Two remained unmodified (controls) and two had their perimeter trenched by pushing a shovel blade to a depth of 40 cm, thus severing most roots that could interfere with annual species. Half of the plots of each treatment were watered monthly with the equivalent to an extra 15 mm of rainfall, starting in May till the end of the experiment in September 1996. Plots were 1 m × 1 m, and sampling was conducted in the central 0.5 m × 0.5 m. Seedling counts were conducted in early July, August and late September. Due to high mortality in some plots during September, biomass was not assessed.
Effects of shrub canopies on vertebrate herbivory
This experiment was established in the grazed area. Ten individuals of A. vesicaria, 10 of M. sedifolia and 10 open areas were selected; five were randomly assigned to the grazing and five to the exclosure treatments. In March 1996 the exclosure plots were surrounded by wire mesh (openings 3 cm) cages, 1 m in diameter, with flaps extending 20 cm on the ground to exclude rabbits. The plots were sampled in September 1996, and in September 1997 using the same arrangements as in the field survey. The material was sorted into species, oven dried at 80°C for 48 h and weighed. We predicted that, if shrubs provide any protection against grazers, the effect of fencing on annual plants growing under shrubs should be less than the effect of fencing in open spaces.
Statistical analyses
We tested all data for normality using the Shapiro–Wilk's test and for variance homogeneity with Bartlett's' test. We performed anova on the data, and, when required, Tukey's Studentized Range test for pairwise comparisons. When assumptions of anova were not satisfied even after applying appropriate transformations, we used the Kruskal–Wallis non-parametric test, and Dunn's non-parametric pairwise comparison. Exploratory data analysis showed that the watering treatment in the field experiment had no effect, so we excluded it from any further analyses, and we only tested for the three contrasts of interest: Removed versus Shrub, Shrub versus Open, and Open versus Trenched.
Results
Soil seed-banks in open spaces and beneath shrub canopies
Overall, the germinable soil seed-bank under A. vesicaria was substantially higher, than in open soil, and their compositions were different, but there were only slight differences between M. sedifolia and open soils (Fig. 1, Table 1). Total number of seedlings, species number, and emergence of grasses, and Other species were higher in A. vesicaria soil than in open soil. Only the number of seedlings of Tetragonia tetragonioides was higher in M. sedifolia soil than in open soil. The total number of seedlings, and the number of seedlings of C. colorata and grasses was lower in M. sedifolia soil than in A. vesicaria soil.
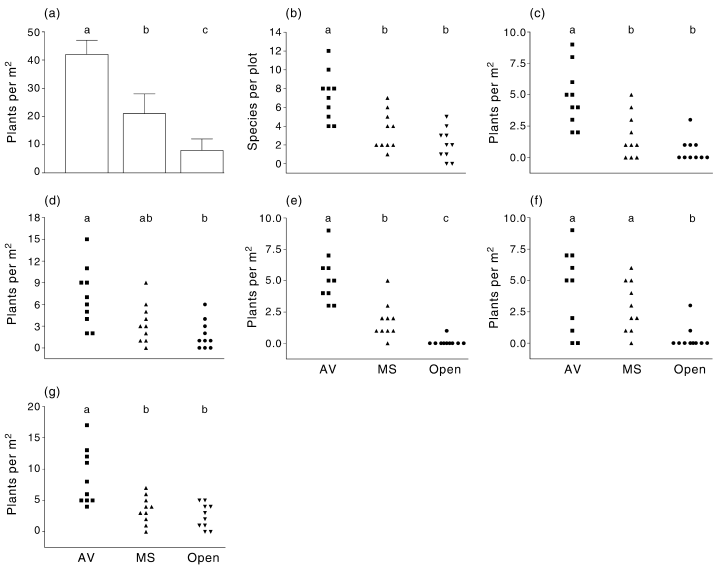
Emerged seedlings from soil collected from open spaces (Open), or under the canopy of Atriplex vesicaria (AV), or Maireana sedifolia (MS). (a) total number; (b) number of species; (c) Crassula colorata; (d) grasses; (e) Tetragonia tetragonioides; (f) others. (a) Whiskers represent 1 SD; (b–f) points represent individual replicates. Different letters indicate a significant difference (anova, Tukey test, P < 0.05 in (a), or Kruskal–Wallis, Dunn test, P < 0.05).
| Variable | P | Tests |
|---|---|---|
| Seed-bank study | ||
| Total numbers | <0.01 | anova/Tukey |
| Species numbers | 0.03 | Kruskal–Wallis/Dunn |
| Crassula colorata | <0.01 | Kruskal–Wallis/Dunn |
| Grasses | 0.04 | Kruskal–Wallis/Dunn |
| Sclerolaena spp. | 0.25 | Kruskal–Wallis |
| Sida corrugata | 0.48 | Kruskal–Wallis |
| Tetragonia tetragonioides | 0.01 | Kruskal–Wallis/Dunn |
| Others | <0.01 | Kruskal–Wallis/Dunn |
| Vegetation study | ||
| Total numbers | <0.01 | anova/Tukey |
| Species numbers | <0.01 | Kruskal–Wallis/Dunn |
| Crassula colorata | 0.19 | Kruskal–Wallis/Dunn |
| Grasses | 0.03 | Kruskal–Wallis/Dunn |
| Senecio glossanthus | 0.58 | Kruskal–Wallis |
| Daucus glochidiatus | 0.31 | Kruskal–Wallis |
| Sclerolaena spp. | <0.01 | Kruskal–Wallis/Dunn |
| Spergularia laevis | 0.03 | Kruskal–Wallis/Dunn |
| Tetragonia tetragonioides | 0.01 | Kruskal–Wallis/Dunn |
Annual plant communities in open spaces and beneath shrub canopies
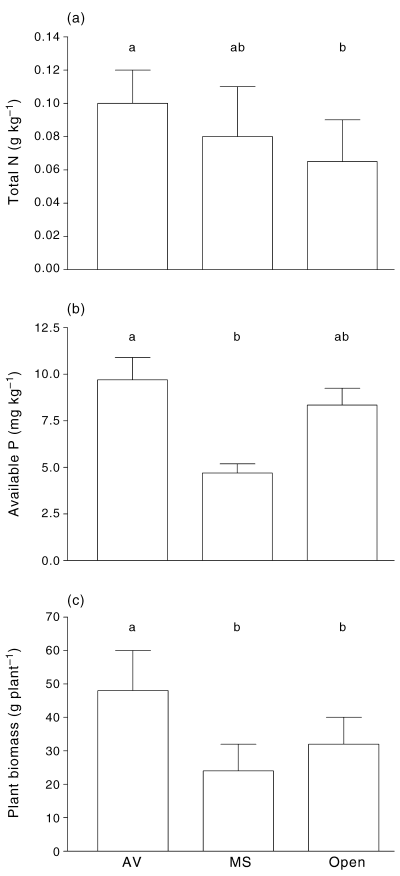
The composition of the annual plant community differed between the three environments (Fig. 2, Table 1). The total number of plants and the abundances of Spergularia laevis were highest under A. vesicaria, intermediate under M. sedifolia, and lowest in open spaces. The number of species, the abundance of grasses and ‘Others’ were higher under A. vesicaria than under M. sedifolia or in open spaces. Sclerolaena spp. was more abundant under A. vesicaria than in open spaces, whereas T. tetragonioides was more abundant under both A. vesicaria and M. sedifolia than in open spaces.
Annual plant communities in open spaces (Open), or under the canopy of Atriplex vesicaria (AV), or Maireana sedifolia (MS). (a) total numbers; (b) number of species; (c) grasses; (d) Sclerolaena spp.; (e) Spergularia laevis; (f) Tetragonia tetragonioides; and (g) others. (a) Whiskers represent 1 SD; (b–g) Points represent individual replicates. Different letters indicate a significant difference (anova, Tukey test, P < 0.05 in a, or Kruskal–Wallis, Dunn test, P < 0.05).
Soil properties in open spaces and beneath shrub canopies
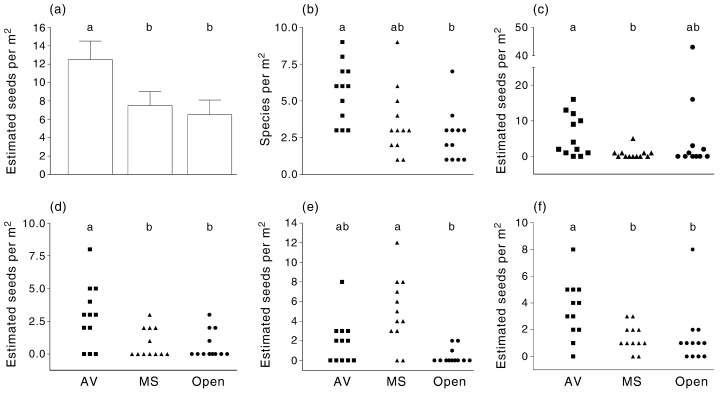
Soil collected under both shrub species was different from the soil in open spaces. There was no difference in pH (P > 0.05), but available phosphorus was lower under M. sedifolia (P = 0.015) whereas total nitrogen was higher under A. vesicaria than in open soil (P = 0.009) (Fig. 3). The biomass of C. annua plants grown in A. vesicaria soil was higher than the biomass of plants grown in M. sedifolia soil or open soil (P < 0.001; Fig. 3).
(a) Total nitrogen and (b) available phosphorus in soil collected from open spaces (Open), or under the canopy of Atriplex vesicaria (AV), or Maireana sedifolia (MS) and (c) biomass of plants of Carrichtera annua grown in a glasshouse in the said soils. Whiskers represent 1 SD. Different letters indicate a significant difference (anova, Tukey test, P < 0.05).
Direct effects of shrubs on the annual plant community
There were few differences between the plots with shrubs present and open spaces (Fig. 4). The number of C. annua was marginally higher (P = 0.053), and total density, and density of the Other category were higher under shrubs (P < 0.05). The removal of the shrub increased the total number of seedlings (P < 0.01), and the abundances of C. annua and T. tetragonioides (P < 0.05). Trenching reduced the total number of plants, and the number of C. annua and T. tetragonioides.
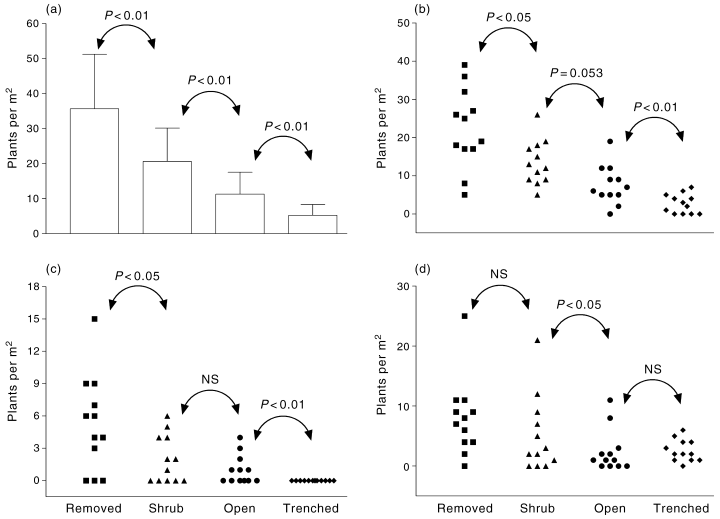
Abundance of annual plants in plots with Atriplex vesicaria removed, shrub present, unmodified open spaces, or trenched open spaces. (a) total numbers; (b) Carrichtera annua; (c) Tetragonia tetragonioides; and (d) other species. (a) Whiskers represent 1 SD. (b-c) Points represent individual replicates. Probability values from planned orthogonal contrasts shown (Dunn tests except for (a), Tukey test).
Effects of shrub canopies on vertebrate herbivory
In both 1996 and 1997 grazing exclosure and shrub presence had an interdependent effect on the total biomass of annual species (Fig. 5, Table 2). In 1996 no individual species had any significant response, but in 1997, grazing exclusion, site and their interaction, had a significant effect on biomass of Danthonia caespitosa (Fig. 5, Table 2). Overall the effects in 1996 and 1997 were similar except that in 1997 the biomass of annual species in the exclosures was higher under A. vesicaria than under M. sedifolia. In general, the proportion of biomass consumed (calculated as the difference between the biomass in exclusion plots and the plots open to grazers, relative to the biomass in the exclusion plots) was always higher in open spaces (0.54 in 1997 and 0.89 in 1997), intermediate under A. vesicaria (0.45 and 0.48, respectively) and lowest under M. sedifolia (0.13 and 0.17, respectively).
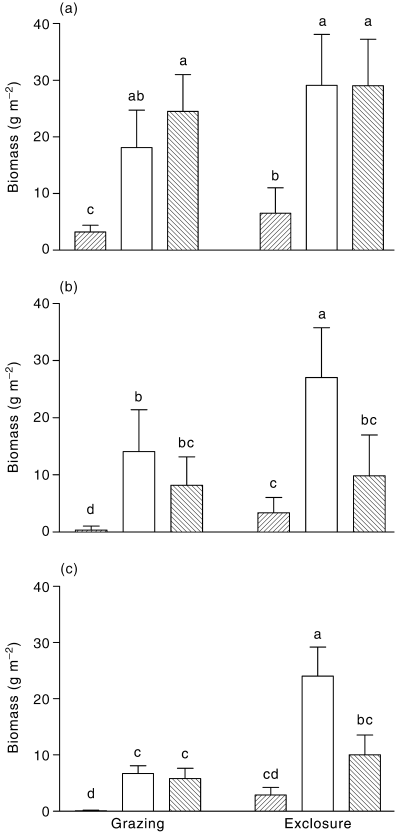
Biomass of annual plants in plots protected from vertebrate grazing or open to grazing, in () open spaces or under canopies of (□) Atriplex vesicaria, or () Maireana sedifolia. (a) Total biomass in 1996; (b) total biomass in 1997; (c) biomass of Danthonia caespitosa in 1997. Whiskers represent 1 SD. Different letters indicate a significant difference (anova, Tukey test, P < 0.05).
| Source | d.f. | SS | MS | F | P |
|---|---|---|---|---|---|
| Total biomass 1996 | |||||
| Site | 2 | 24.520 | 12.26 | 5.87 | 0.008 |
| Grazing | 1 | 0.972 | 0.972 | 0.47 | 0.501 |
| Interaction | 2 | 10.314 | 5.157 | 2.47 | 0.042 |
| Residual | 24 | 50.126 | 2.088 | ||
| Biomass of Danthonia caespitosa 1997 | |||||
| Site | 2 | 966 | 483 | 12.6 | 0.0002 |
| Grazing | 1 | 496 | 496 | 12.9 | 0.0015 |
| Interaction | 2 | 323 | 162 | 4.21 | 0.0270 |
| Residual | 24 | 920 | 38.3 | ||
| Total biomass 1997 | |||||
| Site | 2 | 1782.54 | 891.27 | 35.5915 | 0.0000 |
| Grazing | 1 | 259.63 | 259.63 | 10.3681 | 0.0043 |
| Interaction | 2 | 190.819 | 5.405 | 3.8100 | 0.0396 |
| Residual | 24 | 600.898 | 25.041 | ||
Discussion
Our results indicate that shrubs may have various effects on herbaceous plants through different mechanisms. We detected patchiness in the distribution of nutrients associated with the presence of shrubs that were able to affect the growth of the dominant annual species in the system. Seed soil-bank heterogeneity was also associated with the presence of shrubs. We also found that direct effects ranged from competition to facilitation. At the same time, shrubs can offer protection against grazing to some of the annual plants. Importantly the results indicate that different shrub species have different effects on the annual plant community. Shrub species affected soil conditions, the seed-bank, the growing community and grazing by large vertebrates. The result of these factors is likely to be a complex array of interactions that may result in heterogeneity in annual plant community composition at the local scale.
Although we detected some differences in the germinable soil seed-bank in the different microenvironments examined, these differences alone cannot explain the differences in annual plant community. The experimental studies conducted give some insight into other mechanisms that contribute to structure annual plant communities but they do not allow us to determine precisely to what extent the incongruence between the germinable soil seed-bank and the growing community reflects different species responses to the different microenvironments. Studies of seedling emergence starting from standardized seed pools and/or mutual transplant experiments will be required to answer this question.
Shrubs may favour seed accumulation through increased in situ seed production resulting from enhanced local conditions (Soriano & Sala 1986; cf. Aguiar & Sala 1994) or through accumulation of seeds transported by animals (Fuentes et al. 1986), surface water runoff (Reichman 1984) or wind (Reichman 1984; Aguiar & Sala 1994). The fact that there were more plants growing under A. vesicaria than M. sedifolia suggests that higher in situ production may contribute to higher seed accumulation. This is consistent with the limited dispersal ability of most arid land species (Ellner & Schmida 1981). However, specific characters of the two shrub species, such as differences in mound heights, or differences in branching structure that may affect deposition of seed carried by wind, can also contribute to these differences.
Although there is accumulated evidence that shrubs and trees in arid lands modify the soil around them (Garcia-Moya & McKell 1970; Charley & West 1975; Gutierrez et al. 1993; Facelli & Brock 2000), we cannot rule out that some of the differences observed in our study were due to deflation of soil surrounding the shrubs due to grazing activity (Rogers & Lange 1971; Andrew & Lange 1986). The two processes may act concurrently, because trampling may dislodge particles that are trapped and accumulate under the canopies of shrubs. If shrub presence alone is directly responsible for the nutrient accumulation, it could be expected that the effect of M. sedifolia would be more intense than that of A. vesicaria, given the longer life span of the former. It is possible, however, that the A. vesicaria mounds may be of similar age to M. sedifolia mounds if seedlings establish more often than not under the canopy of other shrubs. However there is no solid evidence to support this.
Although shrub structure may determine the patterns of accumulation of organic debris and fine particles (DeSoyza et al. 1997) these mechanisms alone could not account for all the differences between the soils under A. vesicaria and M. sedifolia. The lower level of phosphorus in soil under M. sedifolia is particularly surprising. A possible explanation may be increased termite activity under M. sedifolia canopies. Termite activity has been reported to increase phosphorus leaching from the soil (Okwakol 1987), but very little is known about the mechanisms involved. Higher termite activity under M. sedifolia was observed during this experiment as well as in another chenopod shrubland in South Australia (Louise Emmerson pers. comm. 1997).
Whatever the mechanisms involved, the growth response of C. annua to the different soils under controlled conditions demonstrates that the soil underneath A. vesicaria is more conducive to plant growth, most likely because of increased nitrogen availability. The lower biomass in the plants grown in the open and M. sedifolia soils may be due to different limiting factors. In the former, nitrogen may have been limiting, while in the latter phosphorus was likely to be highly limiting.
Overall our results suggest that shrubs contribute to the creation of patchiness in the distribution of nutrients at a level that affects the growth of annual species in the system. The importance of redistribution of resources in arid landscapes by surface water movement has been recently discussed by Ludwig and Tongway (1997) and Aguiar and Sala (1999). Facelli and Brock (2000) suggested that plant-driven patchiness is at least equally important and may have substantially different consequences, because it often results in the uncoupling of different resources (i.e. nutrients and light).
The experimental removal of A. vesicaria resulted in higher plant density, showing that the presence of a living A. vesicaria may restrict establishment or survivorship of some annual plants. The absence of any effect of water addition on annual plant density does not support competition for water as the acting mechanism. Swank and Oechel (1991) found that the reduction of light by a shrub canopy limited photosynthetic activity of annuals, and canopy removal increased annual seedling emergence and growth. Desert annual plants usually have high saturation levels (Werk et al. 1983) and radiation reductions in the order of 75%, such as those measured for saltbush (Lenz 1999) can seriously reduce resource acquisition (Jackson & Caldwell 1992), but these levels are unlikely to result in seedling mortality. The shade in the shrub microenvironment is more likely to affect the germination of some annual species either directly (Guterman 1993) or through changes in soil temperature (Mott 1972; Plummer & Bell 1995; Moro et al. 1997; Facelli & Chesson, unpubl. data).
Surprisingly, trenching decreased the abundance of seedlings. Trenching was originally included to assess root competition between shrubs and annuals for water and nutrients (see Swank & Oechel 1991), and was expected to enhance annual plant establishment. Our results actually indicate some facilitation of the shrub on annual plants. A possible reason is that hydraulic lift may be arrested by the severing of A. vesicaria roots (Dawson 1993). Recent research suggests that water can move along roots in any direction, and leak into drier soil at any point were the root is permeable (Burgess et al. 1998). Because of lower resistance to water movement, shrubs should deplete first the areas closer to the shrub, creating a concentric gradient of soil water potential that could result in lateral hydraulic lift. Root structure studies (Facelli, unpubl. data) showed that A. vesicaria in this area has an extended (at least 2.5 m radius) shallow (approximately∼ 20 cm) root system, which is consistent with the proposed explanation. On the other hand it must be noted that the experimental addition of water had no effect. However, the effects of pulses of water (as the one experimentally imposed) may be quite different from a more constant supply produced by hydraulic lift (Harris & Facelli, in press).
Our results indicate that shrubs can offer protection against grazing to some of the annual plants. Given the destructive effect of grazing on the annual plant community (Meissner & Facelli 1999) this protective effect may be of great importance for the persistence of sensitive species. Both shrub species reduced the proportion of biomass consumed, particularly M. sedifolia. There are at least two possible, non-exclusive, mechanisms that can account for the different effects of the two shrub species. Maireana sedifolia has a low-hanging, dense canopy, with rigid branches that may prove to be a better physical barrier than the more open, less rigid structure of A. vesicaria. It is also possible that higher rates of herbivory under A. vesicaria are produced by more frequent visitation by grazers. The preference of sheeps for A. vesicaria over M. sedifolia is well documented (Crisp 1978), and grazers could be also attracted to these shrubs by the higher availability of annual plants, which are usually the most favoured food item.
Conclusion
Our results show a complex array of facilitatory and inhibitory effects of the shrubs on the annual plant community. Although soil conditions under one of the species (A. vesicaria) were more conducive to the growth of the dominant species, our results also indicate that canopy presence had an inhibitory effect on annual species, probably through reduced light availability. Importantly the results indicate that different shrub species have different effects on the annual plant community. Shrub species affected soil conditions, the seed-bank, the growing community and grazing by large vertebrates.
Perhaps the most surprising result is the seemingly facilitatory effect of shrub roots on the annual plants growing outside the canopy. This is contrary to our expectation that the canopy was the site where the facilitatory effect of the shrub would be found (Hunter & Aarssen 1988; Valiente-Banuet & Ezcurra 1991; Guterman 1993). It is quite likely that the strength of some of these effects may vary in time and space. In drier years, when establishment or survivorship into the spring are severely water limited, facilitation under canopies may be more important than inhibition. Emmerson and Facelli (1996) found that in a drier year there were higher abundances of annual plants under M. sedifolia, whereas there was little evidence for this during 1996, when rainfall was slightly above average. During years of low rainfall, M. sedifolia shrubs may create a microenvironment with less water stress compared with A. vesicaria because of the dense canopy restricting light and, hence, evaporation (Jordan & Nobel 1979). The strength of the interaction may also change throughout the same year. It is likely that facilitative processes may not be important in the initial establishment phase in wet years, but as temperature increases and the soil dries out, the microenvironment created by the shrub may be more favourable for survival than in the desiccating open areas.
Our results also suggest that there is a spatial component to the intensity of the various interactions. The spatial component is likely to interact with changes in the nature of the predominant interaction through time. It has been pointed out (Callaway et al. 1991; Holmgren et al. 1997) that shade may have either a competitive or a facilitative effect, depending on water availability. Also the effect of shade is likely to change at different positions underneath the canopies. Similarly, extended root systems may produce a negative or a positive effect depending on water availability, root system structure and distance from the shrub.
The overall higher abundance of annual species under shrubs may be important for the long-term persistence of several species. During stressful periods or under heavy grazing, some annual populations may be unable to replenish their seed-banks in open spaces, and individuals growing under shrub canopies may contribute to maintain the population in the long term. The decline of A. vesicaria even under moderate grazing (Crisp 1978) may have important consequences for annual plant populations, given that it seems to provide a better environment than M. sedifolia. However, the ability of the latter to provide refugia against vertebrate grazing may be critical for the persistence of some annual plant populations. The development of a comprehensive understanding of the dynamic and spatial components of these interactions seems to be a major challenge for future research.
Acknowledgements
We thank D. Bigham, P. Chesson, K. Dunbar, L. Emmerson, G. Hastwell and R. Sinclair for help at different stages of this project. The manuscript was improved thanks to comment by G. Hastwell and J. Prider. The Nicolson family allowed access to Middleback Station for the field work. This project was partially supported by a grant from the Australian Research Council and a Holtz Memorial Fund Grant, and is a contribution to the Middleback Field Centre Research Program.




