Elicited ponto-geniculo-occipital waves by auditory stimuli are synchronized with hippocampal θ-waves
Abstract
Abstract The study demonstrated that ponto-geniculo-occipital (PGO) waves are phase-locked with hippocampal θ-waves during rapid eye movement (REM) sleep. A possible mechanism for influencing a PGO wave generator by hippocampal θ-waves was hypothesized. The generator is known to receive afferent inputs such as auditory input. To test this hypothesis, the temporal relationship between hippocampal θ-waves and elicited PGO waves (PGOE) by auditory stimuli was analysed. The analysis showed that PGOE were synchronized with θ-waves, but did not reset the phase of a θ-wave. Because the occurrence of PGOE and spontaneous PGO waves shared the same phase-dependency on θ-waves, the results strongly suggest that the PGO wave generator was driven by θ-waves, as originally hypothesized.
INTRODUCTION
Ponto-geniculo-occipital (PGO) waves are observed in the pons, the lateral geniculate nucleus (LGN), and the occipital cortex. A PGO wave generator is believed to be located within the pontine caudolateral peribrachial area. Hippocampal θ-waves (hereafter known simply as θ-waves) are observed as rhythmic extracellular field potentials of 3–8 Hz in cats. The medial septum is involved in the generation of θ-waves. A close relationship between PGO waves and θ-waves has been demonstrated electrophysiologically.1,2 We have also shown previously that PGO waves tend to be phase-locked with θ-waves.3 We hypothesized a possible mechanism for influencing a PGO wave generator by hippocampal θ-waves. The generator is known to receive afferent inputs such as auditory input. Actually, a PGO-like wave is elicited by auditory stimuli, which is called an elicited PGO (PGOE) wave. To test our hypothesis, we analysed the temporal relationship between hippocampal θ-waves and PGOE waves.
MATERIALS AND METHOD
Three adult cats (bodyweight range 3.3–5.5 kg; CAT1–CAT3) were used in the study, and standard techniques were used for polygraphic recording.4 Twisted bipolar electrodes (stainless, 250 μm φ; gap between tips, 0.5–1.0 mm) were implanted in the LGN (A 5.8–6.9, L 9.0–9.4, D 4.0–4.9) and hippocampus (A 4.0–4.2, L 4.4–6.1, D 4.5–5.0) for recording PGO and θ-waves, respectively.2–4 The cats could move freely in a sound-attenuated room illuminated by dim lighting. Tone stimuli (2000 Hz, 90 dB, 20 ms duration at intervals of 20 s) were applied at an arbitrary phase in reference to a θ-wave by elaborately equipped small speakers (1 cm distant from ears). The PGOE waves were defined as those with latencies between 40 ms and 200 ms.5 All experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee.
RESULTS AND DISCUSSION
We investigated the time intervals between a negative peak of a PGOE wave and the preceding and succeeding adjacent positive peaks of a θ-wave; these intervals were denoted by tep and tes, respectively (Fig. 1a). tep and tes were distributed unimodally, as shown in Fig. 1b, whereby the values for tep were 0.099 ± 0.054 s (mean ± SD) for CAT1, 0.092 ± 0.051 s for CAT2, and 0.080 ± 0.047 s for CAT3; and the values for tes were 0.088 ± 0.053 s for CAT1, 0.088 ± 0.049 s for CAT2, and 0.112 ± 0.049 s for CAT3. These results suggest that PGOE waves tend to be phase-locked with θ-waves rather than phase-resetted because no systematic correlation between tep and the period of θ-wave (tep + tes) was observed. In addition, the same analysis was performed for PGO waves, whereby values for tp were 0.113 ± 0.037 s for CAT1, 0.086 ± 0.047 s for CAT2, and 0.087 ± 0.049 s for CAT3; and values for ts were 0.078 ± 0.033 s for CAT1, 0.088 ± 0.043 s for CAT2, and 0.116 ± 0.048 s for CAT3. For all cats, there was no significant difference between the corresponding intervals tp/tep and ts/tes. In conclusion, PGOE waves were phase-locked with θ-waves and shared the same phase-locking tendency with spontaneous PGO waves. The results lead us to speculate that the PGO wave generator is driven by θ-waves, which is at least supported anatomically.6
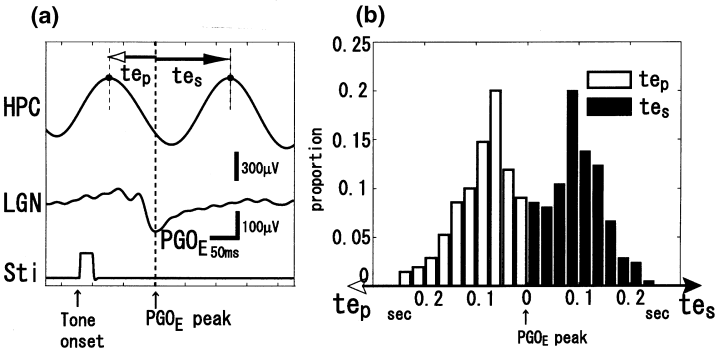
. (a) Individual example of data collected for one trial and variables measured. Time intervals between negative peaks of an elicited pontogeniculo-occipital (PGOE) wave and the preceding (tep) and succeeding (tes) adjacent positive peaks of hippocampal θ-waves. HPC, hippocampus; LGN, lateral geniculate nucleus; Sti, auditory stimuli. (b) Histograms of tep and tes (bin width: 0.025 s).




