Cognitive resource model for the information-processing of task-irrelevant visual stimuli
Abstract
Abstract In order to elucidate possible factors that effect P3s to task-irrelevant visual stimuli (non-target P3s), we made a normative visual event-related potential study with button-press tasks in four different conditions in which two factors (the number of colors of non-target stimuli, and the shape of the stimuli) were manipulated without any change in task nor target/non-target ratio. The peak distributions of non-target P3s (centrally peaking) were significantly different from those of task-relevant P3s (parietally peaking) in all conditions. The amplitude of non-target P3 decreased as the variety of colors of the non-target stimuli increased. The amplitude of non-target P3 to colored solid circles was larger than that of non-target P3 to colored Stroop stimuli. Between each condition, task- relevant P3 and reaction time showed no significant difference. Both the variety of non-target stimuli and the shape of the stimuli were shown to have effects on the amplitudes of non-target P3s without any alteration in task-relevant P3s. It is suggested that the amplitude behaviors of non-target P3s partly reflect the amount of cognitive resource allocated for each different kind of task-irrelevant visual stimuli.
INTRODUCTION
Oddball paradigm, in which subjects are requested to perform certain mental tasks when particular stimuli are presented among consecutive presentations of other irrelevant stimuli, is known to elicit P3b event-related potential. P3b to task-relevant stimuli is reported to reflect cognitive resource allocation required to update working memory.1 The amplitude of P3b is known to decrease when two different types of tasks are loaded concurrently within a session, and this phenomenon is explained by decreased allocation of resource from a limited pool.2
The term cognitive resource means energetical or control systems that modulate cognitive processes or stages of processing.3 This concept helps us to understand several aspects of information-processing.
P3s are also elicited by unexpected and unrecognizable non-target rare stimuli, namely ‘novels P3’ in visual modality‚4 and ‘P3a’ in auditory modality‚5 which are thought to represent cognitive updating after orienting response.6 P3 to non-target rare stimuli which are easy to recognize is reported to have distinct generating mechanism.7
Positive waveform, peaking at the central region, is also observed around 300 msec after presentations of non-target, task-irrelevant, frequent auditory stimuli. This positive waveform to non-target auditory stimuli is reported to share several features, including latency, amplitude and scalp topography, with P3a.8 This waveform does not appear when no task is demanded, therefore this waveform may be the result of an attentional shift towards the stimuli, and reflect some aspects of the classification process.8
In visual modality, task-irrelevant frequent stimuli also elicit positive waveforms around 300 msec after the onset of stimuli.9 These ‘non-target visual P3s’ may occur when no task is demanded.10 Non-target visual P3s may occur, with decreased amplitudes, in patients with dementia of Alzheimer type.11
To our knowledge, however, few studies have been chiefly concerned with non-target visual P3s, and no outstanding principal cognitive model is raised as to the processing of task-irrelevant frequent visual stimuli. Saito reported that the amplitudes of non-target visual P3s to Stroop stimuli were significantly decreased compared with those of non-target P3s to colored circular disks, suggesting that the amplitudes of non-target visual P3s could be manipulated by certain parameters which are related to the information processings of task-irrelevant frequent stimuli.12
In order to elucidate possible parameters that may effect non-target visual P3s, we performed a normative study in which two factors (the variety of non-target stimuli, and the shape of stimuli) were manipulated without any change in task nor target/ non-target ratio.
SUBJECTS AND METHODS
Subjects
Nineteen right-handed healthy unmedicated volunteers (12 males and seven females), with no history of neurological or psychiatric disorders, participated in the present study. The mean age was 28.8 (range 23.7–56.3, SD 7.34) years. The educational level was 17.4 (15.0–18.0, SD 0.99) years. No corrected right-handed person was included. Binocular visual acuity of each subject was above 0.6.
All volunteers provided written informed consent after the procedures has been explained, and were studied in accordance with institutional guidelines.
Recording system
A color display, controlled by a stimulus-generating PC (NEC PC-9821Ne2; NEC, Tokyo, Japan), was placed 100 cm from each subject’s nasion.
Non-polarizable Ag/AgCl electrodes were placed on Fpz (ground), Fz, Cz, Pz, Oz, T3, T4, right and left earlobes, 2 cm above right external ocular angle, and 2 cm below left external ocular angle (for electrooculograph (EOG)). Impedance levels of all leads were less than 5 kOhm at the beginning and at the end of each session. Potentials at Fz, Cz, Pz, Oz, T3, T4 were referred to the linked earlobes. Scalp potentials, EOG, and button-press currents were amplified by an electroencephalometer (Nohon-Kohden EEG5214; Tokyo, Japan) with a time constant of 0.3 s. Bandpass filter was set to an upper cutoff frequency of 50 Hz and a lower one at 0.1 Hz. Potentials were digitized by an A/D converter with its sampling rate of 500 Hz. The resolution of potentials was 12 bit. Potentials were then stored to a recording PC (NEC PC-9801Vm21). The stimulus-generating PC and the recording PC were connected through RS–232C interfaces.
Each recording sweep began 100 msec before the onset of each presentation and continued for 1022 msec.
Each sweep data containing potential exceeding ± 60 μV at any one of active leads were eliminated from off-line averaging.
Stimuli and tasks
Stimuli were either colored solid circles (conditions 1, 2, and 3) or colored characters representing the names of colors, which are the Japanese version of Stroop stimuli13 (condition 4).
In condition 1, red-colored solid circles (targets, probability of occurrence 25%) were presented among blue ones (non-targets,75%). In condition 2, targets (25%) were presented among blue non-targets (37.5%) and green non-targets (37.5%). Condition 3 contained targets (25%) among blue, green, and yellow non-targets (25% each). In condition 4, red-colored Stroop stimuli (targets, 25%) were presented among blue, green, and yellow Stroop stimuli (non-targets, 25% each). In every stimulus of condition 4, the color of character and the name of color represented by the character was different. In condition 4, each target stimulus was arranged to be preceded by a stimulus whose color and shape was different from the target, to prevent any kind of priming.14
Visual angle of stimuli were 2.3°. Each stimulus was presented for 900 msec in quasi-random order. The span of interstimulus interval (ISI) was determined randomly (range 1500–1900 msec) at each presentation of stimulus. During ISI, a fixation cross appeared at the center of the display, with its visual angle of 0.5°.
Each subject, seated deep in a chair in a dimly lit electrically shielded room, was requested to press a button held at their right hand, as soon as possible when a red-colored stimulus (target stimulus) was presented.
The order of conditions 1–4 was determined randomly. Subjects rested for 3 min between each session.
Peak identification and statistical analyses
Each P3 peak was identified as the most prominent positive peak occurring 220–410 msec after the onset of each stimulus. The mean potential of pre-stimulus period (– 100 ms to 0 ms) defined the baseline for calculating each P3 amplitude.
Data were stored in Lotus 1-2-3 files and processed on DrSPSS for Windows (SPSS Japan Co., Tokyo, Japan).
Because our data (shown later) do not completely fit Gaussian- nor t-distribution, we employed repeated-measure analysis of variance (ANOVA) with Huynh-Feldt’s adjustment of degrees of freedom (d.f.), to avoid false-positive results.15 (All ANOVA cited below are repeated-measure ANOVAs with Huynh-Feldt’s adjustment of d.f.).
Scalp distributions of P3s were tested with ANOVA following the procedures of scaling the voltages by vector length.16 Amplitudes and latencies of P3s, and reaction times were tested with ANOVA. The effects that produced significant difference in ANOVA were also tested with multiple comparison tests (least significant difference test, Bonferroni’s test).
RESULTS
Figure 1 shows grand-averaged potentials recorded in each condition.
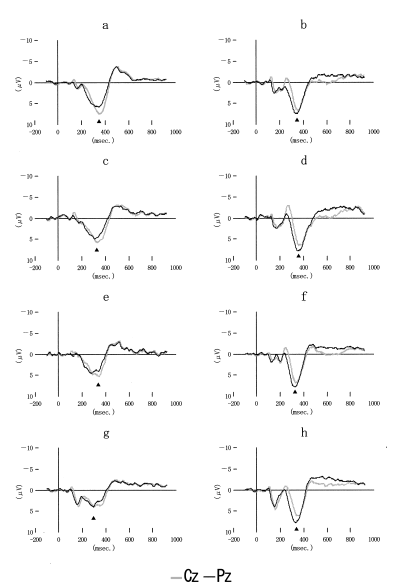
Grand-averaged scalp potentials at Cz (gray lines) and Pz (black lines) for non-target (a, c, e, g) and target (b, d, f, h) stimuli in condition 1 (a, b), 2 (c, d), 3 (e, f), and 4 (g, h), respectively. Non-target stimuli were painted with blue (a), blue or green (c), blue or green or yellow (e, g). The shapes of stimuli were solid circles (a, b, c, d, e, f) or Stroop stimuli (g, h). The color of target (red) and its probability of occurrence (25%) were not changed in all conditions. Arrowheads (▴) indicate the peaks of P3s.
P3s to task-irrelevant frequent stimuli (non-target P3) were observed in all conditions in all subjects. The mean loadings of vector-scaled peak amplitudes of P3s in all conditions in all subjects (Fz, Cz, Pz, Oz, T3, T4) were (0.24, 0.40, 0.53, 0.51, 0.24, 0.32) to target stimuli, and (0.37, 0.46, 0.43, 0.31, 0.23, 0.31) to non-target stimuli, respectively. The ANOVA on the vector-scaled amplitudes of P3s in all electrode sites (Huynh-Feldt’s epsilon = 0.508) showed significant effect of electrode sites × task-relevance (F = 34.693, P < 0.001), while no significant difference was pointed out between conditions (electrode sites × conditions, F = 1.415).
Table 1 shows the mean amplitudes (Cz) and peak latencies of non-target P3s in different conditions. The ANOVA on the amplitudes of non-target P3s at Cz (Huynh-Feldt’s epsilon = 0.853) showed significant difference between conditions (F = 7.656, P < 0.001). Multiple comparison (least significant difference test) showed the maximal amplitude of non-target P3 in condition 1 and the minimal one in condition 4 at two electrode sites (P = 0.023 at Cz, P = 0.019 at T3). The ANOVA on the latencies of non-target P3s (Huynh-Feldt’s epsilon = 0.961) showed significant difference between conditions (F = 4.264, P = 0.010), while multiple comparison (LSD test and Bonferroni’s test) showed no significant difference in latencies of non-target P3s between each condition.
| Condition 1 | Condition 2 | Condition 3 | Condition 4 | |
|---|---|---|---|---|
| Amplitude (Cz, μV) (mean ± SE) | 8.9 ± 5.1 | 6.4 ± 4.0 | 7.5 ± 4.9 | 5.6 ± 3.0 |
| Latency (msec) (mean ± SE) | 347 ± 41.6 | 337 ± 36.6 | 319 ± 34.9 | 314 ± 43.3 |
As shown in Table 2, task-relevant P3s and reaction time exhibited no significant difference between each condition (paired t-tests).
| Condition 1 | Condition 2 | Condition 3 | Condition 4 | |
|---|---|---|---|---|
| Amplitude (Pz, μV) (mean ± SE) | 10 ± 3.6 | 11 ± 3.9 | 9.8 ± 3.1 | 10 ± 3.6 |
| Latency (msec) (mean ± SE) | 326 ± 34.1 | 334 ± 42.3 | 334 ± 40.0 | 333 ± 30.9 |
| RT (msec) (mean ± SE) | 349 ± 73.3 | 352 ± 61.6 | 358 ± 70.8 | 363 ± 64.6 |
The amplitudes of non-target P3s were larger than those of task-relevant P3s in four of the 19 subjects (condition 1) and in one subject (condition 4), respectively. In condition 1, two subjects exhibited frontally distributed non-target P3s which were larger in amplitude than task-relevant P3s.
DISCUSSION
Non-target visual P3 and information processing
As seen in other studies‚9–12,17 non-target visual P3s were observed in the present study. One possible explanation of the presence of non-target P3 is that non-target visual P3 is a mere positively peaking trough which occasionally appears between other negatively peaking significant potentials. However, there are two reasons that non-target P3s are worth studying. First, P3s to non-target stimuli were elicited in all conditions in all subjects. If the presence of non-target P3s were occasional, only a limited number of subjects would have exhibited non-target P3s in limited conditions, but the present results showed otherwise. Second, as mentioned above, non-target P3s could be observed in many other studies. Although other studies did not focus on non-target P3s, findings in those studies clearly show non-target visual P3s. Therefore, we think that non-target visual P3 is a consistent potential, although it is not clear whether non-target P3 has subcomponents.
In auditory modality, P3s to frequently occurring stimuli do not appear when no task is being demanded‚8 when attention is distracted to puzzle-solving or reading to ignore auditory stimuli,18 or when 100% simple reaction task is loaded.19 Although we did not perform a recording condition in which no task was demanded, it is reported that non-target frequent visual stimuli may elicit P3s when no task is required. For example, P3s were shown to be elicited by flashes with varying probability of occurrence when no guessing task was demanded in the first report of P3.10 Therefore, it is reasonable to think that non-target visual P3 represents the automatic information processing which is not intentional but not a mere ignoring process.
The centrally peaking distribution of non-target visual P3, which was shown by vector-scaled ANOVA to be significantly different from the distribution of visual P3b in the present results, partly resembles the fronto-central distribution of P3a and novels P3. P3a and novels P3 are elicited by specially designed non-target stimuli which cause orienting responses4,5 (i.e., rare deviant non-targets, unrecognizable non-targets, or attention-attracting non-targets), and these P3s are prone to decrease when such stimuli are repeated, while our non-target visual P3s were elicited by frequently occurring non-target stimuli. Taken together, non-target visual P3s may represent distinct mechanism from those associated with P3b, P3a, or novels P3.
Parameters effecting non-target visual P3
We manipulated two factors (the variety of non-target stimuli, and the complexity of stimuli) without any change in task nor target/non-target ratio. Conditions 1, 2, and 3 had different numbers of non-target color (variety manipulations). Conditions 3 and 4 had difference in the shapes of stimuli (shape manipulation), while the task-irrelevant colors were identical in these conditions. Those manipulations seemed to effect exclusively task-irrelevant P3s, because there were significant alterations in the amplitudes of non-target visual P3s with no changes in the amplitudes and latencies of task-relevant P3s or reaction times between four conditions.
Our results suggest that the amplitudes of non-target visual P3s tend to decrease as the number of non-target colors increase, and as the shapes of stimuli were changed from circles to Stroop stimuli.
The difference in the shapes of stimuli between conditions 3 and 4 could be subdivided in two aspects: difference in complexity, and difference in the variety of color–figure combinations. Both aspects may be related to the alteration of the amplitude of non-target P3 in conditions 3 and 4, although it remained unknown which aspect is more closely related to this alteration.
No significant difference was observed between the amplitude and the latency of non-target P3 in condition 2 and those in condition 3. We cannot deny the possible effects caused by yellow non-targets, which were brighter (35.5 cd/m2) than red (10.3 cd/m2) or blue (4.00 cd/m2) ones.
Although we did not manipulate the stimulus modality, the probability of target occurrence or the tasks, these factors may presumably alter the distributions, amplitudes, or latencies of non-target P3s.9,10
The latency behavior of non-target P3s (shortest in condition 4) was not significant in multiple comparison. However, our manipulations may possibly have had effects on non-target stimuli to make non-targets ‘deviant’, because the latency of task-irrelevant P3 is reported to be shorter as stimuli become more deviant‚20 and because the difference between conditions in the latencies of non-target P3s was pointed out by ANOVA in our results.
In condition 1, no significant difference was observed between the amplutude of task-relevant P3 and that of non-target P3. However, it is questionable whether a mere comparison of the amplitudes of P3b and non-target P3 gives meaningful information because the peak distributions are distinct from each other. There may be several underlying mechanisms working concurrently in producing the amplitude of non-target P3 as large as that of P3b in condition 1. First, non-target P3s to visual stimuli tend to be larger than those to auditory stimuli.9 Second, non-target P3s tend to be larger when reaction-time tasks are demanded, compared to those when count-tasks are loaded.9 For these two reasons, we employed visual reaction-time tasks. Third, our tasks were easy for our educated subjects. In single task situations, the amplitude of P3b is reported to be decreased when easy tasks are loaded.7 Fourth, orienting response may possibly be present in some subjects, because two subjects exhibited frontally distributed non-target P3s which were larger than task-relevant P3s in condition 1 (mentioned above). This mechanism can also explain merely part of the amplitude behavior of P3s in condition 1, because orienting response may habituate when presentations are repeated‚4–6 and because only some of the subjects showed frontally peaking non-target P3s.
Conclusions and possible mechanism
In conclusion, non-target visual P3 is suggested to be a consistent event-related potential which possibly reflects the processing of task-irrelevant, frequently presented visual stimuli. The amplitude of non- target visual P3 is influenced by either the variety of non-target colors or the shape of stimuli.
As well as other P3 (P300) components‚1,2,6 the amplitude behavior of non-target visual P3 could be explained by the resource allocation model. Figure 2 shows our hypothetical cognitive resource model for the amplitude of non-target visual P3, in which a limited quantity of cognitive resource for the processing of task-irrelevant frequent stimuli is divided and allocated for each kind of color and each kind of shape (character). In our model, the amount of each division is thought to be correlated with the amplitude of non-target P3, although the correlation might not be strict because of confounding factors such as individual difference, stimulus modality, and tasks.
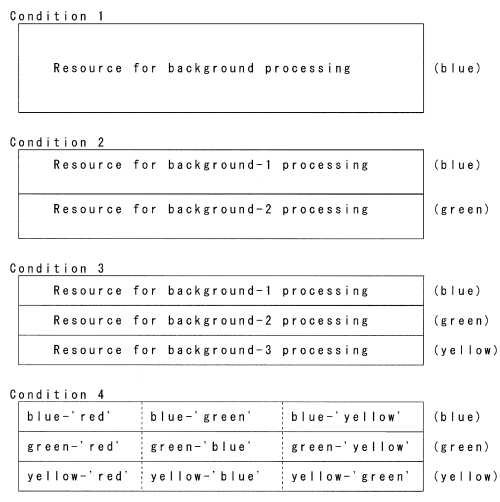
The hypothetical model of cognitive resource allocated for the processing of task-irrelevant frequently presented visual stimuli. A limited amount of cognitive resource is divided as the number of non-target colors increases (conditions 1, 2 and 3), and further divided for each kind of character (condition 4). The amount of resource in each division might have some relationship to the amplitude of non-target P3.
This hypothetical model could sufficiently explain the changes of amplitudes of non-target P3s in different conditions. By this possible mechanism, target/background ratio of resource allocation might be appropriately increased in everyday circumstance in which many irrelevant stimuli exist.
Further studies should be directed to determine whether resource allocation model for non-target P3 can explain psychopathological phenomena (e.g., disturbance of maintaining attention or ‘sustain’ element of attention21).




