Identification of mRNA-binding proteins during development: Characterization of Bufo arenarum cellular nucleic acid binding protein
Abstract
Ultraviolet irradiation was used to covalently cross-link poly(A)+RNA and associated proteins in eggs and embryos of the toad Bufo arenarum. Four major proteins with apparent sizes of 60, 57, 45 and 30–24 kDa were identified. It was observed that the same mRNA-binding proteins were isolated from eggs to gastrula and neural stages of development. The 30 kDa polypeptide, p30, appeared as the main ultraviolet (UV) cross-linked protein in the developmental stages analyzed. By means of polyclonal antibodies, it was determined that this polypeptide has a cytoplasmic localization and it was detected in liver, eggs and embryos. The presence of p30 was also analyzed by western blot during oogenesis and development. The 30 kDa polypeptide was present in all stages analyzed but it could not be detected in stages I–II of oogenesis. At the neural stage, the relative amount of p30 began to decrease, reaching its lowest levels after stages 26–30 (tail-bud in Bufo arenarum). On the basis of purification, immunoprecipitation and western blot assays the 30 kDa protein was identified as the Bufo arenarum cellular nucleic acid binding protein.
Introduction
The translational control of maternal mRNA is a major regulatory mechanism for gene expression during oogenesis, oocyte maturation and the first stages of embryogenesis (Standart 1992; Wickens 1992; Wormington 1993). Two main mechanisms have been proposed to contribute to this control. The first is through regulation of mRNA polyadenylation (Jackson & Standart 1990; Richter 1991; Bachvarova 1992). The second one involves the regulation of assembly and disassembly of mRNA in messenger ribonucleoprotein particles (mRNP) (Spirin 1966; Standart & Jackson 1994; Wormington 1994). Several studies showed that translation is not controlled by alterations in mRNA (Davidson 1986). It is widely assumed that proteins associated with these maternal mRNA mediate repression of translation (Richter 1987).
In Xenopus oocytes, many protein components of mRNP particles have been identified (Darnbrough & Ford 1981) and characterized with regards to their capacity to bind mRNA (Richter & Smith 1984; Dearsly et al. 1985; Kick et al. 1987; Richter 1987; Murray et al. 1991). In particular, the abundant Y-box protein, FRGY2, was able to inhibit mRNA expression, although this inhibition was not selective (Richter & Smith 1984; Ranjan et al. 1993; Bouvet & Wolffe 1994). Whether homologous proteins bind to stored maternal mRNA in other organisms remains unknown.
It has been proposed that at the appropriate time of development, the mRNA-binding proteins would be displaced by other specific proteins (Standart 1993) or modified and released, leading to translation activation (Murray et al. 1991; Walker et al. 1996). Nevertheless, the details of the molecular mechanisms of maternal mRNP assembly/disassembly and mobilization into polysomes have not been elucidated yet.
The aim of the present study was to identify and characterize RNA-binding proteins that associate with poly(A)+RNA in eggs and embryos of the toad Bufo arenarum. Using ultraviolet (UV) cross-linking and RNase digestion assays, we have identified the major poly(A)+RNA-binding proteins with apparent molecular masses of 60, 57 and 30–24 kDa. A 45 kDa polypeptide was also observed in the neural stage. By means of polyclonal antibodies, we characterized the subcellular tissue and developmental distribution of the 30 kDa polypeptide (p30) in Bufo arenarum. Through purification, immunoprecipitation and western blot analysis, p30 was identified as the Bufo homologue of Xenopus cellular nucleic acid binding protein (CNBP).
Materials and Methods
Collection of cells and embryos and isolation of germinal vesicles
Bufo arenarum specimens were collected in the neighborhood of Rosario City, and kept in a moist chamber at 15°C until used.
Oocytes were staged following the classification proposed by Dumont (1972) for Xenopus laevis. Stages I/II, III/IV and V/VI oocytes from unstimulated B. arenarum specimens were manually isolated from pieces of ovary in 0.1 mol/L sodium phosphate buffer (pH 7.2) containing 10 mg/mL collagenase, 10 mg/mL hyaluronidase and 20 mmol/L EDTA. Ovaries were stirred gently at room temperature for 60 min. Oocytes were collected and washed with 10% Barth solution (8.8 mmol/L NaCl; 0.1 mmol/L KCl; 0.24 mmol/L NaHCO3; 0.082 mmol/L MgSO4; 0.033 mmol/L Ca(NO3)2; 0.02 mmol/L CaCl2; 0.75 mmol/L Tris-HCl, pH 7.6).
Ovulation was induced by injecting a female in the dorsal lymphatic sack with an homogenate of homologous hypophysis. After 12 h at 18–22°C, animals were pithed and eggs were collected from ovisacs, hydrated in 10% Barth solution and dejellied with 1% thioglycolic acid, pH 8, in 10% Ringer. Eggs were inseminated with a sperm suspension obtained by mincing testes in 10% Barth, cultured in 10% Barth at 15–22°C and staged according to Del Conte and Sirlin (1951).
Germinal vesicles from 20 stage VI oocytes were separated manually under stereomicroscope from pieces of ovary in 1:5 relationship medium (87 mmol/L KCl, 17 mmol/L NaCl, 10 mmol/L Tris-ClH, pH 7.4, 100 μmol/L phenylmethylsulfonyl fluoride (PMSF)). The remaining ooplasmic material was used for preparing the cytoplasmic fraction. Both fractions were freeze dried and used for immunological assays.
Extract preparations
Unfertilized eggs and embryos were homogenized in four volumes of HB buffer (10 mmol/L MgCl2, 50 mmol/L KCl, 0.1% Triton X-100, 100 μmol/L PMSF, 60 g/mL polyvinyl sulfate (PVS), 20 mmol/L Tris-ClH, pH 7.6). In some experiments, 50 μg/mL leupeptin and 50 μg/mL pepstatin A (both from Sigma Chemical Co., St Louis, MO, USA) were used. Homogenization was carried out in an Omnimixer homogenizer (Omni Int., Wetesbury, USA) at 4°C during 3 min at low speed. Cellular debris including yolk and pigment were removed by centrifugation at 40 000 g at 4°C for 30 min.
Isolation of cross-linked poly(A)+RNA–protein complexes
Ultraviolet cross-linking assays were performed essentially as described (Swiderski & Richter 1988). Protein extracts (2 mg) were placed on ice and irradiated with UV light (2 × 105 erg/mm2) using a germicide UV lamp or a 254 nm UV lamp. When heparin was used, 5 mg/mL (final concentration) was added 15 min before irradiation.
Irradiated extracts were adjusted to a final concentration of 60% formamide, 0.5 mol/L NaCl, 0.5% sodium dodecyl sulfate (SDS) and heated for 5 min at 60°C. Yeast tRNA was added (13 μg/mL) and the samples were precipitated with three volumes of absolute ethanol at − 20°C overnight. The UV cross-linked RNA–protein complexes were pelleted at 12 000 g for 30 min at 4°C, resuspended and poly(A)+RNA–proteins were isolated by affinity chromatography on oligo(dT)-cellulose. Samples were digested with a cocktail of RNases (10 mmol/L Tris-HCl pH 7.6, 1% aprotinin, 2 μg/mL leupeptin, 2 μg/mL pepstatin A, 125 U/mL RNase T2, 0.1 mg/mL RNase A and 500 U/mL RNaseT1, all from Sigma Chemical Co.) during 30 min at 37°C and denatured at 100°C in the presence of 1% SDS and 70 μmol/L β-mercaptoethanol. Analysis of proteins was carried out on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), using 12% polyacrylamide gels (Laemmli 1970) and visualized by silver staining (Rabilloud et al. 1994).
Protein concentrations were estimated according to Lowry et al. (1951), using bovine serum albumin (BSA) as standard.
Antibody preparation
Antigen solution, containing 50 μg of the four major proteins with mRNA binding potential, was injected into rabbits to obtain polyclonal antibodies (Harlow & Lane 1988). Injections were repeated monthly, and from the third stimulation onwards, the anti-poly(A)+RNA-binding proteins titer was checked. Antibodies were reactive mostly for p30. To prevent cross-reactions, antibodies against p30 were affinity-purified. Briefly, ≈ 5 μg of purified poly(A)+RNA-binding proteins were resolved in SDS-PAGE and blotted overnight onto a nitrocellulose membrane (0.2 μm pore size; Bio-Rad, Richmond, CA, USA) at 0.8 V/cm. The piece of filter containing the polypeptide of 30 kDa was cut and treated essentially as described by Lewis et al. (1992). The affinity-purified antibodies (anti-p30) and pre-immune serum were stored in aliquots at − 70°C until used.
Western blotting and immunoprecipitation
After SDS-PAGE, proteins were transferred to a nitrocellulose membrane. The membrane was treated with blocking solution: 5% non-fat dried milk in phosphate-buffered saline (PBS; Johnson et al. 1984). Anti-poly(A)+RNA-binding proteins antibody diluted 250-fold in blocking solution was then added (Harlow & Lane 1988) and incubated for 1 h. Three washes were carried out with blocking solution. The membrane was incubated with anti-rabbit immunoglobulin G (IgG) coupled to peroxidase (Sigma Chemical Co.) diluted 1500-fold in blocking solution for 1 h. After washing, peroxidase activity was detected by incubating the sheet in diaminobenzidine (DAB) solution (100 μL of 20 mg/mL DAB in 0.5 N HCl, 7.5 μL of 30% H2O2, in 20 mL final volume of 100 mmol/L Tris-HCl buffer pH 7.2).
Immunoprecipitation experiments were carried out essentially as described by Pellizzoni et al. (1996). Briefly, anti-Xenopus CNBP antibodies (gift from Dr Pierandrei-Amaldi) were incubated with protein A-Sepharose CL-4B in 250 mL of Ipp150 buffer (10 mmol/L Tris-HCl pH 7.6, 150 mmol/L KCl, 0.1% (v/v) Nonidet P-40) on ice for 2 h with rotation. Following three washes with Ipp150 buffer, 50 μL of the 0.5 μg/μL purified poly(A)+RNA-binding proteins pool were added to the beads and brought to 0.25 mL with Ipp150 buffer. After 2 h on ice with rotation, the beads were washed four times with Ipp150 buffer. Supernatants and immunoprecipitated proteins, released from the beads by heating for 10 min at 80°C in the presence of SDS-gel loading buffer, were analyzed by SDS-PAGE and visualized by silver staining.
Microinjection in fertilized eggs, scintillation counting and fluorography
Seventy embryos at the early blastula stage (eight divisions) were injected in the blastocoele with 60 nL (5 μCi/μL) of S35-methionine (specific activity of 1230 Ci/mmol; New England Nuclear, Boston, MA, USA). Embryos were then cultured for 1 h in Barth solution and transferred to 10% Barth solution until the required stage. Staging was according to Del Conte and Sirlin (1951).
Embryo extracts were loaded in Whatman GF/A fiber discs and the radioactivity associated to proteins was measured as described (Mans & Novelli 1961).
Aliquots of extracts were also dissolved in 10 mL scintillation liquid and counted in a 1214 Rackbeta Walac liquid scintillation counter (Pharmacia, Uppsala, Sweden).
The UV cross-linked proteins from injected embryos were analyzed on polyacrylamide gels as described earlier. After electrophoresis, the gels were soaked with dimethylsulfoxide (DMSO) and 2,5-diphenyloxazole (PPO). They were dried and fluorographed with Kodak X-O mat AR film at − 80°C.
Protease treatment
One hundred microliter (50 μg of total proteins) of crude extracts were incubated for different times at 37°C with or without chymotrypsin. The final ratio of protease/total protein was 1:20. Adding PMSF at a final concentration of 1 mmol/L stopped the reaction. The treated extracts were directly analyzed by 12% polyacrylamide gel electrophoresis and the proteins were visualized by western blot.
Results
Detection of mRNA binding proteins
The UV cross-linking assays were used to identify proteins that bind poly(A)+RNA. Unfertilized eggs and embryos in the gastrula and neural stage from B. arenarum were homogenized, centrifuged and the supernatant was irradiated using a germicide UV lamp for different periods of time or with a 254 nm UV lamp for 15 min. Poly(A)+RNA was purified by affinity chromatography in oligo(dT) cellulose. The bound fraction was expected to contain, together with poly(A)+RNA, proteins that have a close interaction with them, the binding having turned covalent by UV treatment. After digestion with RNase, the fractions were analyzed by SDS-PAGE. Four major protein bands were detected by silver stain, their apparent molecular weights being estimated as 60 kDa (p60), 57 kDa (p57), 30 kDa (p30) and 24 kDa (p24). As for irradiation time, 15 min with germicide lamp proved to be the most suitable period in our working conditions (Fig. 1).

. Identification of poly(A)+RNA-binding proteins in Bufo arenarum oocytes. Poly(A)+RNA-binding proteins were isolated as described in the Materials and Methods. Fractions were analyzed by SDS-PAGE on a 12% polyacrylamide gel and silver stained. Proteins were isolated from 40 oocytes under different UV cross-linking conditions: Without UV irradiation (lane 1), 5 min irradiation with 15 W germicide lamp (lane 2), 15 min with 15 W germicide lamp (lane 3) and 15 min with 254 nm UV lamp (lane 4). Total egg proteins from extracts irradiated during 0, 5 and 15 min with 15 W germicide lamp were also analyzed (lanes 5–7). The molecular masses of marker proteins are indicated on the left.
To ascertain the degree of specificity involved in protein–mRNA interactions, the effect of the non- specific polyanion heparin on the binding of proteins on mRNA was tested. Heparin could also function as a RNase inhibitor. An increase of p30 binding was observed, without significant differences in p60 and p57 binding (data not shown).
Poly(A)+RNA-binding proteins during development
Poly(A)+RNA-binding proteins were identified in unfertilized eggs and embryos at early the gastrula and neural stages. In all cases, protein patterns obtained by SDS-PAGE followed by silver staining were very similar, p30 being the main protein detected in all stages analyzed (Fig. 2A,B). We therefore focused on this protein for further analysis.

. Identification of poly(A)+RNA-binding proteins in B. arenarum eggs and embryos. Analysis of proteins was carried out by SDS-PAGE on a 12% polyacrylamide gel and silver staining. (A) Proteins were isolated from 100 eggs (lane 1) and 100 embryos at gastrula stage (lane 2). (B) Proteins were isolated from 100 embryos at neural stage (lane 1); mRNP from 300 embryos at neural stage were also analyzed (lane 2). The molecular masses of marker proteins are indicated on the left.
We tried to discern whether p30 was actively synthesized in embryos or maternally inherited. Embryos in the early blastula stage were microinjected with 35S-methionine in the blastocoele and then cultured until the neural stage. After homogenization, 35S-methionine incorporation into proteins was measured. About 65 and 70% of the injected radioactivity was incorporated into proteins in the early gastrula and neural stages, respectively. Poly(A)+RNA-binding proteins from these stages were purified as described and analyzed by SDS-PAGE, silver stain and autoradiography (Fig. 3A,B). The 30 kDa polypeptide was again detected by silver staining. However, it showed no radioactive signal. Then, despite significant incorporations of 35S-methionine into total proteins, no de novo synthesis of p30 was observed (Fig. 3B), although this polypeptide was clearly present (Fig. 3A). These results indicate that most p30 is maternally synthesized.
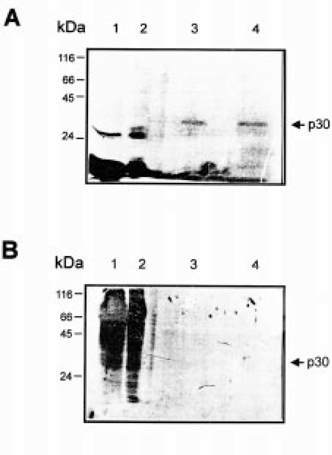
. Poly(A)+RNA-binding proteins during development. (A) Embryos at early blastula (8-division) stage were microinjected with 35S-methionine as described in the Materials and Methods. Total protein (25 000 c.p.m.) from embryos at neural stage (lane 1) and gastrula stage (lane 2) and poly(A)+RNA binding proteins purified from five embryos at neural stage (lane 3) and 20 embryos at gastrula stage (lane 4), respectively, were analyzed by SDS-PAGE on a 12% polyacrylamide gel and silver stained. (B) Fluorography from SDS-PAGE showed in A. Time of exposure was 2 weeks. The molecular masses of marker proteins are indicated on the left.
Immunological analysis of p30
The presence of p30 was analyzed on pure poly(A)+RNA-binding protein samples and on crude extracts by western blot. When immunodetection assays were carried out on purified proteins, the main band identified always showed a relative mobility corresponding to a molecular size of 30 kDa. However, in crude extracts p30 was almost not detected and the main immunoreactive protein showed a relative mobility of 20–24 kDa (p24). If protease inhibitor leupeptine and pepstatin A were added during extract homogenization, p30 rather than p24 was observed (Fig. 4A), suggesting that p24 is a specific proteolytic product of p30. Limited proteolysis assays were carried out to confirm this possibility. Egg extracts were processed in the presence of protease inhibitors and then incubated for different periods of time with or without chymotrypsin. Western blots showed an increase of p24 associated to a p30 decrease upon incubation with chymotrypsin (Fig. 4B). Similar results were obtained when limited proteolysis assays were carried out on UV-irradiated crude extracts (data not shown). These data agree well with observations on protease inhibitor treatments, and support the notion that the 24 kDa band detected by western blot was a cleavage product of p30.
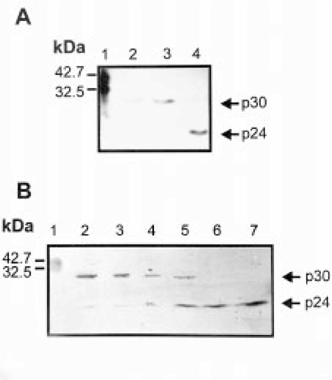
. Identification of p24 as a proteolytic product of p30. (A) Total proteins obtained from eggs in the presence (lane 2, 25 μg; lane 3, 50 μg) or in the absence (lane 4, 50 μg) of protease inhibitors were analyzed by SDS-PAGE on a 12% polyacrylamide gel, transferred to nitrocellulose membranes and probed by western blot with the anti-p30 antibodies. Prestained molecular weight standards were applied (lane 1). (B) Total proteins obtained from eggs were subjected to proteolysis during 0, 5, 15, 30, 60 and 120 min (lanes 2–7). Fifty micrograms of total protein were applied to each lane of a western blot probed with anti-p30 antibodies. Prestained molecular weight standards are shown on lane 1.
Subcellular localization, tissue-specific expression and developmental behavior of p30
To determine the subcellular localization of p30, nuclear extracts from stage VI oocytes were prepared from manually collected germinal vesicles. Nuclear and cytoplasmic extracts from 20 oocytes were then subjected to SDS-PAGE. The intracellular distribution of p30 was established by western blot. Results clearly showed that p30 is present only in the cytoplasmic fraction (Fig. 5A).

. Subcellular localization (A), tissue-specific expression (B), and developmental behavior (C) of p30. Total proteins were analyzed by SDS-PAGE on a 12% polyacrylamide gel, transferred to nitrocellulose membranes and probed on western blot with the anti-p30 antibodies. (A) Purified poly(A)+RNA-binding proteins (5 μg) were used as positive control (lane 1). The ooplasmic fraction (lane 2) and nuclear fraction (lane 3) from 20 oocytes were analyzed. (B) Lysates (50 μg of total proteins) from kidney, oocytes, liver, heart, serum, sperm and lung were applied to lanes 1–7, respectively, and western blotted. (C) Total protein (25 μg) from stages I/II, III/IV and V/VI of oogenesis (lanes1–3), celomic oocytes (lane 4), mature oocytes (lane 5) and embryos at 1-, 8-, 9-, 14-, 19- and 21-division stage (lanes 6–11) were applied to each lane.
Expression of p30 was analyzed in different tissues of B. arenarum specimens. Homogenates of liver, heart, lung, kidney, eggs, and sperm, together with serum were assayed by SDS-PAGE; proteins were transferred to nitrocellulose membranes and p30 was revealed by immunoreaction. This protein was detected in liver, serum and eggs and was absent in the other tissues analyzed (Fig. 5B).
The characterization of p30 during oogenesis and early embryo development was also performed by western blot. The polypeptide was not detected in stage I/II of oogenesis, being present from stage III/IV of oogenesis until the tail-bud stage (Fig. 5C). Results obtained showed that p30 is present up to the last stages of oogenesis, and during embryogenesis until the neural stage. From this stage onwards, a slight decrease in the relative amount of the protein was observed, reaching its lower level in the tail-bud stage.
Identification of p30 as the B. arenarum CNBP protein
The biochemical properties of p30 suggest that this polypeptide may be related to the X. laevis CNBP described by Pierandrei-Amaldi’s group (Pellizzoni et al. 1997). To evaluate this possibility, immunoprecipitation experiments were carried out on purified poly(A)+RNA-binding proteins using anti-Xenopus CNBP rabbit antiserum. The polypeptide of 30 kDa was immunoprecipitated by the CNBP antiserum, but not by pre-immune serum. No other poly(A)+RNA-binding protein present in the sample was immunoprecipitated by anti-Xenopus CNBP (Fig. 6A). On the other hand, western blots of B. arenarum eggs, embryos and liver extracts, using anti-Xenopus CNBP, showed the same pattern as those obtained with anti-p30 antibodies, recognizing both the native p30 and p24 proteolytic form. Pre- immune serum was not reactive against p30 (Fig. 6B). These results indicate that the polypeptide of 30 kDa identified in this work is the B. arenarum homolog of Xenopus CNBP.
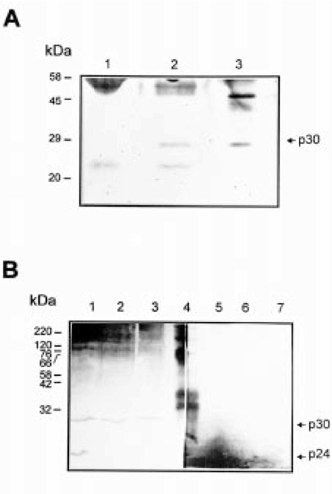
. Identification of p30 as the CNBP protein. (A) Immunoprecipitation analysis. Purified poly(A)+RNA-binding proteins from oocytes were immunoprecipitated with a pre-immune serum (lane 1), or with an anti-Xenopus CNBP antiserum (lane 2). Poly(A)+RNA-binding proteins (5 μg) were used as positive control (lane 3). Samples were analyzed by SDS-PAGE on 12% polyacrylamide gel and silver stained. Molecular mass markers are indicated on the left. (B) Western blot analysis. Total protein (50 μg) from oocytes (lanes 1,5), embryos at neural stage (lanes 2,6) and liver (lanes 3,7) were applied to each lane of a western blot probed with an anti-Xenopus CNBP antibody (lanes 1–3), or with a pre-immune serum (lanes 5–7). Pre-stained molecular mass markers are shown on lane 4. The molecular masses of marker proteins are on the left.
Discussion
We report here the identification of the major proteins associated with poly(A)+RNA in eggs and embryos of the toad B. arenarum.
Proteins with apparent sizes of 60, 57 and 30–24 kDa were identified by means of UV cross-linking and RNase treatment. In the case of extracts from neural stage embryos, we also found a 45 kDa polypeptide to be cross-linked to poly(A)+RNA under our experimental conditions. As crude extracts become less turbid in later developmental stages because of a lower content of yolk platelet, the finding of a 45 kDa polypeptide in these embryos could be related mainly to increased photocross-linking efficiency.
Isolation of the mRNA-binding proteins was dependent on the UV irradiation period and the UV wavelength used (Fig. 1), although independent of the presence of heparin. Moreover, no proteins were detected by SDS-PAGE when UV irradiation (Fig. 1) or prior RNase treatment were omitted (Fig. 2B), suggesting that the proteins identified in the present work actually interact with RNA. It is important to note that protein–RNA cross-linking was shown to occur in the absence of exogenously added mRNA. Therefore, the ratio of poly(A)+RNA to their associated proteins was kept under conditions that closely mimic those occurring in vivo, indicating that UV cross-linking is a reliable procedure to identify proteins that specifically interact with poly(A)+RNA in B. arenarum eggs and embryos.
Differences in the prevalent types of RNP proteins present in oocytes and somatic cells have been reported (Swiderski & Richter 1988), suggesting a turnover of RNP during differentiation. Likewise, maternal mRNA stored in the oocytes is mostly inactive (Davidson 1986). It has been suggested that mRNA-binding proteins prevent translation in oocytes, and selective replacement of these proteins by others during development allows specific mRNA to engage translation (Standart 1993). However, we observed that the same photocross-linked proteins were isolated throughout eggs to the gastrula and neural stage. The finding that similar sets of proteins are associated with mRNA in different developmental stages in B. arenarum raises the question as to whether these proteins are translational regulators. Therefore, translational regulation could not be a consequence of the displacement of these mRNA-binding proteins by other specific proteins.
The 30 kDa polypeptide described in the present paper appeared as the main UV cross-linked protein in the developmental stages analyzed (Fig. 2). When immunoassays were performed in crude extracts with anti-p30 antiserum, a reactive band of 20–24 kDa was found instead of the 30 kDa polypeptide. The present data indicate that both polypeptides represent different forms of the same protein as a result of specific proteolytic cleavage.
In the course of oogenesis, the major activities occurring within the oocyte are the synthesis of RNA and the deposition of yolk. The process of vitellogenesis begins at stage III and decreases to very low levels at the last stage of oogenesis (Dumont 1972). During this period the oocyte is transcriptionally active, although its own contribution to protein synthesis is small compared with those reaching the oocytes from outside. We studied the presence of p30 during oogenesis and early development by western blot. The 30 kDa protein was present from stage III of oogenesis to eggs but it could not be detected in stages I–II. We still do not know whether p30 is synthesized in the oocyte or elsewhere in the animal body and transported by blood to the oocyte. Our data show that most p30 is not synthesized de novo in embryos and is present in blood and liver but not in other tissues. It seems reasonable to assume that p30 is synthesized in the liver and imported by the oocyte from stage III of oogenesis. However, we do not have conclusive evidence about this hypothesis so far.
According to their molecular sizes, p60 and p57 could be related to FRGY2 or to proteins that bind the 5′UTR region of L1 mRNA (Cardinali et al. 1993). It is widely known that mRNA for ribosomal proteins (rp-mRNA) is about 10% of total mRNA. Moreover, a similar 5′UTR region is present in other mRNA encoding proteins related to the synthesis and function of the translational apparatus (Loreni et al. 1993; Jefferies et al. 1994; Terada et al. 1994). Together, these classes of mRNA represent about 15–20% of animal cell mRNA (Meyuhas et al. 1996). Therefore, as we do not add exogenous RNA to our preparations prior to UV irradiation, it is likely that the poly(A)+RNA-binding proteins described here are the rp-mRNA-binding proteins reported by Pierandrei-Amaldi’s group (Cardinali et al. 1993).
The 31 kDa rp-mRNA-binding protein was identified as the Xenopus homolog of human CNBP (Pellizzoni et al. 1997), its sequence being highly conserved within all the animal species analyzed. Bufo p30 shares some properties with that polypeptide: mRNA-binding potential, molecular size, proteolytic cleavage to yield a 24 kDa product, cytoplasmic localization and presence in liver, oocytes and embryos. On the basis of immunoprecipitation and western blot assays using specific anti-Xenopus CNBP antibodies, we conclude that p30 is the B. arenarum homolog of Xenopus CNBP (Fig. 6A,B).
Cellular nucleic acid binding protein was originally reported as a transcription factor involved in the cholesterol biosynthetic pathway (Rajavashisth et al. 1989), the regulation of CT elements (five imperfect direct repeats of the CCCTCCCCA sequence) of the c-myc oncogene (Michelotti et al. 1995), and on the human β-myosin heavy chain gene expression (Flink & Morkin 1995). However, CNBP has been shown to be a cytoplasmic protein (Warden et al. 1994; Fig. 5A), suggesting that it may interact preferentially with RNA.
It has been shown that Xenopus rp-mRNA begins to be synthesized and accumulated at the blastula stage, but is mostly kept unused as light mRNP that is mobilized into polysomes only at the tail-bud stage (Pierandrei-Amaldi et al. 1982; Baum & Wormington 1985). Moreover, it has been proved that the 5′UTR of rp-mRNA is involved in its translational regulation during Xenopus development (Mariottini & Amaldi 1989). We have observed that the relative amount of p30 was much reduced in the 19-division stage compared with early stages and it was not detected in the 21- division stage at all. These data suggest a relationship between the amount of protein and the activation of translation of rp-RNA. Nevertheless, the specific functions of either CNBP or p30 still remain unclear. Work is currently in progress to address these questions.
Acknowledgements
We gratefully acknowledge Dr Paola Pierandrei-Amaldi, Istituto di Biologia Cellulare CNR of Roma, Italy and her associates for the generous gift of antibodies against the Xenopus-CNBP.
This work was partially supported by Grants from Fundación Antorchas, CONICET, ANPCyT, and Universidad Nacional de Rosario. N.B.C. and M.O.C. are staff members and J.F.P. is a Fellow of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. J.F.P. and D.M.B. contributed equally to this work.




