Assessment of tissue damaging effects of mixed micellar absorption enhancers on the intestinal mucosa of common carp (Cyprinus carpio), African catfish (Clarias gariepinus) and rainbow trout (Oncorhynchus mykiss) as a consequence of enhanced intestinal absorption of sGnRH-a
Abstract
Non-ionic surfactant–polyoxyethylene sorbitan monooleate (Tween-80) and oleic acid are food and pharmaceutical ingredients for oral and parenteral delivery and generally regarded as safe (GRAS). They have the potential as an intestinal absorption enhancer for the development of oral drug delivery systems. However, their safety in terms of mucosal integrity has yet to be evaluated. Therefore, the purpose of the present work was to study the tissue damaging effects of Tween-80, oleic acid and of their mixed micellar formulation (oleic acid + Tween-80). This was investigated at 2 h and 24 h after rectal delivery and compared with the topical effect of polyoxyethylene 9 lauryl ether (Polydocanol). The same experiment was carried out on three species with distinct feeding habits: the common carp, Cyprinus carpio (L.); the African catfish, Clarias gariepinus (Burchell); and the rainbow trout, Oncorhynchus mykiss (Walb.). Based on the findings of this study it was concluded that Tween-80 (4%), or its mixed micelle with oleic acid (0.6%) can be considered as a safe formulation, inducing only a moderate alteration of the intestinal mucosa, comparable to the effect of an isotonic saline. By contrast, in the three species, the same dose of Polydocanol, or of its mixed micelle with oleic acid, induced severe tissue damage of the intestinal mucosa, still present after 24 h. Mixed micelles of oleic acid with Tween-80 were also demonstrated as increasing the intestinal absorption of the salmon gonadotropin releasing hormone analogue (sGnRH-a) in catfish, C. gariepinus, and consequently stimulating GtH II secretion. This effect was compared with the action of other drugs considered as intestinal absorption enhancers.
Introduction
Oral delivery is potentially the most useful for mass treatments and long-term therapy of aquatic organisms. Methods of oral delivery such as oral vaccination (Dunn et al. 1990) and oral spawning induction (Roelants et al. 1995) are under investigation. However, oral delivery can only be an attractive alternative for injection or implantation if sufficient absorption is achieved. The natural intestinal systemic absorption of macromolecules, such as protein antigens or peptide hormones, is too limited to obtain a cost-effective oral delivery form. However, the intestinal permeability for proteins and peptides can be enhanced dramatically (Hertz et al. 1991; Breton et al. 1998; Roelants et al. 2000; Mikolajczyk et al. 2001). For this study we selected Tween-80 (FDA Ref. 172.840), a detergent proven to be a potent intestinal absorption enhancer (Roelants et al. 2000; Mikolajczyk et al. 2001). Moreover, this compound meets all specifications of the US federal regulations and food chemistry code (HMSO 1962) and is currently used as a food additive and in pharmaceutical products for internal use. To assess its safety, a study was carried out on three teleost species to define the damaging effects of Tween-80 and its mixed micelles with oleic acid on the integrity of the intestinal mucosa. Another nonionic surfactant, Polydocanol, had been involved as a negative control for a comparison with potential tissue damage. Although Polydocanol has been proven to be a powerful enhancer of intranasal growth hormone in rats (Fisher et al. 1991) and rectal eel calcitonin (Morimoto et al. 1984) absorption, its membrane-damaging effect has been well documented (Chandler et al. 1991).
Additionally, the combination of Tween-80 and oleic acid in a mixed micellar delivery system was used in the present study in order to enhance the intestinal uptake of a salmon gonadotropin-releasing hormone analogue (sGnRHa) in catfish and to compare its action with the effect of several other surfactants. This was estimated by the ability of sGnRHa to stimulate the secretion of the maturational gonadotropin (GtH II).
Materials and methods
Experimental design
Experiments were performed in three laboratories on three fish species with different feeding habits: common carp, C. carpio (warmwater omnivorous, stomachless, Cracow), rainbow trout, O. mykiss (cold water carnivorous, Rennes) and African catfish, C. gariepinus (warmwater carnivorous, Leuven). All experiments on the assessment of the topical effect were performed following the same protocol. Analysis of histological preparations was done in Leuven by one person only.
Chemicals
Polyoxyethylene sorbitan monooleate (Tween-80), Polyoxyethylene 9 lauryl ether (Polydocanol) and chicken egg white trypsin inhibitor type II were purchased from Sigma Chemical Co (St Louis, MO, USA); sodium oleate from BDH Laboratory Supplies (UK); EDTA disodium salt from J.T. Baker B.V.; and domperidone from Pharmaceutical Janssen (Beerse, Belgium). The salmon gonadotropin-releasing hormone analogue used in this study was (Des-Gly10, D-Arg6, Trp7, Leu8, Pro9)-LHRH ethylamide from Bachem Feinchemikalien AG (Switzerland). Oleic acid (pharmaceutical grade) was a gift from Mosselman Ltd (Belgium). Polyoxethylene triglyceride (Atlas G-1284), polyoxyethylene castor oil (Arlatone 650), polyglycerol ester of fatty esters (SCS 2064), and polyoxyethylene (10) oleyl alcohol (Brij96) were provided by ICI Europe Ltd (Everberg, Belgium). All formulations used were freshly prepared in a phosphate-buffered saline (PBS) (pH=7.4; 0.058 M) just prior to the experiments; all treatment groups in the different experiments received the same volume of vehicle of 1 mL/kg bw.
Animals
Mid-February, 40 male carp (body weight of 900 ± 165 g, mean ± SD) originating from the Experimental Fish Farm of the University of Agriculture in Cracow, were netted from an outdoor stocking pond and brought into the Department of Ichthyobiology and Fisheries laboratory to be anaesthetized in an ethylene glycol monophenyl ether solution (0.15 ml/L), weighed and tagged. Groups of 20 males were placed in seven 1000-L flow-through basins and acclimated for 3 days at 20.0 ± 0.5 °C at an ambient photoperiod (13 h dark : 11 h light). The fish were not fed before and during the experiment. The absorption enhancer preparations were rectally introduced using a polyethylene tube fitted to a syringe.
At the end of March, 42 immature rainbow trouts, O. mykiss (average weight ± 300 g) originating from the INRA fish farm in Drennec, France, were transferred to the laboratory in Rennes. They were acclimated for 3 days to 10–12 °C and an ambient photoperiod (12 h dark : 12 h light) in two 1-m2 plastic tanks supplied with recirculated water. After anaesthesia in an ethylene glycol monophenyl ether solution (0.3 ml/L), they were individually tagged and the absorption enhancers rectally introduced using a polyethylene tube fitted to a syringe.
The catfish, C. gariepinus, were raised in a recirculated water system in the Katholieke Universiteit van Leuven (K.U.L.) aquaculture laboratory at a temperature of 26 ± 1 °C and a photoperiod of 12 h dark : 12 h light. The same environmental conditions were applied during the experiment. Before the experiment began, the fish were starved for 3 days. For the experiment on absorption enhancement, 60 catfish weighing 787 ± 287 g (mean ± SD) and for the assessment of the damaging effect 62 catfish (220 ± 7 g) were netted and anaesthetized in ethylene glycol monophenyl ether (0.5 ml/L) for tagging, weighing and rectal intubation of the absorption enhancers.
Treatment protocols
To establish the mixed micelle of the non-ionic surfactants (4%) and oleic acid (or sodium oleate) (0.6%) the components were vigorously mixed by a sonicator. Single components of these delivery forms were treated in the same way and delivered at the same dose. Rectal intubation was achieved by insertion of 6 cm of polyethylene tubing (Habia, I.D. 1.2 mm) attached to an injection needle and tuberculin syringe into the intestine via the anus.
Enhancement of sGnRHa absorption
For the experiment on the absorption enhancement of sGnRHa (Table 1), catfish starved for 48 h were removed from their holding facilities and anaesthetized in a solution of 2-phenoxyethanol (0.5 ml/L) for drug delivery or blood sampling. Just prior to rectal intubation the fish were intraperitoneally injected with domperidone (5 mg/kg). Blood samples (200 μL) were collected at 0, 2 and 4 h post-treatment from the caudal vessel, mixed with 5 μl of Na-citrate (4%), centrifuged and the plasma stored at −20 °C until assay. Plasma GtH II quantification was carried out by RIA as previously described (Schulz et al. 1993).
Assessment of the tissue-damaging effect of the absorption enhancers on gut mucosa
For this part of the study the same protocol was used for all species. However, in African catfish, one additional experiment was carried out in which oleic acid was replaced by sodium oleate. From each assigned treatment group (n=6), three fish were decapitated for tissue sampling at 2 h after drug delivery, with another three fish 24 h later. For the tissue sampling the delivery device was inserted once more in the same position and the intestines surgically excised at a point of 1.5 cm from the tip of the delivery tubing. All formulations were tested in groups of three animals in a non-randomized way.
The tissue was fixed in Bouin-Hollande and dehydrated in a series of ethanol of increasing concentration; the fixed tissue was embedded in paraffin. Six-micrometer slices were stained with haematoxylin and eosin. The sections were histologically graded on an eight-point scale as presented in Table 2. Intermediate scores were given if the mucosal appearance could not be assigned unambiguously to one of the stages of the scale. All scoring was performed in a blind fashion by one observer.
Statistical analysis
The Wilcoxon test was used to compare pre-treatment and post-treatment plasma GtH II levels within experimental groups. Comparison of plasma GtH II concentrations between experimental and control groups were made using the Mann–Whitney U-test. The Statistical Analysis System (SAS) was used to perform the two-way ANOVA and the NPariway procedure was used to investigate possible differences in intestinal mucosa alteration between different species, between different times after delivery and between several drug forms.
Results
Intestinal absorption enhancement of sGnRHa (Fig. 1)
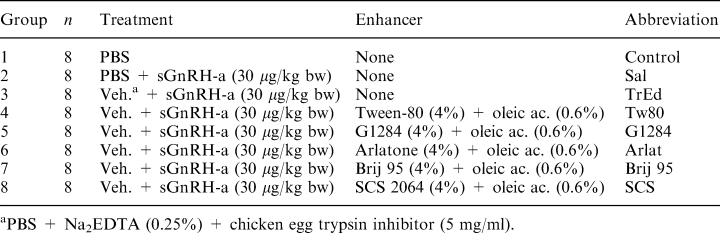
Effects of 30 μg/kg bw of sGnRHa, delivered rectally, in domperidone (5 mg/kg bw) pretreated African catfish, C. gariepinus, on the release of maturational gonadotropin (GtH II) as indicated in Table 1. The mean plasma levels of GtH II are expressed as bars; lines represent the upper SEM range (n=8). Symbols for significance difference: #= significantly different from control group, ●=GtH II values significantly different from the SAL group, x = significantly different from the Tween-80 group ▮= mean GtH II values significantly different from the TrED group. Ovulation ranges having the same letter are not significantly different. Treatment groups are described under Table 1
Micelle of Tween-80 or polyoxyethylene triglyceride (Atlas G-1284) with oleic acid enhanced the uptake of sGnRHa, as after 2 and 4 h significantly higher plasma GtH II levels were obtained as compared to rectal delivery of sGnRHa in PBS. On the basis of the ability to stimulate GtH II secretion or induce ovulation, in the African catfish, Arlatone, Brij 85 and SCS 2064 mixed micelles with oleic acid appeared to be less effective absorption enhancers than Tween-80 with oleic acid or G-1284 with oleic acid mixed micelles. The absorption enhancement effect of Na2EDTA (0.25% w/v) + trypsin inhibitor (5 mg/ml) was also demonstrated, as at 4 h GtH II levels were significantly higher as compared to the rectal delivery of sGnRHa in the saline vehicle.
Tissue-damaging effects of the Tween-80/oleic acid absorption enhancers and comparison with the polydocanol/oleic acid micelles: the three species model (Fig. 2; Table 3)
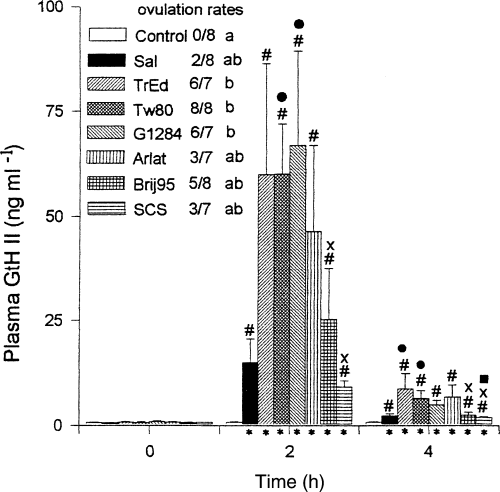
The effect of the Tween-80/oleic acid absorption enhancers and comparison with the Polydocanol/oleic acid micelles on the integrity of intestinal mucosa in three fish species expressed as histological index of damage. Con = intact fish (control); Sal = phosphate-buffered saline; Pd=4% w/v Polydocanol in PBS; Tw=4% w/v Tween-80 in PBS; Ol=0.6% w/v oleic acid in PBS; Ol/Pd = 0.6% w/v oleic acid +4% w/v Polydocanol in PBS; Ol/Tw = 0.6% w/v oleic acid + 4% w/v Tween-80 in PBS; So=0.6% w/v sodium oleate in PBS; So/Pd = 0.6% w/v sodium oleate + 4% w/v Polydocanol in PBS; So/Tw = 0.6% w/v sodium oleate + 4% w/v Tween-80 in PBS. Statistics are displayed in Tables 2 and 3
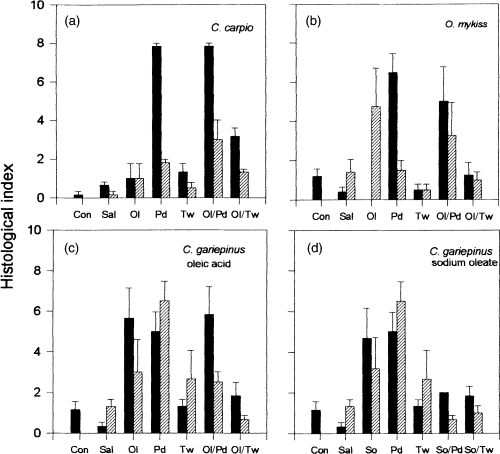
There was no species difference in the damaging effect observed (P > 0.1193) and a clear treatment/species interaction could be established at 2 h (P < 0.0023) and 24 h (P < 0.0145) after delivery of the different forms of absorption enhancers. It was found that Polydocanol (P < 0.0001) and the mixed micelle of oleic acid with Polydocanol (Ol/Pd; P < 0.0001) had a clear damaging effect on the intestinal mucosa as compared to the untreated group 2 h after treatment. Comparison of the histological index 24 h after treatment with the untreated control indicated that there is a long-lasting damage of the intestinal mucosa by 0.6% oleic acid (P < 0.004), 4% Polydocanol (P < 0.0042) and their mixed micelle (P < 0.0139). The mixed micelle of Tween-80 (4%) and oleic acid (0.6%) induced a moderate alteration of the intestinal mucosa which completely recovered after 24 h.
In all three species, Tween-80 alone and in combination with oleic acid had little or no effect on the gut mucosa 2 h after administration; the gut mucosa recovered almost fully after 24 h. On the contrary, oleic acid alone, Polydocanol alone and in combination with oleic acid induced rapid damage of the gut mucosa, which was long-lasting and seemingly irreversible since it was still clearly visible after 24 h.
Common carp, C. carpio (Table 4 panel A; 2Fig. 2a)
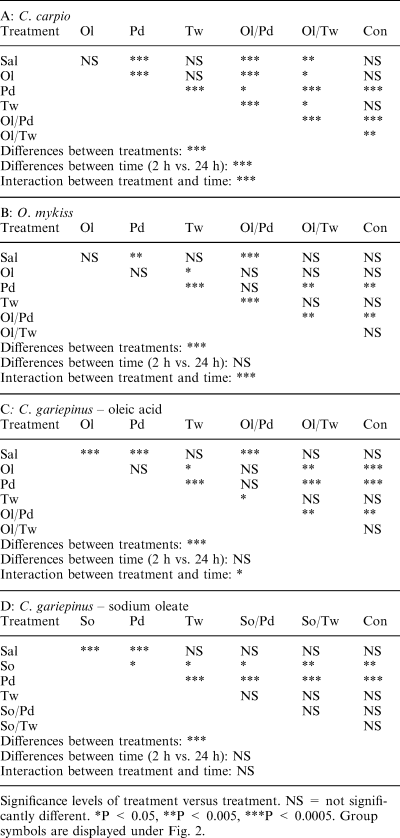
In carp, the saline vehicle (PBS), Tween-80 (Tw) and oleic acid (Ol) did not have a negative effect on the intestinal mucosa integrity as compared to the untreated control. On the other hand, Polydocanol (Pd), the mixed micelle oleic acid + Polydocanol (Ol/Pd) and the mixed micelle oleic acid + Tween-80 (Ol/Tw) had clear damaging effects on the intestinal wall – the significant levels being P < 0.0001, P < 0.0001 and P < 0.0014, respectively. Comparison with the saline treatment (PBS) confirmed these observations. Polydocanol (P < 0.001), the mixed micelle Ol/Pd (P < 0.0001) and the mixed micelle Ol/Tw (P < 0.0043) had negative effects on the intestinal mucosa. This effect of the mixed micelle Ol/Tw was less severe than that of the Ol/Pd micelles (P < 0.0001). In both cases the addition of oleic acid increased the topical effect of the surfactants. The injured intestinal mucosa was, however, able to recover since the histological indices were significantly lower at 24 h when compared with 2 h after treatment (P < 0.0001).
Rainbow trout, O. mykiss (Table 3 panel B; 2Fig. 2b)
The microscopic anatomy of the trout gut showed that, in comparison with the untreated control group, there was a severe alteration of the mucosal integrity by Polydocanol (Pd; P < 0.0026) and its mixed micelle with oleic acid (Ol/Pd; P < 0.0026). Comparison with the isotonic saline (PBS) control group confirmed this observation, the significance levels being P < 0.001 for Pd and P < 0.006 for the Ol/Pd treatment. Oleic acid alone (Ol), Tween-80 and their mixed micelle had no effect. The histoindices 2 h and 24 h after treatment were not significantly different. However, there was a significant interaction between the treatment and recovery time.
African catfish, C. gariepinus (Table 3 panels C, D; 2Fig. 2c,d)
It was demonstrated (Table 3 panel C; 2Fig. 2c) that oleic acid (P < 0.0009), Polydocanol (P < 0.0001) and the mixed micelle Ol/Pd (P < 0.0005) caused a significantly damaging effect on the intestinal mucosa as compared with the untreated control group. Similarly, the histological index after oleic acid treatment (Ol), Polydocanol treatment (P < 0.0001) and treatment with their mixed micelle (P < 0.0005) scored a significantly higher histological index of mucosal damage than did treatment with the isotonic saline (PBS). Oleic acid, Tween-80 and their mixed micelles did not affect the integrity of the intestinal mucosa. The ANOVA also confirmed that, in the African catfish, there was a clear damaging effect of the treatment on the intestinal mucosa (P < 0.0001). At 2 h and 24 h after treatment the histological indices were not significantly different. However, there was a significant interaction between treatment and recovery time. Although Polydocanol caused severe damage of the catfish intestinal mucosa, its mixed micelle with sodium oleate (So/Pd) did not significantly (Table 3 panel D; 2Fig. 2d) alter the mucosal integrity.
Discussion
The present study demonstrates that a Na2EDTA + trypsin inhibitor formulation or mixed micelles of non-ionic surfactants with oleic acid can efficiently increase the systemic bioavailability of sGnRHa in catfish, C. gariepinus. This confirms our previous results obtained in African catfish as well as in other fish species (Breton et al. 1998; Roelants et al. 2000). However, in the development of drug formulations with absorption enhancers, safety studies are required to assess the reversibility of effect on the mucosal structure at the intestinal delivery place. This is a first study to evaluate enhancer safety in terms of the effect on the mucosal integrity in fish.
In mammals, a damaging effect on the small intestinal mucosa has already been reported for sodium deoxycholate, sodium lauryl sulphate polyoxyethylene glycol, NSAID, ethanol and bile salts. The NSAIDs frequently cause an inflammation of the small intestine (Bjarnaso and Peters 1989; Moghal and Jafarey 1989). Acute ethanol intake causes an increase in number of damaged villi and denaturation of the villi tops (Millan et al. 1989). Bile acids were reported to induce mucosal damage in the small intestine; the acids cause increased cell loss at the tops of the villi and a shortening of the villi. These morphological changes probably result in an increased permeability of the mucosa (Teem and Phillips 1972; Ammon et al. 1985; Erickson 1988). There are also reports of an inhibitory influence of lipoidal components on the membrane-damaging effect of detergents. Feldman et al. (1973) observed an increased absorption rate of salicylate in the averted jejunal segments of the rat due to 10 mM taurodeoxycholate (TDC), which was accompanied by an alteration in the mucosal membrane. When an additional 3 mM oleic acid or 1 mM monoolein was added, an alteration of the mucosal membrane could be prevented.
From the present study, however, it appeared that the oleic acid/Polydocanol-based mixed micellar absorption enhancers induced a more severe epithelial alteration than did each component separately. Sodium oleate, on the other hand, had a clear protective effect. The data of the histoindices of our investigation also showed that there is a recovery from damage after 24 h. A re-epithelization was observed for treatment groups in which losses of epithelial cells were discovered 2 h after drug delivery. The presence of cuboidal or flattened cells, which could be explained by cell damage, is not a direct manifestation of epithelial cell damage but the result of a restitutional process. Epithelial cell loss is quickly followed and compensated by restitution, e.g. migration of immature cells from the crypts to the intercryptal surface, thus restoring the epithelial defect. Henrikson et al. (1989) described this process after several types of epithelial cell damage. Van Hoestenberghe et al. (1991) described a recovery to apparently normal in rats, but with cuboidal cells 2 h after epithelial cell loss.
On the basis of the findings reported in the present study, a strategy for further investigations can be proposed. In future research, drug bioavailability will have to be studied in relation to the alteration in mucosal structure. Since Tween-80, in contrast to the Tween-80/oleic mixed micelles, caused no microscopically observable modifications of the intestinal mucosa in carp, it would be interesting to measure in a similar study the absorption-promoting effect of both formulations.
Despite the clear enhancement of intestinal absorption of sGnRHa in catfish when using the mixed micelle of Tween-80/oleic acid, our study clearly showed that in carp, catfish and trout this formulation has only a moderate (carp) or no negative effect on the gut mucosa integrity (trout and catfish) 2 h after administration. An earlier study, which correlated the absorption-promoting capacity with membrane-damaging effect, also indicated that a direct relation does not exist between intestinal absorption and the topical effects on gut integrity (Merkus et al. 1992).
Since no damaging effects were observed 24 h after treatment (in carp, trout and catfish), it was concluded that there is no irreversible damage to the intestinal mucosa induced by this formulation. From the view point of biosafety it is an interesting formulation to be used in intestinal drug delivery systems. An original technology was recently developed in order to transform the liquid form of the above-mentioned formulation into solid form, more suitable for food processing (Roelants et al. 1997).
Acknowledgements
The authors wish to thank Mosselman Ltd, Brussels, Belgium and ICI Europe Ltd, Everberg, Belgium for kindly providing free product samples. The research was partially funded by a grant from the Polish government, the Catholic University of Leuven and INRA. The visit of R.I. to Poland was supported by a Belgium – Poland Scientist Exchange Grant from the Flemish Ministry of Education.