The effect of temperature on Aeromonas hydrophila infection in goldfish, Carassius auratus
Abstract
The effect of temperature on Aeromonas hydrophila infection in goldfish, Carassius auratus, was studied using A. hydrophila strain A-3500. After comparison of four different infection methods, subcutaneous injection was selected. Different test temperatures were also tested and higher mortality was observed at 17 and 25°C during a 15-day period. SDS-PAGE analysis of outer membrane proteins prepared from A. hydrophila cultured at 10, 17, 25 and 32°C in formulated salt water showed different protein profiles. For example, a 40-kDa band was found only at 17 and 25°C. Phagocytic rates of A. hydrophila by goldfish macrophages at 10, 17, 25 and 32°C were 20.46 ± 2.07, 16.15 ± 1.39, 15.94 ± 1.85 and 22.22 ± 2.49%, respectively. The results indicated that temperature affects both the cell membrane structure of A. hydrophila and phagocytic activity of goldfish macrophages, resulting in varying fish mortality when infected at different temperatures.
Introduction
Aeromonas hydrophila is one of the most important microbial pathogens affecting cultured fish worldwide. This organism has been reported to causes mass mortalities in several species, including carp (Cyprinus carpio L., Labeo rohita Ham.), snakehead gourami (Channa striatus Bloch or Channa punctatus Bloch) and catfish (Clariasgariepinus Burchell or Clarias batrachus L.), and is the aetiological agent of several diseases including haemorrhagic septicaemia, asymptomatic septicaemia, ulcerative infections, tail rot, and fin rot. A. hydrophila can grow at temperatures ranging from 4 to 42°C (Palumbo et al. 1985) and can produce a variety of toxins (Ljungh and Wadstrom 1982) which may be related to virulence in fish and other hosts.
O’Reilly and Day (1983) reported that protease production by A. hydrophila was more efficient at 20°C than at higher temperatures such as 25–37°C. A higher proteolytic activity was found at 28°C than at 37°C in strains of A. hydrophila from both clinical and environmental origins. However, there is no information available on the optimum temperature required for this bacterium to infect fish.
Goldfish (Carassius auratus L.) culture is popular in Japan for both commercial and recreational purposes. In recent years, severe epizootics with mortality reaching almost 100% occurred among goldfish of all ages in western Japan. The goldfish epizootics occur in the spring and autumn when the water temperature ranges between 15 and 25°C; however, there is no information about the possible causative agent of disease in goldfish. The objective of this study was to investigate the optimum temperature for infection of goldfish by Aeromonas hydrophila.
Materials and methods
The bacterium and its culture
Aeromonas hydrophila strain A-3500 originally isolated from a diseased eel (Anguilla japonica Temmick & Schlegel) in Shizuoka prefecture, Japan, was passaged in golden carp (Cyprinus carpio) several times to enhance virulence. The strain was cultured in nutrient broth (Nissui) at 25°C for 24 h. Cultured cells were lyophilized and stored at – 80°C until used.
Preparation of bacterial culture
Bacteria were cultured in nutrient broth at 25°C for 24 h. Bacterial cells were harvested by centrifugation at 3000 g for 20 min and washed twice in physiological saline (0.85% NaCl, PS). Washed cells were resuspended in formulated water (FW) containing 0.60% NaCl, 0.50% KCl, 0.10% CaCl2.2H2O and 0.20% MgCl2.6H2O in distilled water (Rahman et al. 1997) and incubated at the temperature to be tested for 24 h prior to the experiments.
Selection of infection method
Bacteria were cultured at 25°C for 24 h prior to infectivity testing. Four methods were used to determine the best method to infect goldfish with A. hydrophila. Fish weighing 10–20 g were obtained from a fish farm in Kochi Prefecture, Japan. The fish were kept in 600-L tanks with well aerated, re-circulating water and fed with commercial food pellets. Different concentrations (3 × 108–3 × 104, 8 × 108–8 × 104, 8.5 × 108–8.5 × 104 and 4.6 × 108–4.6 × 104) of bacteria were made up in PS and injected intraperitoneally, intramuscularly or subcutaneously into five groups of goldfish each containing six fish. In addition, five groups of goldfish that each contained five fish were immersed into different concentrations of a bacterial suspension for 30 min. Injected fish were monitored for 15 days in water maintained at 25°C; mortality was recorded daily. Infection was confirmed by re-isolating bacteria from the kidneys of dead fish using nutrient agar (Nissui) and performing a slide agglutination test on the bacteria using anti-A. hydrophila rabbit serum. The median lethal dose (LD50) was calculated by the method of Reed and Muench (1938).
The effect of temperature on infection
Bacteria were cultured at 10, 17, 25 and 32°C for 24-h infectivity testing. Fish were acclimatized to temperatures of 10, 17, 25 and 32°C for 21 days prior to being infected with A. hydrophila. For each temperature, five goldfish groups each containing four or five fish were injected subcutaneously with 1.9 × 108–1.9 × 104 CFU/fish. Injected fish were monitored at specific temperatures for 15 days and mortalities were recorded. Infection was confirmed and the LD50 was calculated as described above.
Preparation of outer membrane protein (OMP)
Cells were washed twice in phosphate buffered saline (PBS) and once in 10 mM Tris-hydrochloride (pH 7.5) and harvested by centrifugation at 3000 g for 20 min. Cells were re-suspended in Tris-hydrochloride and sonicated at 50 W, for 30 s, four times on ice. After sonication the suspension was mixed with Sarkosyl for solubilization of the OMP then incubated at 25°C for 30 min. After incubation the suspension was centrifuged at 4000 g for 20 min and the supernatant was collected. After centrifugation at 45 000 g for 45 min the pellet was collected and stored at −70°C until used.
Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE)
OMP samples were analysed by SDS-PAGE according to Laemmli (1970) using a 14% separation gel and 4% stacking gel. The proteins were solubilized in sample buffer containing 10% (w/v) SDS, 3% (v/v) 2-mercaptoethanol, 10% (v/v) glycerol, 1 M Tris (pH 6.8) and 0.025% bromophenol blue, and the solution was heated at 98°C for 6 min. The protein bands were visualized by staining with Coomassie brilliant blue R-250. The molecular mass of the protein bands was estimated from the migration distance of a protein marker (Daichi, 015RKT).
Preparation of rabbit antiserum
Rabbits weighing about 2 kg each were used for preparing antiserum. Injection schedules included two or more subcutaneous injections (1 ml antigen solution plus 1 ml Freund’s complete adjuvant) at 3-week intervals. The antigen used was formalin-killed cells (FKC) of A. hydrophila strain A-3500. Harvested cells were washed twice in PS by centrifugation and 0.5% formalin was added to the suspension which was incubated for 48 h at 20°C. The cells were harvested by centrifugation and washed twice with PS. The FKCs were suspended in PS at a concentration of approximately 10 mg ml–1 and stored at 4°C until used.
Western blotting
The method of Towbin et al. (1979) was followed. The samples separated by electrophoresis were transferred onto nitrocellulose membranes. The membranes were blocked with 5% skimmed milk (dried) in PBS and incubated overnight at 4°C. After overnight incubation the membranes were washed twice in PBS containing 0.5% Tween-20, immersed in antiserum with PBS, and incubated at 37°C for 2 h; the bound antibodies were detected with an Immune Staining Kit (Konica).
Phagocytosis assay
Goldfish weighing about 20–25 g were purchased from a carp farm in Kochi Prefecture, Japan. The fish were maintained in a 600-L tank with re-circulating well-aerated water. The fish were fed commercial food pellets. Before the experiment, the fish were acclimatized for 6 weeks in waters at test temperatures. Twenty goldfish (five fish for each temperature) were euthanized and dissected longitudinally from the ventral side. The head kidney was removed and washed with 1 ml Eagle’s minimum essential medium supplemented with 10% fetal bovine serum (MEM-10). The kidney was then cut into small pieces in MEM-10 and kept for a few minutes. The cell suspension obtained was centrifuged at 2000 g for 2 min and the cells were then collected. Collected cells were washed three times by centrifugation at 2000 g for 2 min with the same medium. The leucocytes were counted by a light microscopy using a haemocytometer and their viability was determined by trypan blue exclusion. The cell suspension was then adjusted to 4 × 106 cells ml–1. To prepare macrophage monolayers, 100 μL of this suspension was added to glass coverslips placed in a 24-well culture plate. Two monolayers were prepared from each fish. After allowing 30 min at 25°C for cells to adhere, nonadherent cells were washed away with MEM. Then 500 μL of bacterial suspensions (5 mg ml–1 in MEM-10) was added to the coverslips. The 24-well culture plates were then incubated at the different temperatures and phagocytosis was permitted over 1 h. Bacterial cells were carefully washed away and the monolayer was fixed with ethanol and stained with Giemsa’s staining solution (Hamaguchi et al. 1989). The number of phagocytosing cells per 200 cells was counted microscopically. The results of hagocytosis were expressed as the phagocytic rate (PR), the percentage of cells showing phagocytosis, the phagocytic index (PI), and the number of phagocytosed A. hydrophila/phagocyting cells.
Results
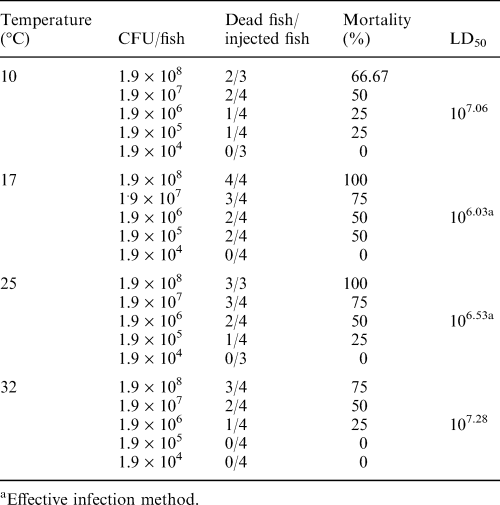
The LD50 of the bacteria determined for the different methods of injection were 106.11 CFU/fish by intraperitoneal, 107.15 CFU/fish by intramuscular, and 106.40 CFU/fish by subcutaneous delivery. The LD50 for immersion delivery was 107.23 CFU/fish (Table 1).
The LD50 of bacteria delivered by subcutaneous injection in fish held at different temperatures of infection were 107.06 CFU/fish at 10°C, 106.03 CFU/fish at 17°C, 106.53 CFU/fish at 25°C and 107.28 CFU/fish at 32°C (Table 2). The virulence of A. hydrophila in goldfish was significantly (P < 0.05) greater at 17°C and 25°C than that at 10°C and 32°C.
OMP fractions obtained from bacterial cells cultured at 10, 17, 25 and 32°C for 24 h showed that a 40-kDa band was present at 17, 25 and 32°C, which was not observed at 10°C. However, the 40-kDa band was also very faint at 32°C (1Fig. 1a). Additional bands at 32 and 25 kDa were present at 17°C but were not detected in preparations of cells grown at any other temperature. Bands at 23 and 30 kDa were present at 25°C but were not found at the other temperatures. A 27-kDa band was observed only in cells grown at 10 and 22°C and a 70-kDa band was only detected at 32°C. Common bands were present at each temperature in the 20–97 kDa region.
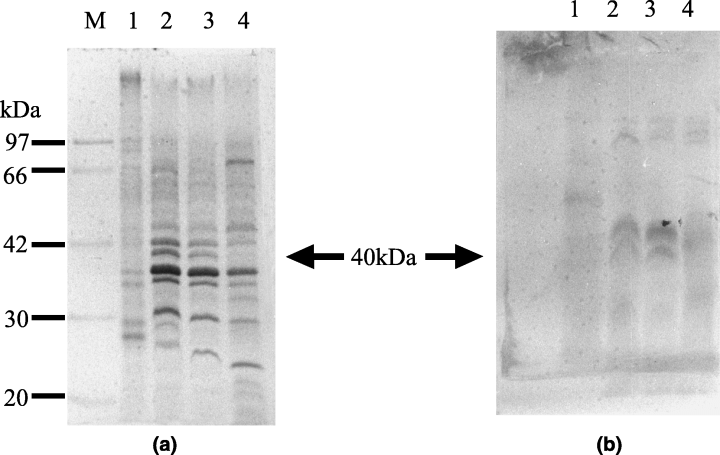
SDS-PAGE analysis (a) and Western blotting (b) of outer membrane proteins of Aeromonas hydrophila incubated at different temperatures. Proteins were stained with Coomassie brilliant blue. Lane 1, 10°C; 2, 17°C; 3, 25°C; 4, 32°C and M, molecular marker. Molecular weights are indicated on the left (kDa)
By Western blotting, a major band at 40 kDa was detected in the bacteria cultured at 17 and 25°C, but not in bacteria grown at 10 and 32°C (1Fig. 1b). There were also bands at 34 and 62 kDa at 10°C. Additionally, the antiserum reacted with three bands in the 85-kDa region in cells grown at 17, 25 and 32°C that were absent at 10°C.
A. hydrophila phagocytosed at 10, 17, 25 and 32°C showed that phagocytic rates were 20.46 ± 2.07, 16.15 ± 1.39, 15.94 ± 1.85 and 22.22 ± 2.49%, respectively. The difference in the phagocytic rate at the different temperatures was shown to be statistically significant at the 5% level by Student’s t-test. However, there was no significant difference in the phagocytic index of bacteria (Table 3).
Discussion
Among the four different infection methods tested, A. hydrophila was more virulent to goldfish when the infection method was by intraperitoneal or subcutaneous injection rather than by intramuscular injection or immersion. The virulence of the bacteria was 10-fold higher when intraperitoneal or subcutaneous injection was the route of infection as compared to infection by intramuscular injection or immersion. The mortality rate was higher after subcutaneous injection at 17 and 25°C, suggesting that these temperatures were optimum for infection of goldfish by A. hydrophila. These findings are supported by Jung and Miyazaki (1995) who reported that epizootics in western Japan occur in spring and autumn when water temperatures are between 15 and 25°C.
SDS-PAGE analysis of the OMPs showed different protein profiles for bacteria cultured at different temperatures. Western blotting showed different reactions of the OMPs obtained at different temperatures. The results of this study indicated that incubation temperature influenced the OMPs of A. hydrophila. The OMPs of gram negative bacteria have been shown to play a vital role in pathogenicity (Buchanan and Pearce 1979; Aoki and Holland 1985; Dooley et al. 1986; Biosca and Amaro 1991). There was significantly greater phagocytic activity of macrophages against bacteria at 10 and 32°C than at 17 and 25°C. Phagocytosis by macrophages is one of the principal defence mechanisms of fish (Wolke 1992) against any invasion by foreign matter. In this study it was clear that at 17 and 25°C, the bacteria were more pathogenic than at 10 and 32°C. Ishiguro et al. (1981) reported that A. salmonicida lost its virulence when cultured at high temperatures. It is possible that A. hydrophila may have lost its virulence at 32°C. At 10°C, the metabolism and potential virulence mechanisms of A. hydrophila are decreased, resulting in the enhanced phagocytosis observed at 10°C.
O’Reilly and Day (1983) demonstrated that stimulation of the extracellular proteolytic activity of A. hydrophila was influenced by temperature. The results of the present study indicate that temperature affects both the cell surface structure of A. hydrophila and the phagocytic activity of goldfish macrophages, resulting in different mortality rates of the fish at different temperatures. It can be concluded that the optimum temperature for infection of goldfish by this bacterium is 17–25°C.
Acknowledgements
The authors are grateful to Dr S. J. Jung for his kind cooperation during this study. The authors are also grateful to Dr Yano of Kyushu University for providing the bacterial strain. The first author is grateful to the MONBUSHO for scholarship support and also the Department of Zoology, Rajshahi University, Bangladesh, for granting him leave during this research. We are also grateful to Dr Scott LaPatra for his critical reading of this manuscript.





