Bacteriological study of shrimp, Penaeus monodon Fabricius, hatcheries in India
Abstract
Shrimp hatcheries often face problems of mortality caused by diseases. To understand the bacteriological status of shrimp, Penaeus monodon Fabricius, hatcheries in India, a study of hatchery water at different points was conducted in several hatcheries located along the east and west coast of India. The species composition of the bacterial flora was also determined. The total plate counts of raw sea water on tryptic soya agar ranged from 102 to 104 ml–1, whereas it ranged from 104 to 106 ml–1 in larval tanks. In the larval tanks, the proportion of Vibrio species ranged from 50% to 73%, as compared to 31% in raw sea water. A mixed bacterial flora was observed in hatchery water; however, in the larval tanks, the flora in the larvae was predominantly made up of Vibrio species. A few of the tested Vibrio isolates were non-virulent to shrimp larvae under experimental conditions. Over 90% of the strains were resistant to amoxycillin, ampicillin, cephalexin, cephazolin, cloxacillin and sulphafurazole. Most strains showed sensitivity to tetracycline, chloramphenicol, and quinolones such as norfloxacin and ciprofloxacin.
Introduction
One of the major problems faced by shrimp hatcheries is that of mortality caused by diseases. A number of microbial agents may be involved in causing mortalities in shrimp larvae. Bacteria, particularly Vibrio species, have been reported to cause larval mortalities in hatcheries in southern and south-eastern Asia. Vibrio harveyi, a luminous species, has been implicated in a number of such cases (Sunaryanto and Mariam 1986; Baticados et al. 1990; Lavilla-Pitogo et al. 1990; Karunasagar et al. 1994). To avoid bacteriological problems, commercial shrimp hatcheries adopt extensive water treatments which include filtration, chlorination and ultraviolet (UV) treatment. The objective of the present study was to assess the bacteriological status of water at different points in a shrimp, Penaeus monodon Fabricius, hatchery and to study the incidence of various bacterial species in hatchery systems.
Materials and methods
The present investigation was carried out in four hatcheries on the west coast and two hatcheries on the east coast of India. These hatcheries draw water from the sea, which is filtered through sand filters, and chlorinated and dechlorinated before distribution to the larval tanks. In some hatcheries, the sea water is treated with UV rays before it reaches the larval tanks.
Raw sea water and water samples from various points in the hatchery such as chlorine-treated water, UV-treated water and water from tanks rearing various larval stages (i.e. eggs, nauplii, zoeae, myses and postlarvae) were collected from 30 cm below the surface using sterile bottles. Total plate count (TPC) was performed by plating serial ten-fold dilutions in tryptic soy agar containing 1% NaCl (TSAS) by the spread plate method. Each sample was plated in duplicate. The plates were incubated at 29 ± 1 °C and observed after 24 h. To determine luminous bacterial counts, plates were observed in a dark room. To determine total vibrio count, a minimum of 50 colonies were selected at random and identified by biochemical tests. Gram-negative, oxidase-positive bacteria showing fermentative reaction in an O/F-test (MacFaddin 1980) and sensitivity to vibriostatic agent 0/129 were identified as Vibrio sp. (Bain and Shewan 1968). The identification schemes of Bain and Shewan (1968), LeChevallier et al. (1980), and Farmer and Hickman-Brenner (1992) were used for identifying bacteria. Vibrios, which are important pathogens of shrimp, were identified to the species level while all other bacterial isolates were identified to the generic level.
Testing the virulence of a few representative isolates
Representative cultures of V. harveyi, V. alginolyticus, V. parahaemolyticus and V. vulnificus were tested for their virulence to P. monodon postlarvae (PL20). The postlarvae were obtained from a local hatchery and acclimatized to laboratory conditions. Two litres of UV-sterilized sea water were taken in 5-L glass beakers and 25 postlarvae were introduced into each beaker. The different Vibrio sp. were grown in brain heart infusion (BHI) broth overnight at 29 ± 1 °C; cells were pelleted by centrifugation, washed and resuspended in phosphate buffered saline (PBS) to the original volume. Ten-fold dilutions of the various cultures were added to the beakers containing postlarvae to give a cell concentration ranging from 107 to 104 ml–1. The larvae were observed for 5 days for any mortalities.
Antibiotic sensitivity test
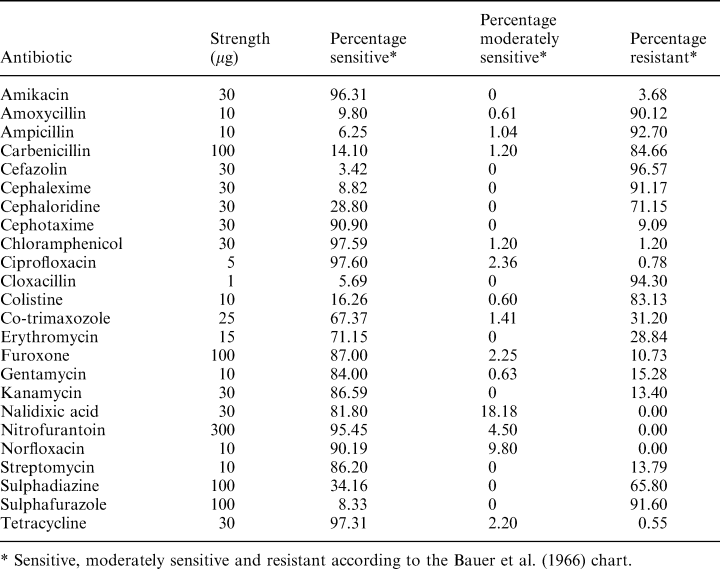
Different bacterial isolates from hatcheries were tested for their sensitivity to various antibiotics. A lawn culture of the bacterial isolates was prepared in TSAS using a young broth culture. Antibiotic incorporated discs (HiMedia, Bombay, India) were then placed aseptically on these plates. After 24 h incubation, the zones of inhibition were measured and classified as sensitive, moderately sensitive and resistant (Bauer et al. 1966).
Results
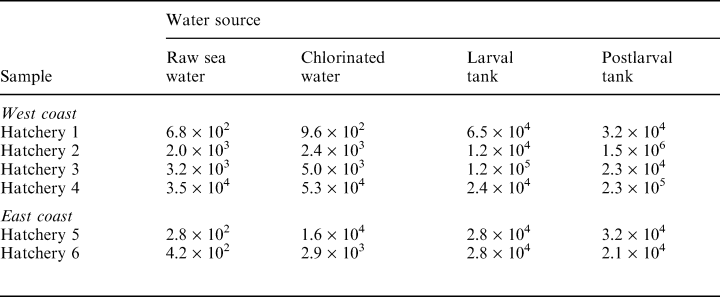
The results shown in (Table 1) demonstrate the bacterial count in raw sea water and in water samples drawn from various points in different hatcheries. Total plate count in raw sea water ranged from 102 to 104 cfu ml–1. Chlorine-treated water also showed counts ranging from 102 to 104 cfu ml–1. In three out of six samples, the counts in treated sea water were over one log unit higher than those in raw sea water. The TPCs in the larval and postlarval rearing tanks ranged from 104 to 107 cfu ml–1.
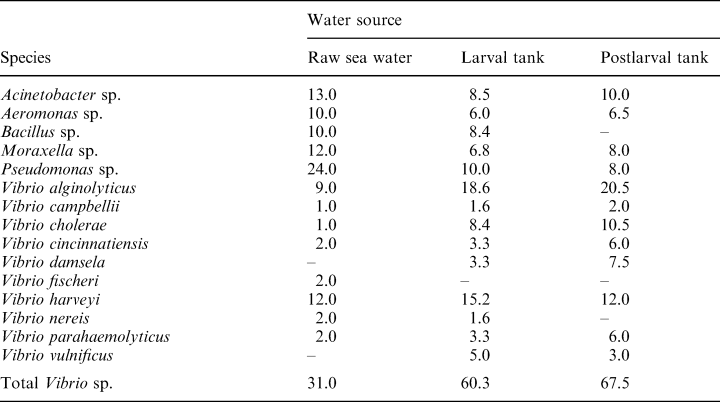
The incidence of various bacterial species in raw sea water, and water from larval and postlarval tanks in hatchery 1 is indicated in Table 2. Raw sea water had Vibrio sp. as the dominant flora, accounting for 31.0% of the total bacteria, followed by Pseudomonas sp. (24.0%) and Bacillus (10.0%); Moraxella, Acinetobacter and Aeromonas sp. made up the remainder. In water from the larva tank, the proportion of Vibrio sp. increased to 60.3%. Vibrio alginolyticus was the dominant species (18.6%), followed by V. harveyi (15.2%) and non O1 V. cholerae. Similar counts were observed in water from postlarval tanks.
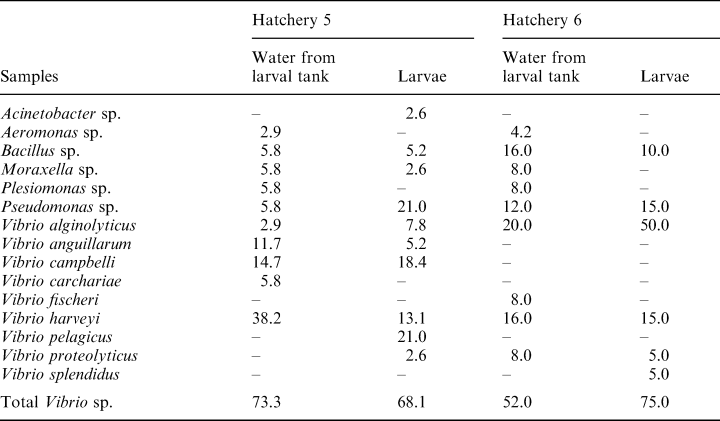
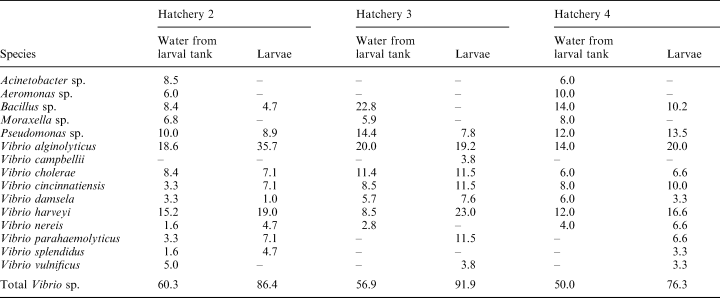
The bacterial composition of water from larval tanks and larval samples from hatcheries 2–6 is indicated in Tables 3 and 4. With the exception of hatchery 5 on the east coast, all other hatcheries had higher proportions of Vibrio sp. in larvae as compared to the larval tank water. The percentage composition of Vibrio sp. in the larval tank water ranged from 50% to 73% while in the larvae the range was from 68% to 92%. In most samples of larvae, V. alginolyticus was the dominant Vibrio sp., followed by V. harveyi. In larval tanks from hatchery 3 on the west coast, V. parahaemolyticus was the dominant flora (23%), followed by V. alginolyticus (19.2%). In hatchery 5 on the east coast, the dominant Vibrio sp. in the larvae was V. pelagicus (21%), followed by V. campbellii (18.4%) and V. harveyi (13.1%). Vibrio alginolyticus accounted for 50% of the flora in larvae, while V. harveyi contributed to 15% of the flora (Table 4) in hatchery 6 on the east coast. In general, the flora in larval tank water and larvae were comparable except for the dominance of Vibrio sp. in larvae. All four species of Vibrio showed the same type of response in the virulence study. Mortality was not observed in larvae even when exposed to a high bacterial load (107 bacteria ml–1). The antibiogram of various hatchery isolates is shown in Table 5. Over 90% of the strains were resistant to amoxycillin, ampicillin, cefazolin, cephalexin, cloxacillin and sulphafurazole. Most strains were sensitive to tetracycline, chloramphenicol, and quinolones such as norfloxacin and ciprofloxacin.
Discussion
A number of factors can influence the microflora in shrimp hatcheries. The natural flora present in sea water may be altered by filtration, chlorination and other treatment measures adopted in hatcheries. Microflora detected in chlorinated water could represent those which survived the treatment and those which were derived from biofilms developing in water pipes and tank surfaces. The results presented in Table 1 show that, in general, chlorine-treated water had bacterial counts similar to those noted in raw sea water. This may be a result of the high organic load in the incoming water, which may render chlorine less effective, or may be caused by recontamination from bacteria surviving as biofilms on tank surfaces. Generally, the water is passed through polyvinylchloride pipes and then stored in cement tanks; shrimp-associated vibrios can form biofilms on such surfaces (Karunasagar et al. 1996).
During larval rearing, different microflora may come into hatchery systems through eggs or through live feeds such as algae and artemia. Because of a higher organic matter content, hatchery water can be expected to have a higher bacterial load in comparison to the incoming water; the results in Table 1 support this view. Generally, water from larval tanks had higher bacterial counts when compared to chlorinated water.
The results in Table 2 suggest that there is not only a quantitative change, but also a qualitative change in the microflora between intake sea water and hatchery water, with a clear dominance of various Vibrio sp. in the hatchery water; this is clearly demonstrated in (Tables 3 and 4). A similar dominance of Vibrio sp. was reported by Groumellec et al. (1995) and Tanasomwang and Ruangpan (1995). Among Vibrio sp., V. alginolyticus was the dominant species associated with larvae, contributing to up to 50% of the flora. This was followed by V. harveyi, V. parahaemolyticus, non 01 V. cholerae and other Vibrio sp. Infections caused by V. alginolyticus were reported in black tiger shrimp, P. monodon, as well as in kuruma prawn, P. japonicus by Lee et al. (1996a,b). However, the present study shows that V. alginolyticus is the most common Vibrio sp. associated with P. monodon larvae. It is possible that certain strains of V. alginolyticus are more virulent than others; therefore, to understand the role of V. alginolyticus in disease in shrimp, it would be important to assess the virulence of the isolates. Although the virulence factors of V. alginolyticus have not been identified, Lee et al. (1997a,b) suggested that alkaline serine protease and other extracellular products may be involved in the virulence. Few representative isolates (n = 6) tested for virulence in the present study showed an LD50 of >107 cells ml–1 for P. monodon postlarvae (PL 20), indicating their low virulence. Nevertheless, the possibility cannot be ruled out that strains with such low virulence might cause infections in a stressed host.
Another important observation is the association of luminous V. harveyi with P. monodon larvae in the present study. The contribution of V. harveyi ranged from 13% to 23% of the flora (Tables 3 and 4). In general, V. harveyi is regarded as an important pathogen of P. monodon larvae; mass mortalities caused by this species were reported by several investigators (Sunaryanto and Mariam 1986; Baticados et al. 1990; Lavilla-Pitogo et al. 1990; Karunasagar et al. 1994). However, even in V. harveyi, virulence might vary with strains (Karunasagar et al. 1994; Pizzutto and Hirst 1995), and therefore investigations on understanding the role of this organism in disease should consider the virulence of the isolated strains. Few representative isolates (n = 5) tested for virulence in the present study showed an LD50 > 107 for P. monodon postlarvae (PL20), indicating their low virulence. Karunasagar et al. (1994) noted that strains of V. harveyi virulent to P. monodon larvae showed an LD50 of ≈103 cells for postlarvae. These results indicate that the strains tested in the present study had very low virulence for P. monodon larvae. The results of this study suggest that populations of V. harveyi with low virulence may be naturally associated with shrimp larvae. Antibiotic resistance of Vibrio sp. which are potentially pathogenic to shrimp is a concern in many countries where shrimp aquaculture is an important industry (Karunasagar et al. 1994). In many hatcheries, it is a common practice to give dip treatment for broodstock, eggs and larvae in antibiotics such as tetracycline, chloramphenicol and furoxone. The results in (Table 5) show that the incidence of resistance to tetracycline (0.55%) and chloramphenicol (1.2%), the two most commonly used antibiotics in hatcheries, is very low. Resistance to furoxone, another commonly used antibiotic, was seen in 10.73% of the isolates. However, 65–91% of strains were resistant to sulphonamide-based compounds such as sulphafurazole and sulphadiazine. Liu et al. (1997) noted that V. harveyi isolated from diseased prawns in Taiwan were resistant to nitrofurantoin, novobiocin and sulphonamide. Although resistance to sulphonamide was observed in the present study, all the isolates tested were sensitive to nitrofurantoin. These results show that testing isolates for resistance to antibiotics would be important before considering chemotherapeutic measures in hatchery systems.
Conclusions
The results of the present study demonstrate that raw sea water supplied to hatcheries contains lower proportions of Vibrio spp. when compared to larval tanks and larvae. Chlorination keeps the bacterial load close to initial levels, and potentially pathogenic Vibrio spp. associated with larval tanks and larvae show very low virulence. Hatchery-associated bacteria showed resistance to certain antibiotics such as sulphonamides.