Effects of hot water treatment, biocontrol agents, disinfectants and a fungicide on storability of English oak acorns and control of the pathogen, Ciboria batschianaEffets de la thermothérapie, d'agents de lutte biologique, de désinfectants et d'un fongicide sur la conservation des glands de chêne pédonculé et la lutte contre le champignon pathogène Ciboria batschiana
Wirkung einer Heisswasserbehandlung, verschiedener Mikroorganismen und Desinfektionsmittel sowie eines Fungizides auf die Lagerfähigkeit von Eicheln und den Fäuleerreger Ciboria batschiana
Summary
enEight biological control agents (BCAs; Clonostachys rosea, Trichoderma harzianum, Trichoderma polysporum, Phlebiopsis gigantea, Bacillus subtilis, Pseudomonas chlororaphis, Streptomyces griseoviridis), five disinfectants and a fungicide (Prochloras-ManganTM) were evaluated for the control of harmful mycoflora on Ciboria batschiana-infected English oak (Quercus robur) acorns during storage at −1°C. All treatments were tested on both hot water-treated (HW) and untreated acorns. HW-treated acorns generally stored better than untreated acorns as a result of elimination of C. batschiana. The HW treatment increased the germination percentage before storage from 60 to 85%. Germination of HW-treated acorns decreased from 85 to 40% after 16.5 months of storage, whereas germination of untreated acorns decreased from 60 to 20% after a similar time. Ciboria batschiana infection of untreated acorns increased from 14% before storage to 55% after 16 months of storage. All disinfectants and BCAs had a positive effect on viability and particularly on control of C. batschiana in untreated acorns. Best control of C. batschiana occurred with C. rosea, MycostopTM (S. griseoviridis), Binab TFTM (T. polysporum + T. harzianum), and P. chlororaphis. For HW-treated acorns, there was only a small effect of BCAs on acorn viability while the fungicide and the disinfectants had no effect. Treatments also affected the saprophytic mycoflora with the HW treatment reducing the frequency of Cladosporium spp. and Papulaspora spp., but enhancing Alternaria spp., Mucoraceae and Penicillium spp. However, when combined with HW treatment, several BCAs significantly reduced the prevalence of these fungi. Also, C. rosea reduced the growth of Fusarium spp. Significant negative correlations (p < 0.001) were found between acorn germination and certain storage fungi such as Acremoniella atra, Cladosporium spp. and dematiaceous mycelia.
Résumé
frHuit agents de lutte biologique (Clonostachys rosea, Trichoderma harzianum, T. polysporum, Phlebiopsis gigantea, Bacillus subtilis, Pseudomonas chlororaphis, Streptomyces griseoviridis), 5 désinfectants et un fongicide (Prochloras-ManganTM) ont été testés pour lutter contre les champignons ayant un effet négatif sur la conservation à−1°C de glands de chêne pédonculé (Q. robur) infectés par Ciboria batschiana. Tous les traitements ont été testés aussi bien sur des glands traités par thermothérapie que sur des glands non-traités. Les glands traités se sont généralement mieux conservés que les glands non-traités du fait de l’élimination de C. batschiana. La thermothérapie a augmenté le pourcentage de germination avant conservation de 60 à 85%. La germination des glands traités a diminué de 85 à 40% après 16 mois et demi de conservation alors que celle des glands non-traités a diminué de 60 à 20% pour une période identique. Le taux d'infection par Ciboria batschiana des glands non-traités est passé de 14% avant conservation à 55% après 16 mois de conservation. Tous les désinfectants et les agents de lutte biologique ont eu un effet positif sur la viabilité des glands et en particulier sur le contrôle de C. batschiana chez les glands non-traités. Les meilleurs résultats vis à vis de C. batschiana ont été obtenus avec Clonostachys rosea, MycostopTM (S. griseoviridis), Binab TFTM (T. polysporum + T. harzianum) et P. chlororaphis. Chez les glands traités par thermothérapie, aucun effet sur la viabilité des glands n'a été observé avec le fongicide et les désinfectants, et seulement un léger effet avec les agents de lutte biologique. Les traitements ont aussi affecté la mycoflore saprophyte, la thermothérapie réduisant la fréquence de Cladosporium spp. et Papulaspora spp., mais favorisant Alternaria spp., des Mucoraceae et Penicillium spp. Toutefois, plusieurs agents de lutte biologique combinés à la thermothérapie ont significativement réduit la prévalence de ces champignons. C. rosea a également réduit la croissance de Fusarium spp. Des corrélations négatives significatives (p < 0.001) ont été obtenues entre la germination des glands et certains champignons de conservation tels que Acremoniella atra, Cladosporium spp. et des champignons du groupe des dématiacées.
Zusammenfassung
deAcht verschiedene Behandlungen mit Biopräparaten (BCAs; Clonostachys rosea, Trichoderma harzianum, T. polysporum, Phlebiopsis gigantea, Bacillus subtilis, Pseudomonas chlororaphis Streptomyces griseoviridis), fünf Desinfektionsmittel und ein Fungizid (Prochloras-ManganTM) wurden auf ihre Wirksamkeit zur Kontrolle der pathogenen Mykoflora auf mit Ciboria batschiana infizierten Eicheln (Quercus robur) während der Lagerung bei −1°C untersucht. Alle Untersuchungen wurden sowohl mit Eicheln, die einer Heisswasser (HW)-Behandlung unterzogen worden waren als auch mit unbehandeltem Material durchgeführt. HW-behandelte Eicheln waren allgemein besser lagerfähig als unbehandelte, da C. batschiana stark reduziert wurde. Die Heisswasserbehandlung erhöhte die Keimrate vor der Lagerung von 60 auf 85%; nach 16,5 Monaten Lagerung bei −1°C fiel die Keimrate auf 40%. Im Gegensatz dazu fiel die Keimrate von unbehandelten Eicheln in einem ähnlichen Zeitraum von 60 auf 20%. Der Infektionsgrad mit C. batschiana erhöhte sich bei unbehandelten Eicheln von 14% vor der Lagerung auf 55% nach 16 Monaten. In nicht wärmebehandelten Eicheln wirkten sich alle Desinfektionsmittel und BCAs positiv auf die Überlebensrate aus und hemmten insbesondere C. batschiana. Die beste Wirkung gegen C. batschiana wurde erzielt mit Clonostachys rosea, MycostopTM (Streptomyces griseoviridis), Binab TFTM (T. harzianum + T. polysporum) und Pseudomonas chlororaphis. Bei HW-behandelten Eicheln hatten die BCAs nur geringe Auswirkungen auf die Überlebensrate, das Fungizid und die Desinfektionsmittel waren wirkungslos. Die Behandlungen beeinflussten auch die saprophytische Mycoflora; die HW-Behandlung reduzierte das Vorkommen von Cladosporium spp. und Papulaspora spp., erhöhte jedoch das Vorkommen von Alternaria spp., Mucoraceae und Penicillium spp. Wenn die HW-Behandlung mit mehreren BCAs kombiniert wurde, reduzierte sich das Vorkommen dieser Pilze jedoch signifikant. Clonostachys rosea reduzierte auch das Wachstum von Penicillium spp. Zwischen der Keimfähigkeit der Eicheln und bestimmten Pilzen wie Acremoniella atra, Cladosporium spp. und pigmentierten Myzelien wurden signifikant (p<0.001) negative Korrelationen festgestellt.
1 Introduction
English oak (Quercus robur L.) is a very important hardwood in Denmark. However, poor storage potential of acorns, resulting from recalcitrant storage behaviour combined with irregular fruiting, hampers planting of Danish seed sources. As the acorns are sensitive to desiccation, they must be stored at a high seed moisture content (MC), and although they can be stored at low temperatures (−3–0°C) viability is lost within 1–3 years (Suszka et al. 1996). It is difficult to establish the minimum safe seed MC for seeds (or critical MC) as factors such as seed maturity and drying rate may influence desiccation tolerance (Pammenter and Berjak 1999). For English oak acorns, the critical MC is 35–40% (von Schönborn 1964; Gosling 1989; Hendry et al. 1992; Suszka et al. 1996).
Fungi are partly responsible for poor storability as their development is favoured by humid storage conditions. The pathogen Ciboria batschiana (Zopf) Buchwald is the most serious problem affecting acorn storage (Delatour and Morelet 1979; Kristensen and Pedersen 1991). Also many other indigenous fungi are associated with acorns (Uros̆evic 1961; Mittal et al. 1990; Schröder 1999), but it is unclear how such fungi affect storability of acorns. Preventive conditions are rather difficult to establish as most storage fungi (e.g. moulds belonging to the genera Penicillium or Aspergillus) are active at relative humidities (RH) of 70–90%. Mycelium and spores survive also dry conditions. However, high humidity generally favours microflora growth, which will reduce aeration of the seeds and perhaps result in production of toxins. Seeds are also more susceptible to pathogens and suffer considerable loss of viability during storage (Mclean et al. 1984; Willan 1985). All together, these factors result in seed rotting and poorer germination.
Thermotherapy by hot water (HW) treatment successfully eliminates C. batschiana before storage (Delatour and Morelet 1979; Kehr and Schröder 1996; Suszka et al. 1996). However, saprophytic fungi and minor pathogens, some of them endophytes (Kehr and Pehl 1993), may survive the treatment, or eventually invade the seeds which are more vulnerable after HW treatment (Grondeau and Samson 1994). Knowledge is scarce about the rate of colonization by various saprophytic fungi and interactions among the microflora under different conditions. Supposedly, changes in microflora related to treatments with thermotherapy may reduce acorn storability (Kehr and Schröder 1996). Some benefit may result if naturally occurring, antagonistic microorganisms survive HW treatment, but in general these microorganisms do not seem to protect the seeds. Consequently, HW treatment is typically followed by a fungicide treatment. In Denmark, pesticide use is prohibited in the state forests as of 1 January 2003, therefore, alternative new approaches such as low-risk disinfectants and microbiological agents are needed. Seed treatments with microbes have been attempted for control of both seed- and soilborne diseases, and some biocontrol agents (BCAs) have been selected for their control of seedling diseases in cool climate, i.e. field crops grown at relatively low temperatures (Knudsen et al. 1997). However, to date, no BCAs appear to have been tested for protecting forest seeds during low temperature storage.
The aim of the present work was to test the impact of different alternative control measures on English oak acorn storability, control of C. batschiana and other fungi affecting viability during low temperature storage.
2 Materials and methods
2.1 Seed material and controls
In October 1999, acorns were collected from nets suspended under street trees in Subregion 1 [CPRO-DLO (Centrum voor Plantenveredelings-en Reproduktieonderzoek, Wageningen) 1996], the Netherlands. The acorns were lightly air-dried before being transported to the State Forest Tree Improvement Station, Humlebaek, Denmark. They were tested for germination and MC. A small sample was stored (at the same conditions as the subsequent treatments) to follow Ciboria development. To remove dead seeds, the acorns were placed in water and after 30 min, floating (dead) acorns were skimmed off and discarded. The remaining acorns were designated as untreated since skimming is a normal sorting procedure before storage. Half of the acorns were HW-treated by immersing them in 800 l of water at 41°C. After about 30 min, the temperature was again raised to 41°C, and the seeds kept at this temperature for 2.5 h before the water was drained off. The seeds were surface dried 2 h on (64 × 100 × 9 cm) net trays, each containing 15 kg of acorns, which represented a sample in the treatments described below. HW-treated and untreated acorns were then treated with disinfectants or BCAs (Table 1).
| Treatments | Untreated | Hot water | Details |
|---|---|---|---|
| OctaveTM fungicide | + | 100 g/100 kg acorns (active ingredient: 50% Prochloras-ManganTM) | |
| 2% AtamonTM | + | + | Treatment for 30 min (active ingredient: sodium benzoate) |
3% H2O |
+ | + | Treatment for 30 min |
| 2% ascorbic acid1 | + | + | Treatment for 30 min |
| Ca(OH)2 solution | + | + | Treatment for 15 min (pH 11) |
| Ozone | + | + | Treatment in water for 20 min, 0.1 p.p.m. |
| SepiretTM (S)2 | + | + | 1.2 ml/kg seed (18 ml per treatment) |
| S + Clonostachys rosea IK7263 | + | + | Peat/wheatbran formulation. 60 g inoculum (1.8 × 108 cfu/g) in 300 ml water |
| S + SupresivitTM 4 | + | 30 g inoculum in 125 ml water | |
| S + Binab TFTM 5 | + | + | 50 g inoculum in 125 ml water |
| S + RotstopTM 6 | + | + | 25 g inoculum in 125 ml water |
| S + MycostopTM 7 | + | + | 50 g inoculum (108 cfu/g) in 125 ml water |
| S + FZB24WGTM 8 | + | + | 2.25 g inoculum (2 × 1010 cfu/g) in 125 ml water |
| S + CedomonTM 9 | + | 105 ml bacterium formulated in rape oil | |
| S + Pseudomonas chlororaphis MA34210 | + | + | 110 ml inoculum (fresh bacterial suspensions) |
- 1Concentrations of H2O2 and ascorbic acid recommended by Klaus Gille, Forstsaatgut Beratungsstelle, Oerrel, Germany. See also Gille and Nowag (1995).
- 2SepiretTM or ‘S’ is a commercial product for seed coating, it is a viscous liquid based on cellulosic binder and organic pigment on a water based system (Seppic, France).
- 3 Clonostachys rosea IK726 was obtained from the Royal Veterinary and Agricultural University, Denmark. It is a near commercial product effective against Fusarium culmorum, Bipolaris sorokinana, Alternaria radicina and Pythium sp. (Knudsen et al. 1995; Jensen et al. 2001). Clonostachys rosea IK726 were applied as a peat/wheatbran formulation (Jensen et al. 2000).
- 4SupresivitTM (Trichoderma harzianum) used to control Pythium, Phytophthora and Rhizoctonia in greenhouse crops (Fytovita C. Ltd, Blatnice, Czech Republic).
- 5Binab TFTM (Trichoderma harzianum + T. polysporum, minimum 100 000 cfu/g). Controls Botrytis, Verticillium, Pythium, Fusarium, Phytophthora, Rhizoctonia, Gaeumannomyces, fairy rings and soil-borne pathogens in general. Also recommended for control of Didymella, Stereum and Fomes (Bayer, Germany).
- 6RotstopTM (Phlebiopsis gigantea). Control of Heterobasidion annosum in forestry (Kemira Agro Oy, Finland).
- 7MycostopTM (Streptomyces griseoviridis) used to control Fusarium oxysporum and Pythium ultimum (Tahvonen 1988; White et al. 1990) (Kemira Agro Oy).
- 8FZB24WGTM (Bacillus subtilis). Recommended as growth promoting candidate for potatoes, tomato and cucumber. Stimulates plant resistance to Rhizoctonia solani, Streptomyces, Fusarium and Pythium (Schmiedeknecht et al. 1998) (FZB Biotechnik GmbH, Germany).
- 9CedomonTM (Pseudomonas chlororaphis MA342). For control of Septoria nodorum, Tilletia caries and Ustilago avenae (Johnsson et al. 1998) (Bio Agri, Uppsala, Sweden).
- 10Supplied by Margareta Hökeberg, R&D Manager (BioAgri).
- 2−10Treated acorns were incubated for 5 days at 15°C before cold storage.
2.2 Experimental design and treatments
Different treatments were applied directly on wet seeds after either the skimming procedure or HW treatment. The fungicide (OctaveTM, Aventis Crop Science, Nordic A/S Copenhagen, Denmark) was applied as a powder and the disinfectants were applied in a water solution (2 : 1 ratio by volume of water to the acorns). The formulations for the biocontrol materials are given in Table 1 along with the target pathogens the BCAs were developed to control. All commercial BCAs were water-soluble powders and were mixed with water before use. The dosage was chosen close to the recommendations of the manufacturer for (smooth) seeds, and the total amount of liquid was adjusted to that which adhered to the seeds. Non-commercial products were amended as suggested by the suppliers (Table 1). After careful stirring, the suspensions were added to 15 kg acorns in a 7800 cm3 plastic bag and the coating was applied by moving and turning the bag for 2 min or until the red sticker (SepiretTM, Seppic, Paris, France) was distributed evenly over the acorns.
Immediately after treatment, the seeds were stored at −1°C, 90–95% RH in open plastic boxes, each containing 15 kg seeds. The biocontrol-treated seeds were incubated in net trays for 5 days at 15°C before being stored. During incubation the trays were covered with plastic to prevent contamination and desiccation, and the trays were stacked criss-crossed to ensure aeration. Afterwards, the seeds were stored in the same way as the disinfected seeds.
Sampling of acorns from the boxes in the storage chamber was performed at random from three to five localities in each box.
2.3 Acorn germination
Germination tests were used to monitor acorn survival during storage. Four replicates of 50 acorns each were placed in paper towel packages after removing the pericarp and one-third of each acorn from the distal end. At this time, the cut surfaces of the acorns were evaluated, and a viability estimate was calculated on basis of the number of sound looking seeds. The acorns were then incubated at 15–18°C in 12 h light and germination was recorded weekly for 6 weeks. Germination was considered to have occurred when the radicle was the same length as that of the acorn. Germination tests were carried out before storage and approximately every fourth months for the first 17 months of storage, plus a final test after 29 months. As preparation of the germination tests was very time-consuming, the treatments were initiated over several weeks. The significance of difference in germination percentage between control and each treatment in the two groups (untreated and HW-treated) were determined using Dunnett's t-test. Data were analysed by pc-sas [release 8.2 (TS2MO); SAS Institute, Cary, NC, USA] for all storage durations (the treatments were not compared with one another). As a measure of speed of germination, mean germination time (MGT) was calculated. MGT is the average time it takes for germinated seeds in a replicate to germinate. MGT was calculated according to Czabator (1962).
2.4 Moisture content
The ends which were cut from acorns used in the germination test (four replicates of 50 acorns each) were oven-dried at 103°C for 17 h to determine their MC (fresh weight basis). The cut-off ends were considered to be representative for entire acorns as the embryo is very homogenous, the embryo axis is small and there is no endosperm. This modification of the ISTA (1999) method is used at the State Forest Tree Improvement Station (unpublished). All MC measurements were assessed simultaneous with the initiation of germination tests.
2.5 Ciboria infection in storage
The Danish Plant Directorate conducted the assays for Ciboria after 0, 4 and 10 months of storage on the three controls: initial unskimmed acorns, the untreated (skimmed) acorns and the HW control acorns. The pericarps were removed from 100 acorns per treatment and they were surface disinfected (1% NaOCl for 10 min), dried and incubated on wet blotter paper at 10°C for 7 days, and acorns which yielded the typical grey mycelial mats of C. batschiana were recorded visually. After 16 months of storage, the authors carried out the Ciboria tests, following the same procedure. Here, combinations of untreated and disinfectants or BCAs (except FZBTM) were tested. To avoid cross-contamination, the samples from each treatment were divided into groups of (i) completely black, (ii) partly black or (iii) healthy looking acorns and incubated in separate containers. Statistical analysis was performed accounting for that the parameters are discrete variables. It was recorded whether or not acorns were infected by Ciboria. Therefore, these results were analysed by logistic regression (corrected for over dispersion) (Collet 1991). Data were analysed by pc-sas [release 8.2 (TS2MO); SAS Institute].
2.6 Survival of BCAs on acorns
To determine survival of the BCAs, samples of three, randomly selected acorns were washed with 30 ml distilled sterile water and 30 glass beads in a vortex mixer (IKA VF2 Janke & Kunkel, Benfleet, Essex, UK) at 2500 rpm for 1 min. Treatments with CedomonTM (Pseudomonas chlororaphis, MA342; BioAgri, Uppsala, Sweden), which cannot be cultured from seeds, were omitted. Series of 10-fold dilutions of the wash water were made and 100 μl aliquots were plated onto PDA (Difco Laboratories, Detroit, MI, USA) amended with 2.2 g/l TritonX-100 and the antibiotics chloramphenicol (0.5 mg/l) and chlortetracyclin (0.25 mg/l). Washings of treatments with FZB24WGTM (FZB Biotechnik GmbH, Berlin, Germany) were spread on Tryptic-soybroth Agar (Difco Laboratories). The tests were performed before acorn storage (after 5 days preincubation at 15°C), and after 4 months of storage.
2.7 Monitoring indigenous mycoflora
Twenty acorns were placed on 20 × 16 cm blotter paper (folded in 2.5 cm pleats) suspended on wire netting in 11 × 17 × 6.5 cm3 plastic boxes. A filter paper supplied the pleated filter paper with water (100 ml) from the bottom of the boxes. The boxes were covered with transparent lids and incubated under near ultraviolet (NUV) light for 8 days at 20°C, 12 h light. There were five replicates of 20 acorns each. The test was carried out for all treatments before acorn storage and after 16 months of storage. Control treatments, treatments with 2%AtamonTM (Tørsleff, Hvidovre, Denmark), and BCAs were also tested after 4 months of storage.
One hundred acorns per treatment were visually inspected to quantify fungus mycelial growth before storage and after 4 and 16 months of storage. The following scale was used to qualify fungal growth: 0, no visible mycelium, 1, scattered mycelium and 2, seed totally covered with heavy mycelium. An average index for each treatment was calculated as ∑(0 × n0) + (1 × n1) + (2 × n2)/100 where n0 is number of acorns given the rating of 0.
As a spot test of the mycoflora diversity, one replicate chosen randomly was tested after incubation. The acorns were moved and placed in exactly the same position for identification of the microflora by stereomicroscopy (von arx 1981; Barnett and Hunter 1972; Hughes 1951; Ellis 1971, 1976; Domsch et al. 1980; Sutton 1980; Ellis and Ellis 1985).
2.8 Mycoflora in relation to seed germination
To investigate the relationship between occurrence of predominate fungi and the percentage of germinated acorns after 4 months of storage, a linear regression analysis performed. Data were analysed by pc-sas [release 8.2 (TS2MO); SAS Institute].
Also, after 10 months of storage a sample of the same acorns was assessed for mycoflora and germination. Seeds with pericarps were first incubated in paper towel packages for 3 weeks and then placed in blotter paper in plastic boxes for 2 days under NUV light. Using this technique, the relationship between presence of specific fungi and the viability of the acorns was determined. Before incubation, to avoid cross-contamination, the samples were placed in the following separate containers (i) completely black acorns, (ii) partly black and (iii) healthy appearing acorns. The test was performed on acorns in the untreated control, HW control, and untreated + ascorbic acid, and HW + ascorbic acid treatments.
3 Results
3.1 Effect of treatments on the moisture content (MC) and acorn germination
When received, the MC of the acorns was 37.9% and germination rate was 49%. Table 2 shows the average MC for four treatment groups before and after storage. HW treatment resulted in an average increase in MC of c. 1.5%, and application of the BCAs reduced MC by c. 3%. The MC of most treatments decreased slightly during storage, for the following treatments below 35% (for treatments see Table 1): untreated control (Fig. 1a), untreated + AtamonTM (Fig. 1b), MycostopTM (Kemira Agro Oy, Helsinki, Finland), Pseudomonas (Fig. 1d), and HW + CedomonTM (Fig. 1e).
| Months of storage | Untreated control and disinfectants1 | HW control and HW + disinfectants2 | Biocontrol agents (BCA)3 | HW + biocontrol agents4 |
|---|---|---|---|---|
| 0 | 39.9 ± 1.1 | 41.6 ± 1.3 | 37.1 ± 0.9 | 38.3 ± 0.8 |
| 3.5 | 39.1 ± 1.0 | 40.4 ± 1.4 | 37.3 ± 1.0 | 37.8 ± 0.7 |
| 8.0 | 38.7 ± 1.1 | 39.4 ± 2.8 | 36.7 ± 1.8 | 36.6 ± 1.0 |
| 14.5 | 38.5 ± 2.8 | 39.0 ± 2.0 | 36.8 ± 2.5 | 38.0 ± 1.5 |
| 16.5 | 38.0 ± 3.6 | 39.2 ± 1.6 | 37.4 ± 1.0 | 37.0 ± 1.9 |
- 1Untreated control, 2% AtamonTM, 3%H2O2, 2% ascorbic acid, Ca(OH)2 solution and ozone.
- 2Hot water-treated (HW) and HW combined with following treatments: OctaveTM fungicide, SepiretTM, 2% AtamonTM, 3% H2O2, 2% ascorbic acid, Ca(OH)2 solution and ozone.
- 3Non-HW-treated acorns treated with following biological control agents: Binab TFTM, MycostopTM, RotstopTM, Pseudomonas chlororaphis MA342, Clonostachys rosea IK726.
- 4HW-treated acorns treated with following BCAs: + Binab TFTM, MycostopTM, RotstopTM, Pseudomonas chlororaphis MA342, Clonostachys rosea IK726, SupresivitTM, FZB24WGTM and CedomonTM.
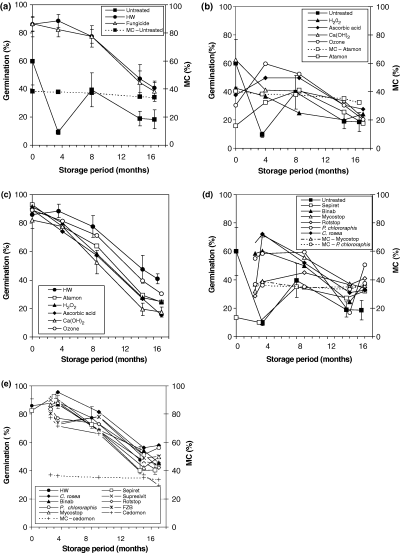
Effect on germination and moisture content of Quercus robur acorns stored up to 7 months at −1°C of (a) untreated, hot water and hot water plus the fungicide (OctaveTM). (b) Disinfectants on untreated acorns. (c) Disinfectants on hot water-treated acorns. (d) Biological control agents (BCAs) on untreated acorns. (e) BCAs on hot water-treated acorns. Vertical bars show standard deviation of the mean. Moisture content (MC) curves are shown for treatments where MC decreased below 35%: untreated control (a), untreated + AtamonTM (b), MycostopTM, Pseudomonas chlororaphis (d) and HW + CedomonTM (e)
Before storage, 60% of untreated acorns germinated, while 86% of the HW-treated acorns germinated. After 16.5 months of storage, the germination capacity of untreated and HW-treated acorns had decreased to 20 and 40%, respectively (Fig. 1a). Germination was very low for untreated control acorns after 4 months of storage.
The different treatments in combination with HW showed some variation in germination, but overall there was in general very little effect of the combined treatments compared with HW treatment alone. No effect on storability resulted from treating HW acorns with the fungicide OctaveTM before storage (Fig. 1a), and none of the disinfectants in combination with HW improved the storability of acorns (Fig. 1c).
All HW-treated acorns survived longer than acorns in any treatments using non-HW-treated acorns (Fig. 1a–e). After 17 months of storage, combined treatments with HW and either C. rosea or P. chlororaphis resulted in significantly higher germination (p < 0.05), but otherwise no significant additional effects were noted on storability using BCAs combined with HW compared with HW alone (Fig. 1e).
For untreated acorns most of the disinfectants displayed a small positive effect (Fig. 1b), however, these differences were not statistically significant. All BCAs had a significant positive effect (p < 0.05) after 4 months of storage, where the germination rates in controls were extremely low (Fig. 1d). After 14.5–15 months of storage significant differences in germination occurred in MycostopTM treatment only and after 16.5–17 months for P. chlororaphis, RotstopTM (Kemira Agro Oy), Binab TFTM (Bayer, Leverkusen, Germany) and MycostopTM treatments.
The cutting test performed on acorns after 29 months of storage showed that acorns treated with disinfectants or chemicals, and untreated control had an estimated viability below 10%. On average, acorns in the HW control and HW combined with BCAs treatments had an estimated viability of 20%. Acorns in these treatments were germinated and the best results were obtained for C. rosea (10%), RotstopTM (9%), SupresivitTM (7%; Fytovita C. Ltd, Blatnice, Czech Republic) and MycostopTM (7%) treatments. Acorns in all other treatments had a germination percentage below 5%. HW control treatment had a germination percentage of 1%, and acorns in the HW + SepiretTM had a germination percentage of 4%.
Regardless of treatment no significant differences occurred in the mean germination time (MGT) of acorns. The average MGT for all treatments and storage durations was 23 days.
3.2 Infection of acorns by Ciboria batschiana
Immediately after removing dead acorns by skimming procedure, 14% of the acorns showed C. batschiana infection while after 16 months of storage 55% were infected. HW treatment of acorns reduced infection to <1% before and after storage (Fig. 2). After 16 months of storage, Ciboria infection rates were determined on non-HW-treated acorns in combinations with the BCAs and with disinfectants (Fig. 3). All treatments, except the H2O2 treatment, reduced Ciboria infection significantly compared with control. The best effects were obtained for acorns treated with C. rosea, BinabTM, P. chlororaphis, and MycostopTM, where the C. batschiana infection levels were reduced 60–70% (Fig. 3).
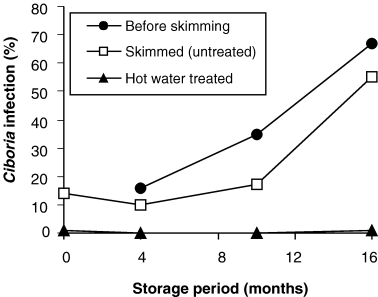
Development of Ciboria batschiana infection of Quercus robur acorns during storage at −1°C for 16 months. Before skimming: unsorted seed lot. Skimmed (untreated): dead acorns have been removed by skimming. Hot water-treated: dead acorns have been removed by skimming, and the remaining acorns have been hot water-treated at 41°C for 2.5 h
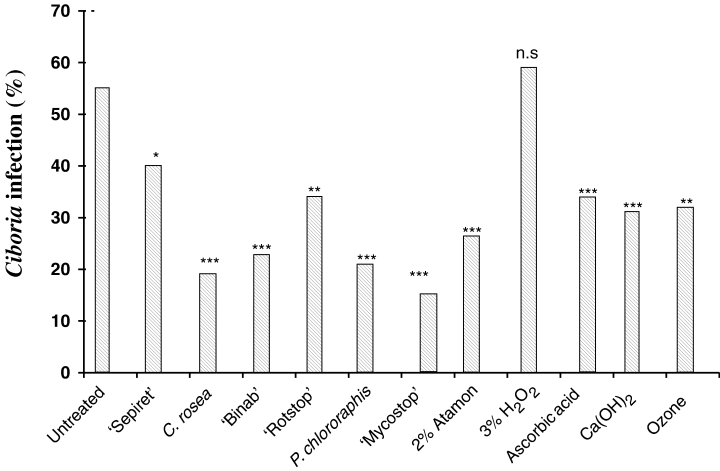
Ciboria batschiana infection after 16 months of storage in acorns which have not been treated with hot water: control, treated with SepiretTM (S), combinations of SepiretTM and biocontrol agents, and disinfectants. The number of asterisk indicates the degree of significance (treatments compared with control). NS, non-significant difference, ***significant at p ≤ 0.001, **significant at p ≤ 0.01 and *significant at p ≤ 0.05
3.3 Survival of BCAs on acorns
Assessment of colony forming units (cfu) of BCAs on acorns after preincubation, but before storage, showed that all the tested BCAs had established themselves on the acorns (Table 3). However, for BinabTM (T. harzianum and T. polysporum) the initial cfu density was close to the detection level (103 cfu/g). After 4 months of storage, the level of cfus was reduced about 10 times for most microorganisms, however, neither the active ingredient in RotstopTM (P. gigantea) was detectable, nor after 16 months of storage was C. rosea.
| Acorn treatments and months (M) of storage | Colony forming units (cfu/g/acorns) | ||||||
|---|---|---|---|---|---|---|---|
| Clonostachys rosea | SupresivitTM | BinabTM | RotstopTM | FZBTM | MycostopTM | ||
| Untreated | 0 M1 | 4.8 × 105 | –3 | 0 | 4.9 × 106 | –3 | –3 |
| 4 M2 | 1.8 × 104 | –3 | 0 | 0 | –3 | 2.5 × 108 | |
| 16 M2 | 0 | –3 | 0 | 0 | –3 | 6.6 × 107 | |
| Hot water- treated | 0 M1 | 3.1 × 105 | 6.4 × 106 | 7 × 103 | 4.6 × 106 | 6 × 108 | 7 × 108 |
| 4 M2 | 1.9 × 104 | 1.2 × 105 | 3.4 × 103 | 0 | 1 × 107 | 5.7 × 107 | |
| 16 M2 | 0 | 7.3 × 104 | 3 × 103 | 0 | 3.5 × 107 | 1.3 × 107 | |
- 1Measurement after 5 days of incubation of treated acorns at 15°C (before storage).
- 2Measurement after 4 or 16 months of storage at −1°C of incubated acorns.
- 3Not tested.
3.4 Mycoflora growth on stored acorns
3.4.1 General observations
Mycelia growth on acorns in all treatments was generally more pronounced immediately before storage compared with the growth after storage (data not shown). Table 4 shows the composition of the prevalent mycoflora 4 months after storage. Most prominent were the taxa Alternaria, Cladosporium, Fusarium, Mucoraceae and Penicillium. A few fungi developed after 10–16 months, e.g. species of Acremonium, Chrysosporium and Oidiodendron echinulatum Barron.
| Treatment | Percentage of acorns with fungi2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acremoniella atra | Alternaria spp. | Cladosporium spp. | Epicoccum purpurea | Fusarium spp. | Muco- raceae | Penicillium spp. | Ulocladium spp. | Sterile mycelia | |
| Untreated | 60 | 30 | 100 | 25 | 50 | 90 | 65 | 30 | 45 |
| Clonostachys rosea | 10 | 0 | 95 | 10 | 10 | 15 | 35 | 15 | 0 |
| BinabTM | 30 | 50 | 100 | 5 | 50 | 55 | 90 | 50 | 30 |
| MycostopTM | 40 | 20 | 65 | 0 | 60 | 35 | 55 | 20 | 15 |
| RotstopTM | 45 | 40 | 95 | 5 | 35 | 60 | 65 | 60 | 35 |
| FZBTM | 45 | 10 | 85 | 0 | 90 | 25 | 70 | 10 | 35 |
| Hot water (HW) | 40 | 70 | 10 | 25 | 55 | 95 | 85 | 50 | 15 |
| HW + fungicide | 15 | 5 | 15 | 0 | 0 | 75 | 10 | 5 | 90 |
| HW + Clonostachys rosea | 0 | 15 | 20 | 15 | 20 | 45 | 0 | 5 | 0 |
| HW + SupresivitTM | 15 | 70 | 10 | 5 | 55 | 45 | 0 | 45 | 5 |
| HW + BinabTM | 25 | 75 | 50 | 20 | 70 | 100 | 0 | 60 | 10 |
| HW + MycostopTM | 10 | 55 | 0 | 0 | 45 | 95 | 75 | 75 | 10 |
| HW + RotstopTM | 5 | 70 | 10 | 5 | 65 | 80 | 0 | 70 | 5 |
| HW + FZBTM | 10 | 80 | 10 | 10 | 60 | 100 | 95 | 30 | 0 |
| HW + Pseudomonas chlororaphis | 5 | 60 | 0 | 0 | 90 | 100 | 0 | 15 | 10 |
| HW + CedomonTM | 0 | 100 | 25 | 0 | 65 | 90 | 100 | 45 | 10 |
- 1Only the most prominent fungi determined.
- 2Twenty acorns per treatment were assayed.
In addition, the following fungi were found on <25% of the acorns in the untreated control after 4 months of storage (data not included in Table 4): Acrotheca, Acrospeira mirabilis, Acremonium/Cephalosporium cf. aurobasidium, Bartalinia, Bipolaris, Botrytis, Calcarisporium, Chaetomium, Chalara, Chrysosporium, Cladotrichum, Codinaea simplex, Colletotrichum, Curvularia, Cylindrium elongatum, Cylindrocarpon, Cylindrocladium, Discosia, Echinobotryum/(Goratomyces stemonitis (Pers. ex Fr.) Morton & Smith), Fumago, Fusicladium, Fusidium, cf. Gonatobotrys, Graphium, Microsphaera alphitoides (Griffon & Maubl.), Monodichtys, Papulaspora, Papularia, Phlebiopsis gigantea (Fr. : Fr.) Mass., Periconia, Pithomyces, Pyrenochaeta, Pythium, Rhinotrichum, Scopularopsis, Septoria, Septocylindrium, Sporotrix, Stemphylium, Subramaniomyces, Subulispora and Truncatella truncata (Lév.) Stey.
3.4.2 Effect of hot water treatment
After 4 and 16 months of storage the percentage acorns overgrown with mycelium was lower in the HW control compared with untreated control (Fig. 4). After 16 months of storage the amount of mycelial growth was reduced by 50% in the HW-treated control compared with the untreated control (Fig. 4). The HW treatment significantly affected, e.g. species of Cladosporium and Papulaspora. However, some Alternaria spp. were often more abundant after HW treatment (Table 4).
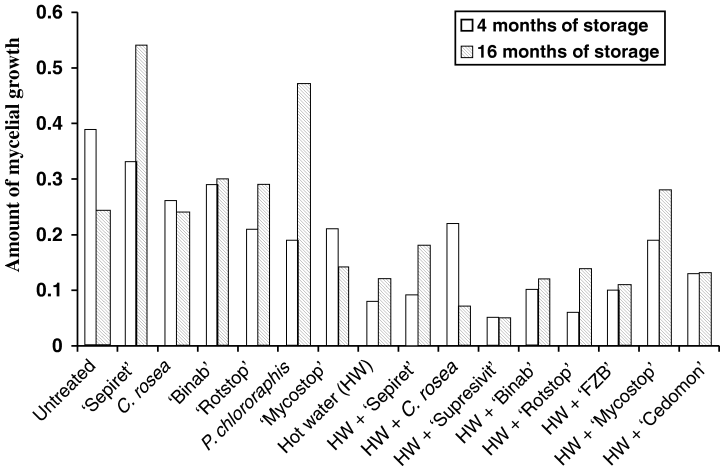
Mycelial growth determined by visual inspection in Quercus robur acorns treated with biological control agents (BCAs) tested on blotter after 4 and 16 months of storage. The histograms show the amount of mycelial growth based on a visual evaluation index of 0–2; where 0, no visible mycelium; 1, scattered mycelium; and 2, seed totally covered with heavy mycelium
3.4.3 Effect of disinfectants
Mycelial growth was, in general, greater after storage when seeds were treated with disinfectants. Thus, all treatments with disinfectants had a higher or comparable percentage of fungus overgrown seeds compared with the respective controls recorded during storage (data not shown).
Only small differences in mycofloral diversity were obtained by using disinfectants after HW treatment compared with HW treatment alone. A few fungi were suppressed: e.g. Acremoniella atra (Corda) Sacc., and Papulaspora sp. compared with HW alone. Otherwise, most of the effect of the combined treatments could be attributed to HW treatment.
3.4.4 Effect of biocontrol treatments
In untreated acorns, visual inspection revealed that SepiretTM provided no protection against abundant growth of mycelia. After 4 months, all BCAs reduced the growth compared with the controls (untreated and untreated + SepiretTM) (Fig. 4). Treatment with the antagonistic fungus C. rosea IK726 suppressed growth of species of Fusarium, Alternaria and Penicillium, before storage and after 4 months (Table 4).
In the combined HW-BCA treatments a group of dematiaceous fungi were suppressed before storage compared with HW alone, including A. atra, Cladosporium sp. and Papulaspora sp. (not shown). In these combined treatments, HW also in some cases stimulated Penicillium sp. and the dematiaceous fungi Alternaria spp. After 4 and 16 months of storage, the picture was very similar (Table 4).
3.4.5 Relationship between fungi and germination capacity
The occurrence of several of the most dominant genera of fungi was tested against germination percentage after 4 months of storage. A significantly (p < 0.0001) high and negative correlation was found between germination rate and species of Cladosporium (r = −0.779), A. atra (r = −0.791) and black/grey sterile mycelia (r = −0.728), respectively. The correlations for Alternaria, Fusarium, Mucoraceae and Penicillium were not significant.
After 10 months, mycoflora and germination rate were tested in the two controls (±HW) and ascorbic acid-treated acorns (±HW). Germination was 29% for the untreated control, 55% for the HW-treated control, 28% for ascorbic acid treatment, and 76% for HW + ascorbic acid treatment.
The mycoflora tested in these four treatments was recorded separately for dead and germinated acorns. Most fungi were more abundant on dead acorns than on germinated (healthy) acorns. Penicillium was almost always present on dead as well as germinated acorns. Cladosporium spp., was prevalent on untreated, dead acorns, whereas species of Cylindrocarpon, Fusarium, Gliocladium/Clonostachys and ‘dark mycelium’ were usually only present on dead acorns (Table 5).
| Taxon | Percentage dead and germinated acorns affected1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Untreated control | Hot water (HW) control | Untreated + ascorbic acid | HW + ascorbic acid | |||||
| Dead | Germ | Dead | Germ | Dead | Germ | Dead | Germ | |
| Acremonium | 22 | 16 | 15 | 17 | 11 | 8 | 16 | 3 |
| Acremoniella atra | 5 | 0 | 5 | 17 | 0 | 0 | 8 | 0 |
| Acrospeira mirabilis | 14 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Alternaria spp. | 7 | 0 | 41 | 3 | 3 | 0 | 17 | 25 |
| Botryotrichum | 1 | 0 | 22 | 0 | 19 | 4 | 9 | 13 |
| Botrytis cinerea | 1 | 11 | 19 | 0 | 1 | 3 | 0 | 0 |
| Chalera | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cladosporium spp. | 26 | 12 | 3 | 0 | 20 | 0 | 1 | 0 |
| Codinaea simplex | 3 | 0 | 1 | 0 | 7 | 0 | 0 | 0 |
| Cylindrium elongatum | 5 | 15 | 7 | 0 | 6 | 4 | 0 | 0 |
| Cylindrocarpon sp. | 12 | 0 | 14 | 0 | 10 | 0 | 24 | 2 |
| Echinobotryum | 0 | 0 | 15 | 0 | 1 | 0 | 0 | 0 |
| Epicoccum purpurea | 0 | 0 | 15 | 0 | 2 | 0 | 3 | 0 |
| Fusarium spp. | 15 | 0 | 8 | 0 | 4 | 0 | 10 | 0 |
| Gliocladium/Clonostachys | 15 | 0 | 0 | 0 | 1 | 0 | 18 | 0 |
| cf. Gonatobotrys | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Graphium spp. | 4 | 4 | 2 | 0 | 2 | 0 | 5 | 0 |
| Mucoraceae | 7 | 0 | 20 | 0 | 7 | 3 | 36 | 12 |
| Oidiodendron echinulatum | 38 | 8 | 0 | 0 | 55 | 25 | 19 | 13 |
| Papulaspora spp. | 9 | 0 | 0 | 0 | 17 | 0 | 6 | 0 |
| Penicillium spp. | 58 | 72 | 97 | 100 | 44 | 68 | 96 | 99 |
| Ulocladium spp. | 1 | 0 | 14 | 0 | 6 | 0 | 15 | 13 |
| Mycelia white (sterile) | 15 | 8 | 0 | 0 | 8 | 17 | 2 | 1 |
| Mycelia grey (sterile) | 18 | 5 | 1 | 0 | 21 | 17 | 9 | 1 |
| Mycelia dark (sterile) | 56 | 4 | 0 | 0 | 55 | 0 | 18 | 0 |
| Unidentified | 0 | 0 | 37 | 0 | 11 | 0 | 26 | 14 |
- 1One hundred seeds assayed in each treatment. Germination was 29% for the untreated control, 55% for the hot water-treated control, 28% for ascorbic acid treatment, and 76% for hot water + ascorbic acid treatment.
4 Discussion
Our study confirms the findings of Delatour and Morelet (1979) and Kehr and Schröder (1996) that C. batschiana is effectively controlled by HW treatment. Without HW treatment, a remarkable increase was recorded in Ciboria infection during the latter stages of storage, when germination capacity had also decreased. In this study, HW-treated acorns germinated better than untreated acorns after 16.5 months of storage at −1°C, 90–95% RH. Some of the tested disinfectants and all the biocontrol agents improved the storability of untreated acorns. This effect seems, particularly for the biocontrol treatments, to be related to Ciboria control.
A diverse saprophytic mycoflora developed during the low temperature storage of the acorns. Some of these fungi such as Acremoniella atra, Alternaria alternata, and species of Fusarium, Papulaspora and Penicillium have been reported as being dominant on acorns during storage (Mittal et al. 1990). Potential pathogenic fungi such as species of Colletotrichum, Graphium, Fusarium and Verticillium (Procházková 1991) seldom occurred.
Hot water treatment affected the abundance of specific saprophytic fungi. However, we did not determine if these fungi reduced germination, or if the saprophytic fungi were associated with poor quality of the acorns. The negative correlation found for germination rate and presence of storage fungi such as A. atra, Cladosporium and dematiaceous mycelia, might be indirectly related to the HW treatment, which obviously had a positive effect on germination. In the study, where the mycoflora was directly related to dead or germinating acorns, it was unclear which fungi killed the acorns. However, dematiaceous mycelia (probably representing Ciboria) were invariably found on dead acorns. Also species of Fusarium, Graphium and Cylindrocarpon were confined almost exclusively to dead acorns. These genera include pathogens of acorns (Uros̆evic 1961). Cylindrocarpon didymum is a weak parasite on seeds with decreased viability after long storage (Werres et al. 1992, 1994). Such fungi were not eliminated by HW treatment. HW treatment does not control Fusarium spp. (Kehr and Pehl 1993).
Domsch et al. (1980) and Kehr and Schröder (1996) suggest that Cladosporium cladosporioides produces plant growth promoting substances which benefits seed viability. However, when forest tree seeds are heavily contaminated with fungi, saprophytes can become opportunistic parasites and cause seed rot (Rees and Phillips 1986). Other fungi influence seed germination or seedling survival, when abundant. Saprophytic fungi such as Mucorales and Penicillium might therefore lower germination capacity. However, we did not test this hypothesis. Here, mycelial growth was generally very sparse on individual acorns and this may have influenced the amount of damage related to specific fungi.
In general, disinfections (for treatments see Table 1) had only a limited effect on fungus growth compared with the effect of thermotherapy, and the fungicide had no effect. The best control occurred after treatment with 3% H2O2 for 0.5 h after HW treatment and treatment with 2% ascorbic acid after HW treatment. However, H2O2 treatment after HW treatment was the least beneficial for germination. For Pinus and Abies seeds, surface sterilization with mercuric chloride, H2O2, sodium hypochlorite or glutaraldehyde, markedly reduced the occurrence of Aspergillus and Penicillium, whereas most of the true pathogenic species were unaffected by any of the test disinfectants (Edwards and Sutherland 1979). None of the H2O2 treatments including 15 and 30% solutions affected the germination capacity of Pinus or Abies seeds (Edwards and Sutherland 1979). In acorns, the radicle often protrudes through the pericarp, and might be harmed by high concentrations of disinfectants.
The efficacy of biocontrol agents is determined by the intrinsic ability to grow into or colonize seed surfaces (e.g. Harman 1991). This ability is strongly affected by the application method and by the physiological, ecological and edaphic interactions that occur immediately after treatment and following seed sowing. Antagonistic fungi might decrease germination under conditions other than those for which they are intended.
In this study, the biocontrol agents (see Table 1) were selected based on criteria such as commercial availability, low temperature tolerance and the recommendation for control of related fungi. However, none of the preparations was recommended for use on acorns or any other crops under such low temperatures as used here (−1°C). Most commercially available biological control products for seed dressing have been developed primarily to control diseases of agricultural crops. Nevertheless, Pseudomonas chlororaphis, MycostopTM (Streptomyces griseoviridis) and Clonostachys rosea showed good effects on seed germination and Ciboria control on untreated acorns. Also in combination with HW, C. rosea and P. chlororaphis seem to be beneficial in suppressing fungus growth.
Some of the results presented here might be specific for the one seed lot which were used in our studies. However, it is interesting that the standard fungicide treatment (OctaveTM, 50% Prochloras-ManganTM) used at The State Tree Improvement Station apparently had no effect. Unfortunately, none of the treatments tested prolonged the viability of the HW-treated acorns significantly. In this seed lot, there was room for improvement, since the viability after 12–13 months was 30% below normal levels for good seed lots at The State Tree Improvement Station (unpublished data). However, the mediocre storability of the tested seed lot might be associated with poor physiological vigour. Also, the initial MC of the acorns was quite low, and this may have limited the growth of fungi on the seed coat. Perhaps some of the treatments would have had a significant effect on a seed lot with higher MC. Conversely, the MC dropped below the critical level for some treatments, which may have negatively affected the viability of the seeds, illustrating that defining and maintaining the optimum MC is difficult. The effect of the critically low MC of acorns in some treatments is difficult to determine. MC below 36% were associated with low viability, but low MC were mostly experienced at the end of the storage period when viabilities are generally low. To further complicate matters, dead seeds hold less water than viable seeds (Bergsten 1987) so perhaps the low MC at the end of the storage period to some degree reflects the low viability of the acorns (this needs to be investigated further). The germination percentages in the untreated control and untreated + AtamonTM treatment were low before the MC decreased. For the untreated + P. chlororaphis and MycostopTM treatments there was no clear relationship between viability and MC, whereas it seems likely that HW + CedomonTM treatment was negatively affected by a low MC.
In conclusion, we have confirmed that HW treatment is an efficient measure against the pathogenic fungus C. batschiana, and that the BCAs tested show promise in reducing the spread of Ciboria, and also control storage fungi. However, more research is needed with different seed lots to optimize of amounts and formulation of seed dressing with beneficial microorganisms before recommendations can be made for putting into practical use.
Acknowledgements
This work was financed by the Danish Forest and Nature Agency. Dr Helmut Junge, Executive Officer, FZB Biotechnik GmbH, Berlin, Germany provided FZB24WG and Dr Margareta Hökeberg, R&D Manager, BioAgri, Uppsala, Sweden provided the CedomonTM as well as fresh Pseudomonas chlororaphis MA342. Authors also thank Henrik Schaumburg, Alsiano A/S for the SepiretTM and Kurt Malmbak-Kjeldsen, Ozon Danmark A/S for providing ozone treatment facilities. Klaus Gille, Forstsaatgut Beratungsstelle, Oerrel, Germany gave advice on the concentrations of disinfectants to use while Henrik Knudsen, the State Forest Tree Improvement Station gave ideas and technical assistance. Dr Anders Ræbild, Danida Forest Seed Centre, assisted with the statistical tests of germination results and Dr David B. Collinge reviewed the article. Furthermore, Nini Leroul, Karin Olesen, Sigrit Diklev and Lene Tjott Müller are gratefully acknowledged for skilful technical assistance.




