Transmission intensity index to monitor filariasis infection pressure in vectors for the evaluation of filariasis elimination programmes
Summary
We conducted longitudinal studies on filariasis control in Villupuram district of Tamil Nadu, south India, between 1995 and 2000. Overall, 23 entomological (yearly) data sets were available from seven villages, on indoor resting collections [per man hour (PMH) density and transmission intensity index (TII)] and landing collections on human volunteers [PMH and annual transmission potential (ATP)]. All four indices decreased or increased hand-in-hand with interventions or withdrawal of inputs and remained at high levels without interventions under varied circumstances of experimental design. The correlation coefficients between parameters [PMH: resting vs. landing (r = 0.77); and TII vs. ATP (r = 0.81)] were highly significant (P < 0.001). The former indices from resting collections stand a chance of replacing the latter from landing collections in the evaluation of global filariasis elimination efforts. The TII would appear to serve the purpose of a parameter that can measure infection pressure per unit time in the immediate household surroundings of human beings and can reflect the success or otherwise of control/elimination efforts along with human infection parameters. Moreover, it will not pose any additional risk of new infection(s) and avoids infringement of human rights concerns by the experimental procedures of investigators, unlike ATP that poses such a risk to volunteers.
Introduction
In 2000, the World Health Organization (WHO) forged a Global Alliance in collaboration with other international agencies in public health and the private sector and launched the Global Programme to Eliminate Lymphatic Filariasis (GPELF) with the aim to eliminate lymphatic filariasis by 2020 (Ottesen 2000; Dean 2002). The principal strategy of GPELF is to interrupt transmission of infection by treating the entire population at risk through community-wide mass drug administration (MDA) (Bockarie 2002), thereby drastically reducing microfilariae (Mf) availability to mosquitoes and preventing transmission of the disease agent (mature Mf as L3) to new hosts through vectors. Filarial transmission depends on more than the two factors, which include microfilaraemia load in the community, vector infection rates, vector densities and man–vector contact. Therefore, transmission can be monitored by quantifying the resting and landing vector densities and vector infection and infectivity rates. The intensity of transmission in filariasis is often based on calculation of the number of infective larvae transmitted per person per year, obtained by dissection of mosquito vectors caught while landing (biting) on human beings. The fifth report of the WHO Expert Committee on filariasis recommended annual biting rate (ABR), annual infective biting rate (AIBR) and annual transmission potential (ATP) as the entomological parameters to measure the transmission intensity for lymphatic filariasis (WHO 1992). Researchers have adopted these parameters to measure the intensity of Wuchereria bancrofti transmission and the influence of control measures, in India (Hati et al. 1989; Reuben et al. 2001), in Africa (McMohan et al. 1981; Jaoko et al. 2001) and in Papua New Guinea (Bockarie et al. 1996, 1998; Kazura et al. 1997). The ATP and AIBR provided useful information for appraisal of impact of intervention measures and for the assessment of the changes in transmission in situations with or without vector control (Bockarie et al. 1996; Das et al. 2001; Reuben et al. 2001; Chang 2002; Sunish et al. 2002). Based on the strong positive correlation between the infection rates in humans and entomological measures before and after treatment, ATP could be used to monitor the efficacy of control measures against lymphatic filariasis (LF) (WHO 1992).
The mosquitoes collected in the resting collection have been used not only to estimate population densities (relative abundance) but also the transmission levels of mosquito-borne diseases. Various sampling methods are used to collect adult vectors of filariasis around the world (Bockarie 2002). All are subject to bias, but landing catches using human volunteers is considered to be the most reliable single method for monitoring populations of anthropophilic species (Service 1977, 1993). But landing collection is time consuming, laborious and increasingly regarded as unethical (Das & Ramaiah 2002), with an increased risk of volunteers being infected with vector-borne diseases such as malaria, multidrug-resistant malaria, dengue, dengue haemorrhagic fever and filariasis. We are therefore looking for new methods to estimate these transmission indices (Subramanian et al. 1989; Lines et al. 1991; Das et al. 1997; Bockarie 2002; Das & Ramaiah 2002) and to validate safe procedures and parameters such as density per man hour (PMH) collection of vectors and transmission intensity index (TII) from resting collections against potentially harmful and more direct parameters such as ABR and ATP, which involve landing collections with a potential risk of infection to human volunteers.
In our longitudinal studies (1995–2000) in nine villages of Tirukoilur area, south India, three intervention projects were implemented (Reuben et al. 2001; Rajendran et al. 2002a,b; Sunish et al. 2002). Here we analyse the data on resting and landing collections to determine the relationship between the two collection methods with respect to the number of female Culex quinquefasciatus caught PMH (per unit time) and the transmission indices (TII and ATP).
Materials and methods
Entomological data were collected from the village level study at Tirukoilur, south India, to determine the impact of annual single mass drug administration (MDA) on lymphatic filariasis, using two drugs [diethylcarbamazine citrate (DEC) + ivermectin (IVM)], with or without vector control from 1995 onwards. There were nine villages and they were randomly allocated to three groups: group A – only MDA with DEC + IVM in 1995 and 1996; group B – MDA integrated with vector control; group C – kept as controls until 1998, then included in the MDA arm (Reuben et al. 2001; Sunish et al. 2002). Data from two experimental studies during the 5-year study period (1995/1996 to 1999/2000), with 23 observation points (yearly data sets) were available from these villages. Five data sets each (yearly averages) were available for three representative villages (between 1995 and 2000), one each from group A, B and C villages on PMH (resting and landing), TII and ATP, covering a 1-year period for each data point (each data point in 1, 2 represents 48–68 h of resting collection; and 144–204 h of landing collection). In addition to these 15 data sets in three representative villages, four more villages were added for entomological observations (one each from groups A and B; two from group C) to measure the impact of new interventions from 1998 (using combination drugs, with DEC, IVM and albendazole), yielding eight more data sets in 1998/1999 and 1999/2000.The details of the study area and the impact on the filarial infection status in human beings and vectors in general terms are described in Reuben et al. (2001) and Sunish et al. (2002).
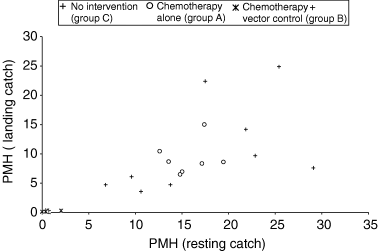
Scatter diagram showing the vector density in resting and landing collections over the years with or without vector control.
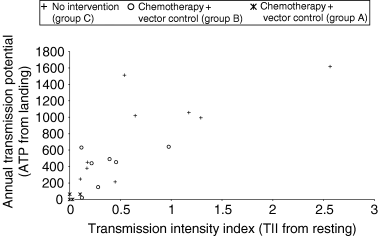
Scatter diagram of transmission indices in three experimental village groups over the years with or without vector control.
During these study periods, the filarial vector Cx. quinquefasciatus and its infection status were monitored, both by resting and landing catches, in order to determine the impact of the various intervention strategies. In each village, resting Cx. quinquefasciatus were collected from 16 human dwellings between 09.00 and 11.00 hours by two well-trained insect collectors, spending 15 min in each dwelling. All potential resting places were thoroughly searched with the help of torchlight and the mosquitoes were collected with an oral aspirator. These collections were carried out at 3- or 4-week intervals. The collections were transported to the laboratory on ice for further identification and dissection, and for determination of the individual vector infection status. From each of the collections, about 100 female Cx. quinquefasciatus were dissected for physiological age and infection with W. bancrofti. They were considered as infective when they harboured infective larvae (L3) in any part of the body (head, thorax and abdomen). These data were used to estimate the TII. The relative density of resting female vectors was expressed as number captured PMH; while TII was derived by multiplying (i) number of females collected PMH; (ii) proportion of females with L3; and (iii) average number of L3/infective females (Krishna Rao et al. 1981). Thus, TII gave the number of L3 availability PMH of collection in the household environment of the village.
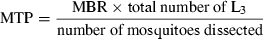
The results of entomological evaluations before and after the interventions which emphasize the relationship between the two collection methods and tested by correlation and regression analysis. The Student's t-test was used for assessing the significance of the correlation coefficient between parameters from resting and landing collections. SPSS/PC+ (version 4.0.1) software was used for the data analyses.
Results
Vector densities (mean PMH for 1-year period) in resting collections ranged from 6.81 to 29.08 in the non-vector control villages of groups A and C, with a mean (SD) of 16.7 (1.5). From the landing collections, the PMH ranged from 3.59 to 24.88, with a mean ± SD of 10.2 ± 1.5 (Table 1). Due to the successful implementation of vector control operations, we collected <1.0 mosquitoes PMH, for both types of collections in group B villages. This is very clear from the scatter diagram (Figure 1), where points lie close to zero. In non-vector control villages (groups A and C), most values were between 10 and 30 PMH. Data points for group A villages were clustered amidst those of group C. One village of group C maintained PMH values at around 10 for 3 years, although the filarial transmission was high (>1000 ATP and >0.5 TII, because of high vector infection). When these density data were analysed for their relationship, PMH for resting and landing collections from the seven villages showed a significant linear association, with r = 0.773 (P < 0.001). The regression equation was Y = 0.13 + 0.59X; where ‘Y’ is landing density (PMH) and ‘X’ is resting density (PMH) with 0.11 as standard error of coefficient.
| Village | Year | Transmission indices | Vector densities (PMH) | ||
|---|---|---|---|---|---|
| TII | ATP | Resting | Landing | ||
| A1 | 1995/96 | 0.975 | 641 | 19.44 | 8.63 |
| 1996/97 | 0.120 | 21 | 15.01 | 6.98 | |
| 1997/98 | 0.115 | 632 | 13.56 | 8.70 | |
| 1998/99 | 0.216 | 440 | 12.60 | 10.47 | |
| 1999/00 | 0.457 | 454 | 17.39 | 15.02 | |
| A2 | 1998/99 | 0.280 | 150 | 14.79 | 6.51 |
| 1999/00 | 0.393 | 491 | 17.13 | 8.37 | |
| B1 | 1995/96 | 0.103 | 63 | 2.03 | 0.32 |
| 1996/97 | 0.000 | 63 | 0.53 | 0.35 | |
| 1997/98 | 0.000 | 0 | 0.69 | 0.09 | |
| 1998/99 | 0.000 | 0 | 0.56 | 0.09 | |
| 1999/00 | 0.000 | 0 | 0.31 | 0.11 | |
| B2 | 1998/99 | 0.000 | 0 | 0.00 | 0.14 |
| 1999/00 | 0.028 | 0 | 0.39 | 0.16 | |
| C1 | 1995/96 | 2.564 | 1617 | 22.84 | 9.70 |
| 1996/97 | 0.539 | 1512 | 9.59 | 6.11 | |
| 1997/98 | 1.173 | 1057 | 10.60 | 3.59 | |
| 1998/99 | 0.646 | 1019 | 6.81 | 4.73 | |
| 1999/00 | 0.169 | 380 | 21.85 | 14.19 | |
| C2 | 1998/99 | 1.292 | 994 | 17.48 | 22.39 |
| 1999/00 | 0.175 | 453 | 25.40 | 24.88 | |
| C3 | 1998/99 | 0.105 | 248 | 13.75 | 4.73 |
| 1999/00 | 0.447 | 213 | 29.08 | 7.61 | |
- TII, transmission intensity index; ATP, annual transmission potential; PMH, density per man-hour.
The transmission parameters were markedly affected by the intervention strategies implemented, namely MDA and vector control. In the villages where vector control operations were carried out from 1995 (village B1), TII values remained at zero level over a period of four consecutive years (1996–2000) (Table 1). The transmission estimated from the landing collection (ATP) followed a similar trend; this index remained at zero level for three consecutive years (1997–2000). The TII values in group A (village A1), where MDA alone was implemented, declined after two MDAs from 0.975 to 0.115 but rose to 0.457 in 1999/2000 after the withdrawal of MDA. In one of the villages of group C (village C1), where no intervention was carried out up to 1999, the TII value ranged from 0.539 to 2.564. From 1999 group C villages were also included in the MDA experiment, transmission levels declined and TII dropped below 0.5. The ATP values in these villages (C1, C2 and C3) declined to 213–453, from 1057–1617 during 1995/1998 (in C1). Thus, of nine data sets in group C villages, three data sets of three villages belonging to the intervention period (July 1999 to June 2000 period) recorded reduced ATP (<453) and reduced TII (<0.5) values, unlike very high values before intervention (1995/1998). There was a significant linear and positive relationship between TII and ATP by correlation analysis: r = 0.809 (P < 0.001). The regression equation was Y = 173.9 + 658.3X; where ‘Y’ is ATP and ‘X’ is TII with 104.5 as the standard error of coefficient. In Figure 2 most points lie near zero or remain very low on both axes (i.e. <0.5 TII and <500 ATP). Even in this clustering, there was a linear pattern of steady decrease of both the parameters simultaneously with interventions. All seven data sets of group B villages over the 5 years (5 years from B1 and 2 years from B2) were below 100 for ATP and below 0.2 for TII. Four of these data sets of ATP and TII (three from a B1 village during 1997/1998, 1998/1999 and 1999/2000 and one from a B2 village during 1998/1999) had zero values for both parameters at the starting point of both axes. In addition to these four, one more value for TII (B1 in 1996/1997) and ATP (B2 in 1999/2000) was zero.
Discussion
Transmission dynamics of LF are complicated as they involve the parasite, the human host and the vector mosquitoes. The relative abundances of competent vectors, their biology and behaviour and the parasite load and its maturity in the vector all influence transmission parameters. Transmission can be reduced or even interrupted by either eliminating the reservoir of microfilariae through community-wide treatment, or by reducing human–vector contact or, ideally, an integrated approach combining both strategies. This is referred to as transmission control (Ottesen et al. 1997). Infection and infectivity of vectors because of Mf load in the community is the vital factor and densities of vectors ranging from 10 to 30 PMH could yield >1000 ATP depending upon the infection rate (high infection in the vectors along with 10 PMH) in one of the group C villages before control measures. This would mean that PMH should be reduced to <2 or even lower to reduce transmission through vector control alone. Thus, the intensity of transmission not only depends on the infection in humans, but also in the vector population, by nurturing Mf to L3 and transferring them to susceptible hosts (Bockarie 2002).The relationship between parasite yield, success rate of ingested microfilariae becoming infective L3 larvae in the mosquito vector, and the density of microfilaraemia in the human host is a very important determinant of transmission efficacy. Cx. quinquefasciatus’ ability to ingest Mf and support their development at ultra-low densities, resulting in effective transmission, was demonstrated by Jayasekera et al. (1991) in Sri Lanka and by Subramanian et al. (1998) in India. Vectors are sufficiently competent to sustain high transmission of >1000 ATP at about 10 PMH, in the current study. There are several quantitative estimates as to the minimum number of infective bites required for successful patent infection (Rajagopalan et al. 1977) and threshold levels of human infection for continued transmission (Ramaiah et al. 1994). Therefore, by monitoring vector densities and vector infection, it is possible to quantify transmission levels. In fact, it is more sensitive as vectors concentrate Mf from the blood meal (Das & Ramaiah 2002).
Frequently used methods include resting catches, landing vectors on human volunteers and light trap collection. Human landing collections are cumbersome, expensive, ethically unacceptable and not feasible in large-scale programmes (Das & Ramaiah 2002). There is an urgent need to find alternative methods that are risk-free and do not involve landing collection of mosquitoes on human volunteers. Resting vector collection in human dwellings and determining their parasite infection status could be a substitute index to landing collections as a monitoring tool. In the seven villages studied, transmission levels in control villages prevailed at >1000 ATP for some years but dropped to one-third level or less with MDA intervention in 1999. At the other extreme, villages with combined interventions maintained zero-level transmission for 3–4 years, although they belonged to the same area and experienced similar transmissions before interventions.
The data from the resting and landing collections from Tirukoilur area, with the above background and significance were grouped by year for different intervention villages and the relationship between the two methods of collections were compared for density (PMH – resting vs. landing) and transmission indices (TII vs. ATP). The coefficient of correlation for the resting and landing density was 0.773 and for transmission, the r value was 0.809 (df = 21). Subramanian et al. (1989) in Pondicherry also recorded a significant relationship between the two densities, with r = 0.938 (df = 46). The R2 value between resting and landing densities was 88% in the Pondicherry study and 60% in this study, leaving a 12–40% variation unexplained in the landing collections from the data on resting collections. However, from the strength of the association with the study in Pondicherry, we used the proportion of resting mosquitoes with L3 larvae (infective vector density) as an index of potential infection pressure, and explored its relationship with human infection parameters (Mf prevalence and intensities) (Subramanian et al. 1989). We obtained the potential infection pressure per hour (TII) from 12 months’ collections for each data point, by incorporating vector densities also into infection pressure. Apart from the positive correlation between resting and biting densities similar to the previous Pondicherry study, we established that TII was positively related to ATP and represents potential infection pressure per unit time (1 h), under varied circumstances with experimental interventions, withdrawals, re-intervention, etc. The TII estimation avoids the infringement of human rights concerns associated with ATP.
Understanding the relationship between the quantum of transmission and corresponding incidence of new infections is essential to plan control strategies and forecast epidemiological trends (Ramaiah et al. 1994). An ATP of 100 was determined as the permissible and safe level (WHO 1977) and taken as criterion (threshold) for the control of onchocerciasis. Although no such estimate is available for LF, Ramaiah et al. (1994) proposed a range of 96–105 ATP and 0.5 TII as the permissible levels of transmission and it was postulated that below these levels no new infections might occur. In our study, these indices were below the suggested threshold levels in those villages where control interventions were implemented, and they became negligible or nil for 3–4 years in the villages where combined control interventions were employed. In stark contrast, the neighbouring control villages maintained transmission levels several times higher. The indices not only declined (ATP and TII) with two MDAs, but also resurged after withdrawal of MDAs, and again dropped with the third MDA. The importance of integrated approach using a package of interventions was advocated in some of the recent studies (Killeen et al. 2000; McKenzie et al. 2002; Utzinger et al. 2002).
A study in Sri Lanka suggested that the examination of recently fed house-resting populations of Cx. quinquefasciatus could be a sensitive method for measuring the prevalence of Mf in the human population (Jayasekera et al. 1991). Blood-meal assays performed on indoor- resting catches showed a high percentage of positives for human beings in comparison with animals and birds (Gowda & Vijayan 1992). Subramanian et al. (1989) have found a quantitative significant correlation between resting and biting density. Earlier workers have analysed the association of landing catch with other methods of collections. A positive correlation was observed for the vectors caught by landing and light trap methods in Tanzania (Lines et al. 1991). In southern Sierra Leone, the number of female Anopheles gambiae caught by light trap was strongly correlated (r ≥ 0.72) with those from corresponding human volunteer catches performed either on the same or on adjacent nights (Magbity et al. 2002). Bockarie et al. (1994) showed that the spray sheet method was more sensitive than the landing catch method at low vector densities. Das et al. (1997) observed a significant correlation between hand catches and insecticide-baited trap collections in terms of mosquito density. In Tanzania Maxwell et al. (1990) found the infective rate to be higher in unfed Cx. quinquefasciatus from light traps than blood-fed specimens from resting catches in the same villages. This is presumably because blood-fed mosquitoes had extruded some of the L3 larvae.
Bockarie (2002) points out that, calculating biting rates from resting catches must be carried out with great caution because the behaviour of Cx. quinquefasciatus varies according to locality. In Rangoon, adults mostly rest and feed outdoors (de Meillon & Sebastian 1967), while in Africa this species exhibits endophagic and indoor feeding behaviour (Service 1982). However, in Pondicherry, south India, this species was endophilic and endophagic and the human blood index was >90% (Rajagopalan et al. 1977). Our findings assume more importance in view of these observations. In Pondicherry, Vanamail et al. (1993) derived a new formula [risk of infection (RI)], which can be used to estimate the biting population from resting catches. They observed a significant relationship of RI with human Mf prevalence. This can also be applied to measure the transmission intensity in an area from the resting vector population.
Hence, because of the various difficulties cited above for carrying out the night landing catches, and to avoid the risk of acquiring vector-borne diseases by human volunteers, it would be prudent if the filarial vectors are collected from the resting vector population in human dwellings, which will provide similar information as landing catches, especially for monitoring and evaluating large-scale control programmes aimed at the elimination of the disease. Both ATP and TII decreased simultaneously with interventions, remained high without interventions in control villages, and returned to higher levels after withdrawal of intervention measures half-way through the programme in the intervention villages, thus demonstrating a positive correlation between them. The TII would appear to serve the purpose of a parameter that can measure infection pressure per unit time in the immediate household surroundings of the man and can reflect the success or otherwise of the control/elimination efforts along with human infection parameters. It does not pose any additional risk of new infection(s), and avoids infringement of human rights unlike in ATP. The validity of TII as an evaluation tool will depend upon the endophilic and endophagic nature of the filarial vector. Therefore studies have to be conducted in different geographical areas to ascertain that both indices positively correlate before adopting only resting collection to monitor the large-scale programmes.
Acknowledgements
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for research and Training in Tropical Diseases (TDR Grant No. 940340 and WHO/CTD/FIL grant no. 990574). The authors are grateful to Dr V. Kumaraswami, Deputy Director, Tuberculosis Research Centre (TRC), Chennai for his constant encouragement and guidance throughout the study. We acknowledge the technical assistance rendered by staff members of CRME (ICMR) Field Station, Tirukoilur. We appreciate the excellent help rendered by Shri A. Venkatesh, Research Assistant, CRME, Madurai, in preparation of this manuscript, particularly in DTP work.




