Susceptibility to development of Mycobacterium ulcerans disease: review of possible risk factors
Abstract
Mycobacterium ulcerans disease, also known as Buruli ulcer (BU), is a disease of subcutaneous fat tissue. BU is prevalent in riverine and swamp areas of the tropical zone in Africa, Asia and South America, and a few scattered foci in Australia. The mode of transmission of M. ulcerans has not been fully elucidated, but inoculation into the subcutaneous tissues probably occurs through penetrating skin trauma. BU has not been linked with HIV infection. Antimycobacterial drug treatment is ineffective, and treatment is surgical. Patients eventually develop scars and contractures, with resulting disabilities, and the disease imposes a large burden on affected populations. The incidence of BU has dramatically increased in West African countries over the last decade. There is an urgent need for research into host and environmental risk factors for BU in order to develop effective strategies to combat this disease. We review possible genetic host susceptibility factors for BU that are relevant in other mycobacterial diseases: natural resistance-associated macrophage protein-1 (NRAMP-1), HLA-DR, vitamin D3 receptor, mannose binding protein, interferon-gamma (IFN-γ) receptor, tumour necrosis factor alpha (TNF-α), interleukin (IL)-1α, 1β and their receptor antagonists; and IL-12. Schistosoma haematobium infection is highly endemic in many BU foci in West Africa, with a striking increase in transmission after river dams were constructed. This observation, and the observations from interaction of schistosomiasis and tuberculosis, have fuelled our hypothesis that schistosomiasis is a risk factor for BU by driving the host immune response towards a predominantly Th-2 pattern, away from a Th-1 preponderant protection against mycobacterial infection. If the latter hypothesis is confirmed, enhanced schistosomiasis control should impact on BU.
Introduction
Buruli ulcer (BU), caused by Mycobacterium ulcerans, has been identified as an emerging infectious disease in West Africa (van der Werf et al. 1999). At an international meeting in July 1998 in Côte d’Ivoire (Asiedu et al. 1998; van der Graaf et al. 1999), the Yamoussoukro Declaration on Buruli Ulcer was made, expressing the concern that little is known about this disease, and called on the international community to support control and research efforts. In 1999 and 2000, the initiative was continued by two WHO meetings to report and evaluate the progress on knowledge and understanding of disease caused by M. ulcerans.
Buruli ulcer was first documented in Australia in 1948 (MacCallum et al. 1948) but reports suggest that the disease was already recognized clinically by Cook in the 19th century in Uganda (Anonymous 1970; van der Werf et al. 1999). In the 1960s, many patients in refugee camps in an area near the Nile River in Uganda, called Buruli, had ulcers which were caused by M. ulcerans. The disease has since become to be known as BU. Since its first description, it has been reported from many predominantly tropical countries, with the highest numbers of patients reported from Africa. Its distribution around the tropical world is patchy, occurring in isolated foci where it may be common. Transmission of the infection is suggested to be by subcutaneous infection with M. ulcerans, an environmental mycobacterium present in soil, shallow water, certain vegetations, or in insects living in or near the water (Portaels et al. 1999). Infection probably results from penetrating injuries (Meyers et al. 1974). Subcutaneous inoculation may result in amplification of bacteria in the subcutaneous fat tissue. Secretion of toxins, one of which has been identified as mycolactone, a polyketide toxin (George et al. 1999), and possibly other toxins as well – e.g. phospholipase C (Gomez et al. 2000), are the major cause of extensive tissue necrosis, resulting in the clinical hallmarks of BU. Inoculation is followed by a latent period without clinical manifestations (stage 0), then a pre-ulcerative subcutaneous nodule appears (stage 1). In Australia, stage 1 lesions consisting of an intracutaneous nodule have been described. Occasionally, patients in stage 1 develop extensive indurated lesions, or oedema (Figure 1). In stage 2, necrosis, the clinical hallmark of BU, sets in. The typically undermined necrotic ulcerative lesions can be easily recognized (Figure 2). Occasionally, the infection has been reported to spread to other tissues like bone. A granulomatous healing response occurs in stage 3 (Figure 3) and finally, in stage 4, fibrosis, scarring, calcification and contractures may result (Figure 4). Treatment of BU is surgical, with excision and skin grafting. Although M. ulcerans is sensitive to various antimycobacterial agents in vitro, such treatment has not been effective in clinical studies. The socio-economic burden of BU for afflicted populations is formidable (Asiedu & Etuaful 1998).
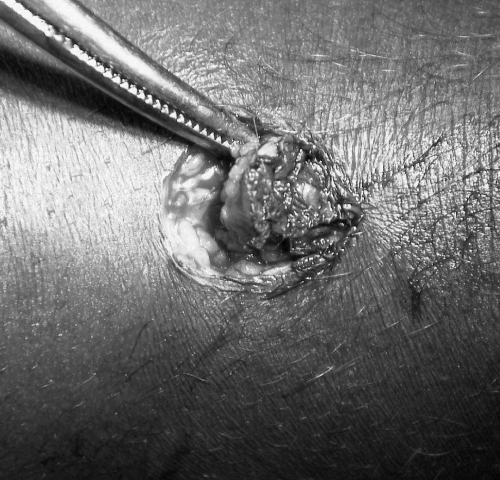
Stage 1 (nodule) is best managed by surgical excision; treatment results are excellent provided that patients report in time.

Stage 2 (ulcer) is highly characteristic, with necrotic slough and undermined ulcer edges.
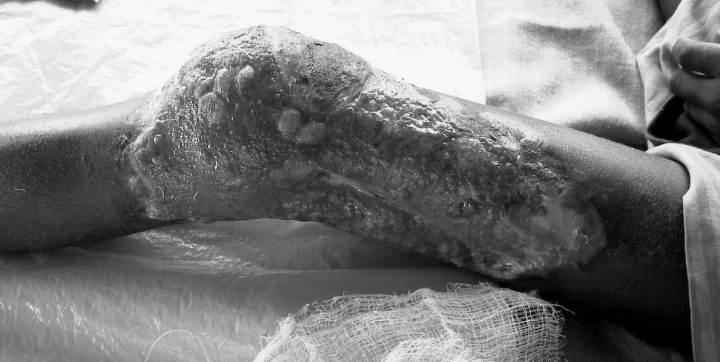
Stage 3 (healing) is often accompanied by development of contractures.
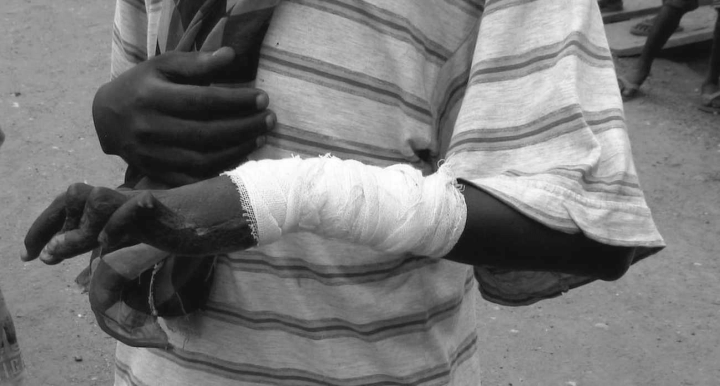
Patients with Buruli ulcer (BU) may have excellent results if surgery is early and adequate, but many patients in endemic areas with poor access to health care are left with scars, contractures, handicaps and disabilities.
Mycobacterium ulcerans can be cultured in vitro at 32 °C albeit with difficulty. Recently, other culture media have been tested which may help to improve the chance of successful isolation, and to investigate the conditions which facilitate the production of toxins (Palomino et al. 1998; Mve-Obiang et al. 1999). In vitro culture may also help to identify new antimycobacterial drugs for the treatment of BU in certain disseminated cases or in complicating osteomyelitis (Portaels et al. 1998; Mve-Obiang et al. 1999). Identification using a polymerase-chain reaction is much more sensitive and more feasible. Several such tests have now been described (Portaels et al. 1997; Ross et al. 1997; Guimaraes-Peres et al. 1999). Restriction fragment length polymorphism of M. ulcerans strains was reported using the pTBN12 probe (Jackson et al. 1995). A 517-base pair fragment of the 16S rRNA gene of M. ulcerans has successfully been amplified and sequenced. Sequence analysis revealed three patterns. Sequences from 10 strains isolated from the African continent appeared identical; one different type was isolated from Mexico, and yet another from Australia (Portaels et al. 1996). Apparently, different endemic areas have regional differences in the genome of M. ulcerans, but the importance of this finding is largely speculative; there are no reports today on differences in virulence associated with differences in the genome.
Of all individuals who have latent tuberculosis infection, only 10% will ever develop clinical disease. We hypothesize that a significant proportion of the population living in an endemic area and who are exposed to M. ulcerans never develop BU, just as is the case with tuberculosis. No data on host susceptibility factors for M. ulcerans are available today, but several risk factors have been described for tuberculosis and leprosy. In this review, we summarize some important results of host susceptibility factors that have been discovered in other mycobacterial diseases, and explore several hypotheses relevant for future research to combat the emerging burden imposed by BU.
Susceptibility for BU: inherited and acquired defense mechanisms
Unlike tuberculosis, the emergence of BU has not been linked with co-infection by the human immunodeficiency virus. This review will therefore not touch upon increased susceptibility to mycobacterial disease and AIDS. Host susceptibility has been studied in a wide range of infections by intracellular microorganisms (McLeod et al. 1995) and in mycobacterial diseases other than BU, notably, tuberculosis (Bellamy 1998, 1999, 2000; Bellamy & Hill 1998; Bellamy et al. 1998a; Goldfeld et al. 1998) and leprosy (Lagrange & Abel 1996; Abel et al. 1998). Occasionally, genetic susceptibility factors have been reported in other mycobacterial diseases such as non-tuberculous mycobacterial (NTM) infections with M. fortuitum, M. chelonae, and M. avium-intracellulare complex (MAC) infections (Levin et al. 1995), and disseminated M. bovis-BCG infection (Frucht et al. 1999; Jouanguy et al. 1999). Conceivably, not only environmental factors determine whether BU will develop. Host susceptibility factors need to be explored to understand factors explaining the development of BU once individuals have been infected with M. ulcerans. These factors can be genetically or environmentally determined. We hypothesize that the immunological balance in T-helper cell 1/2 subset (Th1/Th2) responses is an important mechanism which can potentially be influenced by vaccines or immunotherapeutic therapies, such as BCG, M. vaccae or interferon-gamma (IFN-γ). One of the environmental factors could be helminthic co-infection, e.g. schistosomiasis which is highly prevalent in many of the areas where BU is increasingly found. In BU, no studies have been reported to date.
Th-1/Th-2 balance
Th1 cells produce IFN-γ, interleukin (IL) 2, and tumour necrosis factor alpha (TNF-α) and promote the production of opsonizing and complement-fixing antibodies, macrophage activation, antibody-dependent cell cytotoxicity, and cell-mediated immunity. Th1 cells are essential for the phagocyte-dependent host response. Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13, provide optimal help for humoral immune responses, including IgE and IgG1 isotype switching and mucosal immunity, through induction of mast cell and eosinophil growth and differentiation and facilitating IgA synthesis. Moreover, some Th2-derived cytokines, such as IL-4, IL-10 and IL-13 inhibit several macrophage functions. Hence it is possible to refer to Th2 cells as responsible for the phagocyte-independent host response.
Th1 and Th2 cells do not derive from distinct lineages, but develop from the same Th-cell precursor under the influence of environmental and genetic factors acting at the level of antigen presentation. The genetic mechanisms that concur in controlling the type of Th-cell differentiation remain elusive (Romagnani 1997). Among the environmental factors are the nature of the antigen itself, its tissue distribution once it has come in contact with the host immune system, and its mode of entry (Constant & Bottomly 1997). The site of antigen delivery influences T cell priming (Constant et al. 2000). Leishmania major parasites delivered to mice via an intranasal route failed to induce the expected Th1-dominated responses and instead preferentially prime for Th2 responses, proving that the pulmonary environment promoted preferential Th2-type differentiation. In the determination of lymphokine-producing phenotype, lymphokines and cytokines play a dominant role. The early presence of IL-4 is the most potent stimulus for Th2 differentiation, whereas IL-12 and IFN-γ favour Th1 development. The inducing effect of IL-4 dominates over other cytokines so that, if IL-4 levels reach a necessary threshold, differentiation of the Th cell into the Th2 phenotype results (Romagnani 1997). IL-4 and IL-12 promote the development into Th2 or Th2 effectors by binding of the cytokines to their receptors. This results in tyrosine phosphorylation of signal transducers and activators of transcription (STATS). IL-12 selectively induces nuclear DNA-binding complexes that contained STAT4 (Jacobson et al. 1996) and targeting of the Stat4 gene results in the inhibition of Th1 responses (Kaplan et al. 1996). IL-4 induces nuclear DNA-binding complexes containing STAT6 (Hou et al. 1994), deficiency of Stat6 by gene targeting results in deficient Th2 responses (Takeda et al. 1996). The role of IL-10 does not easily fit into the paradigm of Th1/Th2 disbalance, as IL-10 is primarily associated with immune suppression, and not with Th2 preponderance. Recent data from Gabon show that IL-10 induced by schistosomiasis haematobium may protect individuals from developing atopy that would be expected to result from schistosomiasis-induced Th2 preponderance (van den Biggelaar et al. 2000).
Mycobacterial disease resulting from immune impairment induced by helminthic co-infection
It is apparent that an imbalance in Th1/2 phenotype immune response coincides with the onset of tuberculosis. Zhang et al. (1995) consistently found that the transition from latent tuberculosis infection to tuberculosis is associated with diminished Th1 but not enhanced Th2 responses in the blood stream and at the site of infection (Lin et al. 1996). True Th2 preponderance has been reported by Surcel et al. (1994). Beyers et al. (1998) also suggested that helminthic infections might influence the Th1/2 balance and enhance the probability for development of latent tuberculosis infection to tuberculosis. Apoptosis of Th1 cells has been reported in experimental mycobacterial infection (Das et al. 1999). During the course of successful treatment of tuberculosis patients, IgE levels appear to drop significantly (Adams et al. 1999). Although there is overwhelming evidence that tuberculosis itself causes transient depression of Th1 cell function, and transient enhanced Th2 phenotype cell function, some conflicting results as regards Th2 enhancement have been reported (Zhang et al. 1995) but these differences might be explained by stage and severity of tuberculosis (Dlugovitzky et al. 1997) or other confounding factors that influence Th1/2 balance, such as age (Shearer 1997) and nutritional state (Dai et al. 1998). In tuberculoid leprosy, Th1-like cells predominate with a resistant immune response, whereas Th2-like cells are dominant in lepromatous leprosy patients with ineffective immunity (Yamamura et al. 1991; Chensue et al. 1994, 1997). Subjects with M. ulcerans infection showed significantly lower production of IFN-γ and IL-12 and significantly higher production of IL-4, IL-5, IL-6 and IL-10 in response to both M. ulcerans and BCG than exposed control subjects (T.M. Gooding & P.D.R. Johnson, unpublished data). Both mycobacteria and helminths – e.g. Schistosoma spp., are characterized by host granuloma formation response. Th1 cells protect against mycobacteria while Th2 cell repertoire protects the host against helminthic infections, e.g. Schistosoma mansoni (Yamamura et al. 1991; Chensue et al. 1994, 1997). Th2 responses can be responsible for reduced resistance against several infectious agents. Th2 cells inhibit development of Th1 cells and macrophage activity, thus avoiding attempts to destroy large parasites through Th1 responses and macrophages, which may be harmful to the host (Romagnani 1997). If schistosomiasis infection drives the immune system towards a predominant Th-2 pattern, these infected individuals may become more susceptible to BU after they have been infected by M. ulcerans. Apart from the Th1/Th2 paradigm, worm infestations may impact on several important post-receptor transduction pathways such as tyrosine kinases and mitogen activated protease (MAP) kinases, notably, ERK 1/2 and p38 MAPkinases (Borkow et al. 2000).
Gene polymorphisms involved in host genetic susceptibility to mycobacterial infections
Prior to the discovery of the tubercle bacillus, the observation that tuberculosis frequently occurred in many members of the same family convinced physicians that it was a hereditary disease. Only 10% of those who become infected with M. tuberculosis will ever develop clinical disease, and only occasionally, an obvious risk factor such as diabetes, advanced age, alcohol abuse, HIV infection, or corticosteroid use can be identified (Bellamy & Hill 1998). Studies of twins have confirmed the importance of genetic factors in determining host susceptibility to infection with mycobacteria and other pathogens, and the concept of genetic susceptibility – and even the presentation of disease – for mycobacterial disease has been widely accepted (Stead 1992; Levin et al. 1995; Abel et al. 1998; Bellamy 1998). The relevant immunological responses have recently been summarized (Foote 1999). A central role is played by the antigen presenting cell (APC) belonging to the cell lineage to which the dendritic cell (in the skin) and the alveolar macrophage (AM, alveolar spaces) and monocyte (peripheral blood) belong. The APC/AM/M drives most of the immune responses after phagocytosis of the invading mycobacteria, and express antigens on its cell surface.
Human leucocyte antigen (HLA)
Most of the earlier work on susceptibility to mycobacterial infection has focused on the human leucocyte antigen (HLA) system. Class I and class II HLA, which are the most polymorphic human genes, and their products are crucial for antigen presentation by macrophages to CD8+ and CD4+ lymphocytes. Both in tuberculosis and leprosy associations have been reported with class I HLA A10 and B8, and with class II antigen DR2 (Bothamley et al. 1989; Todd et al. 1990; Brahmajothi et al. 1991). But HLA associations have not been consistent with all forms of tuberculosis; one study could only associate smear-positive tuberculosis with HLA DR2, subtype DR15 in linkage disequilibrium with DQ5 (Bothamley 1998). In a study among Cambodian tuberculosis patients the HLA-DQB1*0503 allele was significantly associated with susceptibility to TB, but the finding of an association between a rarely occurring allele and tuberculosis might be attributed to only one family with the allele having a high number of tuberculosis patients. The large number of immuno-modulatory genes such as that for TNF-α within the MHC might account for some of the associations between HLA antigens and infectious diseases (Bellamy & Hill 1998).
NRAMP-1
The natural resistance-associated macrophage protein-1 of APC/AM/M, the so-called NRAMP-1, is critical in handling antigen presentation. The gene coding for this protein is located on chromosome 2 (Vidal et al. 1993). Failure in antigen presentation has been observed in association with four different NRAMP-1 gene mutations appeared to be associated with development of clinical tuberculosis, in a case–control study in The Gambia, in West Africa. Individuals with the disease-associated genotypes were over four times over-represented among the tuberculosis cases compared with those with the most common NRAMP-1 genotype (Bellamy et al. 1998a). NRAMP-1 gene polymorphism has also been identified in the susceptibility to leprosy (Lagrange & Abel 1996; Abel et al. 1998).
Interleukins, especially, IL-12; adhesion molecules and cell surface molecules
Cell surface molecules like ICAM-1 are involved in the cell–cell cross talk, and CD40/CD40L interactions, and CD86 are involved in the induction of IL-12 production (Alderson et al. 1993). Loss or mutation of the IL-12 receptor molecule has been identified as a risk factor for susceptibility for mycobacterial infections in humans (Altare et al. 1998; de Jong et al. 1998) but IL-12-independent pathways for INF-γ production may be important (Frucht et al. 1999). One paper addressed the possible role of IL-1 (Bellamy et al. 1998b).
INF-c
APC/AM/M produce IL 12 and 18, which stimulate natural killer cells to produce INF-γ; and CD4+ cells of TH-1 phenotype, which produce IL-2 and INF-γ; TH-2 cells are inhibited by INF-γ to produce their specific repertoire of IL-4 and IL-5 production; and CD8 cytotoxic cells are also stimulated by increased production of INF-γ to increase INF-γ production. By the increased INF-γ signals, the APS/AM/M respond by activating inducible nitric oxide synthase (iNOS), an enzyme that drives the production of nitric oxide which is the effector mechanism of macrophage killing. Susceptibility to infection by NTM has been reported in association with a mutation in the INF-γ receptor gene (Levin et al. 1995; Jouanguy et al. 1999; Levin & Newport 1999), but associations with tuberculosis or BU have not been reported (Table 1).
Vitamin D3, VDR, and TNF-a
Vitamin D acts on macrophages by binding to a cytoplasmic receptor that translocates to the nucleus and acts as a transcription factor: activation of APC/AM/M is promoted by INF-γ-driven induction of a hydrolase which converts 25-OH2D3 into the active form of vitamin D: 1,25-OHD3 (Rook et al. 1986; Grange et al. 1995). Vitamin D3 up-regulates the macrophage further and is considered an important immunomodulatory factor. Tuberculosis is more common among Asian immigrants with vegetarian diets in London (Strachan et al. 1995), and the level of vitamin D3 appeared to correlate with disease severity of tuberculosis in Indonesian patients (Grange et al. 1985). In a case–control study in The Gambia, individuals with a genotype associated with high levels of vitamin D3 were under-represented among tuberculosis patients (Bellamy et al. 1999). In a case–control study in London, among Gujarati Asians, both nutritional deficiency for vitamin D and certain gene polymorphisms for VDR, especially genotype TT/Tt and genotype ff (with virtually undetectable blood levels for vitamin D3) were significantly associated with tuberculosis (Wilkinson et al. 2000). Lipoarabinomannan (LAM) is universally present as a cell wall component in all mycobacteria, and is an important trigger for early and later events within the APC. Induction and release of TNF-α is triggered by exposure to LAM. In a later stage of the protective immune response to mycobacterial invasion, granuloma formation plays an important role to protect the infected individual from spread of the disease. TNF-α stimulates human macrophages to restrict growth of M. tuberculosis (Hirsch et al. 1994) and T-cells and TNF-α are essential for granuloma formation (Bean et al. 1999). Granuloma formation develops in BU during the process of healing of necrotic ulcers (Lytton & Lavett 1974; Hayman & McQueen 1985; Hayman 1993), and disseminated BU and osteomyelitis (Hofer et al. 1993) might conceivably be associated with inherited defects in granuloma formation.
Mannose-binding protein gene, and other relevant gene polymorphisms
Bellamy (2000) proposed an integrated and balanced approach for further research into genetic susceptibility to tuberculosis, and showed that NRAMP-1, mannose-binding protein, and VDR gene polymorphisms could explain to a large degree the genetic susceptibility to tuberculosis in man. X-chromosome-linked susceptibility factors might explain the male preponderance for tuberculosis observed in many epidemiological studies.
Discussion
The potential host susceptibility factors as described above – both inherited (Table 1), and induced by the infectious environment – are all hypothetical for BU. None of these factors have been substantiated by any experimental data. But we feel that these possibilities should be explored as answers to the questions raised here will help to design future intervention research. If inherited factors determine the host response to a large extent, and not co-infections from the environment, future intervention research will need to focus on detection of highly susceptible individuals followed by physical protective measures. Protective clothing such as long pants, and wearing of boots during high-risk activities such as farming, or exposure to highly contaminated environments, have been shown to be beneficial in at least one study (Marston et al. 1995). If, on the other hand, much of the host susceptibility is determined by immune shifts that are induced by helminthic co-infections (Bundy et al. 2000), interventional research needs to be set up with antihelminthic drugs – e.g. praziquantel, oxamniquine and perhaps artemether (Doenhoff et al. 2000) – may be the best option, as campaigns to reduce worm infestations by elimination of intermediate snail hosts have not met with much success in the past.
Conclusion
Increased susceptibility to BU might be genetically driven, or be dictated by environmental factors, e.g. co-infection with schistosomiasis. It is very well possible that both inborn and environmental factors determine the overall chance to develop BU. Future research should elucidate these possibilities as they will dictate the direction of future research for treatment and prevention.





