Plant chitinase/lysozyme isoforms show distinct substrate specificity and cleavage site preference towards lipochitooligosaccharide Nod signals
Summary
The ability of 16 chitinases from seven different plant species to hydrolyze a collection of several structurally related lipochitooligosaccharides (LCOs) of Rhizobium was analyzed. It was found that the enzymes differed to a large extent in their activity on different LCOs. Differences were attributed to (i) the relative activity on different LCOs as substrate (e.g. sulfated versus non-sulfated LCOs); (ii) the relative cleavage site preference on a given LCO molecule (hydrolysis of either the second, third or fourth glycosidic bond from the non-reducing end of the molecule); and (iii) the stereochemistry of the reaction (retention or inversion of the anomeric configuration). A graphic representation of the different substrate specificities resulted in a ‘fingerprint’ that is characteristic for a given enzyme or a family of related enzymes. By comparing the LCO-fingerprint of unknown enzymes with those obtained for already characterized proteins, it is possible to identify new glycosyl hydrolases. The high diversity of substrate specificity found among plant chitinases may reflect variations in the natural substrates of the enzymes, such as substitutions on the chitin moiety of fungal cell walls or, in plants, the presence of putative endogenous substrates related to LCOs.
Introduction
During the development of the nitrogen-fixing symbiosis between legumes and rhizobia, lipochitooligosaccharides (LCOs, Nod factors) produced by the bacterial partner play a crucial role as mitogens that trigger cell division and root nodule formation. LCOs consist of a backbone of generally four to five N-acetyl glucosamine residues, with the N-acetyl group at the non-reducing terminal sugar residue replaced by an acyl chain of variable structure. Additional substitutions are found at both the reducing and non-reducing terminus. In different Rhizobium species, LCOs are modified differently, a feature that largely contributes to the host specificity of their activity ( Dénariéet al. 1996 ;Schultze & Kondorosi 1998). The actual concentration of these signal molecules at the root surface is influenced by hydrolytic enzymes present in the rhizosphere ( Heidstra et al. 1994 ;Staehelin et al. 1994a, 1995 ). In light of the structural similarity of LCOs and chitin, LCOs have been tested as substrates for certain chitinases and it was found that the structural modifications of the molecules influence their stability ( Staehelin et al. 1994a , b). Plants produce a large number of different chitinases, often in response to pathogen attack, elicitor treatment or plant hormones ( Boller 1987;Collinge et al. 1993 ;Stintzi et al. 1993 ). Chitinases have been shown to possess antifungal activity in vitro ( Schlumbaum et al. 1986 ) and to enhance resistance against certain fungal pathogens when overexpressed in transgenic plants ( Broglie et al. 1991 ;Grison et al. 1996 ;Lin et al. 1995 ;Vierheilig et al. 1993 ). However, other studies showed that strong enhancement of the expression of a single chitinase gene was not sufficient to increase resistance against fungal pathogens ( Neuhaus et al. 1991 ), nor did reduced expression lead to significant alteration in disease susceptibility ( Samac & Shah 1994). The synergistic effects of chitinases and β-glucanases on fungal growth in vitro ( Mauch et al. 1988 ) and in vivo ( Jach et al. 1995 ;Zhu et al. 1994 ) suggest that, to be effective, chitinases have to act in concert with other elements involved in plant defence.
In addition to their apparent role in plant defence, chitinases have been hypothesized to play a role in plant development, such as flower development, leaf senescence or embryogenesis ( De Jong et al. 1992 ;Hanfrey et al. 1996 ;Leung 1992;Lotan et al. 1989 ;Neale et al. 1990 ). They might produce or interact with hitherto unidentified developmental signals. Since a temperature-sensitive embryogenic mutant of a carrot cell line was rescued both by certain chitinases ( De Jong et al. 1992 ;Kragh et al. 1996 ) and by Rhizobium-derived LCOs ( De Jong et al. 1993 ), it was suggested that plants produce endogenous oligosaccharide signals related to LCOs.
Plant chitinases have been classified according to their primary structure ( Hamel et al. 1997 ;Meins et al. 1994 ;Neuhaus et al. 1996 ), whereby classes I, II and IV belong to family 19 and classes III and VI to family 18 of the glycosyl hydrolases ( Henrissat 1991;Henrissat & Bairoch 1993). Plant chitinases often display lysozyme activity ( Beintema & Terwisscha van Scheltinga 1996;Boller et al. 1983 ;Brunner et al. 1998 ). Although unrelated with respect to the amino acid sequence, similarity in secondary structure between plant chitinases and lysozymes from animals and phage has been reported ( Holm & Sander 1994;Monzingo et al. 1996 ). Some enzymes, identified as chitinases by sequence analysis, show higher activity on bacterial peptidoglycan than on chitin as substrate and were accordingly designated as lysozymes ( Brunner et al. 1998 ).
The work presented in this paper was initiated in order to estimate which classes of chitinases would be able to degrade the rhizobial LCO nodulation signals. In the course of these studies, it turned out that the various enzymes showed striking differences in their activity and their sensitivity towards different modifications of the LCO structure. By using a collection of different LCO molecules as substrates, it was possible to attribute to each enzyme a characteristic fingerprint of cleavage specificity. The significance of these findings with respect to potential substrate structures in planta and ex planta is discussed.
Results
Distinct substrate specificity of different classes of plant chitinases
It has been shown previously that structural modifications of lipochitooligosaccharides, such as the length of the chitooligosaccharide chain or the presence of a sulfate group, influence their hydrolysis by chitinases isolated from the roots of Medicago sativa and Vicia sativa ( Staehelin et al. 1994a ). These enzymes were most likely class I chitinases, as indicated by their molecular weight of 30 KDa, their chitin-binding ability and the induction by ethylene. The enzymes from the two different plant species displayed a similar specificity of LCO degradation ( Staehelin et al. 1994a ). At the same time, it remained open whether the cleavage specificity on different LCOs is an intrinsic property of the LCO structure or whether it is variable with different types of chitinases. To address this question, we compared 16 different plant chitinases, belonging to classes I to VI, from seven different plant species for their ability to degrade various LCO molecules. LCOs used were the pentameric sulfated NodRm-V(C16:2,S), the tetrameric sulfated NodRm-IV(C16:2,S), the tetrameric non-sulfated NodRm-IV(C16:2) and the trimeric NodRm-III(C16:2), all derived from Sinorhizobium meliloti and carrying a C16:2▵2,9 acyl chain ( Lerouge et al. 1990 ;Schultze et al. 1992 ;Staehelin et al. 1994a ).
In a first series of experiments we tested whether class III chitinases would differ in their specificity from class I chitinases. Enzyme preparations from tobacco were used, since from this species five different classes of chitinases were available in purified form ( Brunner et al. 1998 ;Heitz et al. 1994 ;Legrand et al. 1987 ). Figure 1 shows representative chromatograms obtained from the analysis of tobacco class I chitinase, Chi32, and two class III chitinases, the acidic enzyme lys28a and the basic enzyme lysb1 ( Brunner et al. 1998 ). The primary structure of chitinases belonging to class III is unrelated to class I chitinases. The basic class I chitinase Chi32 from Nicotiana tabacum hydrolyzed NodRm-V(S) at a single site, the third glycosidic bond from the reducing end ( Fig. 1a), as was previously found for the chitinases purified from Medicago and Vicia ( Staehelin et al. 1994a ). The same cleavage site was observed when NodRm-V(S) was incubated with the acidic class III chitinase lys28a ( Fig. 1b). However, on prolonged incubation lys28a was able to degrade the lipotrisaccharide product NodRm-III further to the lipodisaccharide NodRm-II ( Fig. 1c), contrary to the class I chitinases which were unable to hydrolyze this molecule ( Staehelin et al. 1994a ; data not shown). Similarly to lys28a, the basic chitinase/lysozyme lysb1 degraded NodRm-V(S) producing the lipodisaccharide and the lipotrisaccharide ( Fig. 1d). However, in that case it appears that the NodRm-II product mainly arose from the hydrolysis of NodRm-V(S) and only in part from the further degradation of the NodRm-III product since the latter is a 10-fold less efficient substrate for lysb1 than the former (see below).
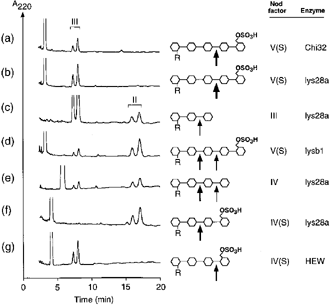
Analysis of LCO degradation by different chitinases/lysozymes.
Representative chromatograms obtained after incubation of LCOs (25 μm) with tobacco chitinases (a–f) and hen egg white lysozyme (g). Incubations were as follows: (a) NodRm-V(S), 1 μg ml–1 Chi32, 1 h; (b) NodRm-V(S), 1 μg ml–1 lys28a, 10 min; (c) NodRm-III, 5 μg ml–1 lys28a, 16 h; (d) NodRm-V(S), 2 μg ml–1 lysb1, 8 h; (e) NodRm-IV, 1 μg ml–1 lys28a, 2 h; (f) NodRm-IV(S), 1 μg ml–1 lys28a, 3.5 h; (g) NodRm-IV(S), 5 μg ml–1 hen egg white lysozyme, 4 h. Different size of arrows indicates relative cleavage efficiency.
The non-sulfated tetrameric LCO NodRm-IV was hydrolyzed by lys28a to give both the dimeric and trimeric products, the quantity of the dimer being twice that of the trimer ( Fig. 1e). The presence of a sulfate group at the O-6 position of the reducing end residue of the LCO NodRm-IV(S) entirely protected the neighbouring glycosidic bond from cleavage by lys28a ( Fig. 1f). Since the sulfate group also protects this bond against degradation by class I chitinases ( Staehelin et al. 1994a ; data not shown), it was possible that sulfation renders this site generally inaccessible for hydrolytic enzymes. However, incubation of NodRm-IV(S) with hen egg white lysozyme demonstrated that the terminal glycosidic bond of this molecule can be hydrolyzed ( Fig. 1g).
Protection of LCOs by 6-O-acetylation at the non-reducing residue
One of the most common substitutions of Nod factors is the 6-O-acetyl group at the non-reducing end ( Dénariéet al. 1996 ). To analyze whether 6-O-acetylation influences the accessibility of cleavage sites, different O-acetylated and non-O-acetylated LCOs derived from S. meliloti were incubated with chitinases. As shown in Fig. 2, the non-sulfated tetrasaccharide NodRm-IV was cleaved by the tobacco class I chitinase Chi32 at the third glycosidic bond to produce a lipotrisaccharide ( Fig. 2a). However, when O-acetylated the LCO NodRm-IV(Ac) was resistant against hydrolysis ( Fig. 2b). Thus, the cleavage at the third glycosidic bond in the tetrasaccharidic LCOs is prevented either by sulfation at the reducing end ( Staehelin et al. 1994a ) or by O-acetylation at the non-reducing end.
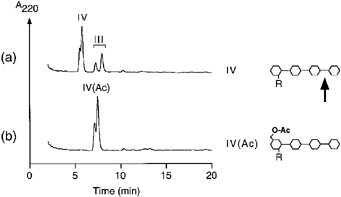
Protection of non-sulfated LCOs by 6-O-acetylation at the non-reducing end against degradation by class I chitinase.
Tobacco chitinase Chi32 (1 μg ml–1) was incubated for 2 h with (a) NodRm-IV and (b) NodRm-IV(Ac).
O-acetylation also affected the hydrolysis of LCOs by other chitinases. Figure 3 shows examples of the degradation of sulfated LCOs by the tobacco class III chitinase lysb1. Whereas the tetrasaccharide NodRm-IV(S) was hydrolyzed to produce the lipodisaccharide NodRm-II ( Fig. 3a), the O-acetylated molecule NodRm-IV(Ac,S) was not a substrate for lysb1 ( Fig. 3c). The pentasaccharide NodRm-V(S) was cleaved by lysb1 at the second and third glycosidic bond ( Fig. 3b). However, upon O-acetylation of the molecule, NodRm-V(Ac,S) was cleaved only at the third glycosidic bond to produce the lipotrisaccharide NodRm-III(Ac) ( Fig. 3d).
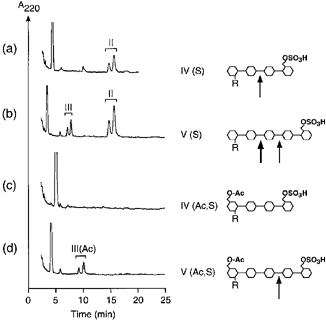
Protection of sulfated LCOs by 6-O-acetylation at the non-reducing end against degradation by class III chitinase.
Tobacco chitinase lysb1 (1 μg ml–1) was incubated for 4.5 h with (a) NodRm-IV(S); (b) NodRm-V(S); (c) NodRm-IV(Ac,S); and (d) NodRm-V(Ac,S). Different size of arrows indicates relative cleavage efficiency.
Characterization of chitinases by LCO degradation
Quantitative analysis of the substrate specificity of the tested chitinases revealed large differences among the enzymes ( Table 1). The specific activity on a given LCO substrate varied by more than four orders of magnitude. For instance, a value of 7900 pkat mg–1 on NodRm-V(S) was measured with lys28a, compared to only 0.41 pkat mg–1 with PR-P. In contrast, on colloidal chitin as substrate, PR-P showed 10-fold higher activity than lys28a. For some enzymes, the presence of the sulfate group on the tetrameric LCO did not significantly affect the activity (lys28a) or even enhanced the specific activity (lysb1, lysb2). In the case of hen egg white lysozyme, which was tested for comparison, the sulfated LCO NodRm-IV(S) was degraded sevenfold faster than the non-sulfated molecule NodRm-IV.
| Nod factor degrading activity apkat mg–1 | chitinase activitynkat mg–1 | |||||
|---|---|---|---|---|---|---|
| Enzyme | Class | NodRm-V(S) | NodRm-IV(S) | NodRm-IV | NodRm-III | 3H-chitin |
| Chi32, tobacco | I | 680 ± 19 | 0 | 2540 ± 15 | 0 | 2050 ± 150 |
| Chi30, alfalfa | I | 5.6 ± 0.8 | 0 | 550 ± 130 | 0 | 250 ± 32 |
| Chi30, vetch | I | 160 ± 30 | 0 | 7400 ± 1400 | 0 | 950 ± 140 |
| Chi30, bean | I | 22 ± 2.0 | 0 | 2400 ± 500 | 0 | nd |
| CH4, sugar beet | IV | 540 ± 190 | 0.94 ± 0.26 | 3800 ± 650 | 0 | 170 ± 20 |
| CH3, sugar beet | IV | 49 ± 17 | 0.19 ± 0.04 | 1900 ± 90 | nd | 80 ± 8 |
| SP2, sugar beet | IV | 2600 ± 420 | 0.23 ± 0.13 | 160 ± 4.0 | nd | 150 ± 28 |
| Chi32, carrot | IV | 3.5 ± 0.2 | 0 | 1.2 ± 0.13 | nd | nd |
| PR-P, tobacco | II | 0.41 ± 0.01 | 0 | 0.24 ± 0.05 | 0 | 110 ± 7 |
| lys28a, tobacco | III | 7900 ± 700 | 455 ± 3 | 620 ± 20 | 16 ± 0.7 | 10 ± 4 |
| AC, chick pea | III | 16 ± 1.2 | 0.79 ± 0.21 | 2.2 ± 0.3 | nd | 210 ± 7 |
| SE2, sugar beet | III | 170 ± 22 | 30 ± 1.3 | 110 ± 3 | 1.0 ± 0.3 | 30 ± 2 |
| lysb1, tobacco | III | 84 ± 0.7 | 79 ± 0.4 | 33 ± 4 | 8.3 ± 2.1 | nd |
| lysb2, tobacco | III | 37 ± 1.1 | 76 ± 4.2 | 31 ± 0.3 | 9.7 ± 2.0 | nd |
| CBP20, tobacco | V | 16 ± 1.9 | 0 | 16 ± 2.4 | 0 | 100 ± 13 |
| Pz, tobacco | VI | 2000 ± 110 | 0 | 15 ± 2.7 | 0 | 0.8 ± 0.2 |
| ChiA, S. marcescens | 11000 ± 800 | 42 ± 5 | 140 ± 16 | 0 | nd | |
| HEW-lysozyme | 2000 ± 20 | 76 ± 9 | 11 ± 0.5 | 0 | nd | |
- aMean values of 3–6 measurements ± SE. nd: not done.
Interestingly, common features were observed among related enzymes isolated from different plant species. None of the class I chitinases tested was able to hydrolyze NodRm-IV(S). Similarly, the structurally related class II chitinase of tobacco (PR-P) did not degrade this molecule. The class IV chitinases from sugar beet and carrot, also related to class I, did not show substantial activity with NodRm-IV(S). None of the tested enzymes belonging to classes I, II or IV was able to degrade the trisaccharide LCO NodRm-III. In contrast, all of the class III chitinases were able to degrade NodRm-IV(S) and, to a lesser extent, NodRm-III.
The enzymes listed in Table 1 were prepared by different laboratories and were stored for different times. Therefore, the absolute values for the specific activity have to be interpreted with caution. Loss of activity in some preparations may explain weak activities on both LCOs and chitin. For example, Chi30 from alfalfa showed a 10-fold lower specific activity on chitin and a fivefold lower activity on NodRm-IV compared to Chi32 of tobacco. On the other hand, the class VI chitinase Pz of tobacco displayed strong degradation of NodRm-V(S) despite relatively little activity on chitin. Therefore, degradation of chitin did not always correlate with degradation of LCOs, and chitinase activity could not be used as a common denominator of enzyme activity. Determination of the Michaelis–Menten constants was not feasible either, due to the limited amounts of both enzyme samples and LCOs.
pH optima were determined for most enzymes, by using those LCOs that were the best substrate for a given enzyme. The pH optimum varied between pH 4 and pH 5. In this range, the maximum activity did not vary by more than 50%. Since the tested LCOs varied substantially in their hydrophobicity (ionic sulfated molecules versus non-ionic non-sulfated LCOs), it was possible that pH differentially influences their degradation rate. To test this possibility, we determined the degradation of NodRm-IV and NodRm-IV(S) by lys28a between pH 3 and 7. An identical activity profile was recorded for the two LCOs (data not shown) demonstrating that the pH optimum is independent of the LCO structure. Hence, the pH of the reaction buffer cannot account for the observed variations in enzyme activity. Similarly, the ion strength of the incubation buffer had relatively little influence on the enzyme activities. The relative order of enzyme activities was not affected when the ion strength of the incubation buffer was varied between 10 and 100 m m (data not shown). By contrast, enzyme stability may have affected the data shown in Table 1, since weak activities were determined upon prolonged incubation times. Therefore, low activities presented in Table 1 are possibly underestimated.
Characterization of chitinases by LCO-′fingerprints′
As shown in Table 1, different enzymes can be characterized by their relative specific activity with the LCOs NodRm-V(S), NodRm-IV(S), NodRm-IV and NodRm-III as substrates. A more detailed picture was obtained when the cleavage site was taken into account. In Fig. 4, the relative hydrolysis of each glycosidic bond is plotted as a percentage of that observed for the least stable bond. This type of analysis eliminated possible variations in the absolute activities and facilitated the comparison of the different enzymes. The graphic representation of the data resulted in what can be considered as a fingerprint characteristic for a given enzyme. For example, the basic class III chitinases/lysozymes lysb1 and lysb2 of tobacco preferentially cleaved the second glycosidic bond in NodRm-V(S), whereas the acidic class III chitinases of tobacco, chick pea and sugar beet (lys28a, AC, SE2) preferentially acted on the third glycosidic bond. All the class I chitinases and the two basic class IV chitinases of sugar beet (CH4 and CH3) preferentially degraded NodRm-IV over NodRm-V(S). In contrast, the acidic class IV enzymes, SP2 of sugar beet and Chi32 of carrot, and the class VI chitinase Pz of tobacco degraded preferentially NodRm-V(S) over NodRm-IV.
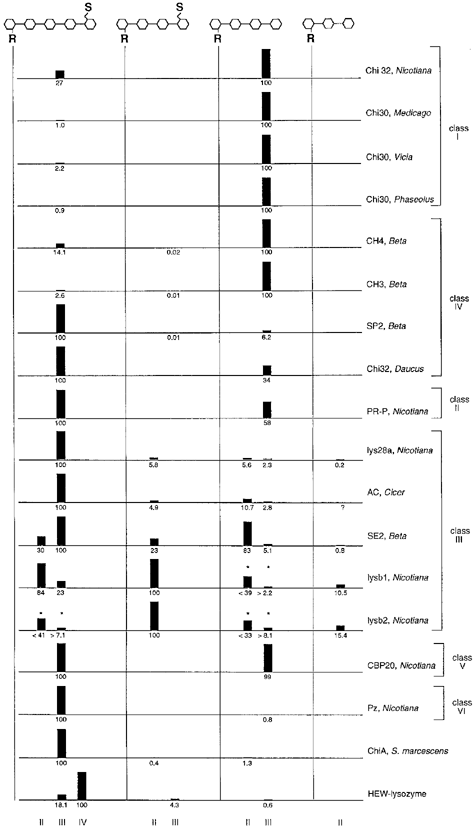
LCO-fingerprinting. LCOs NodRm-V(S), NodRm-IV(S), NodRm-IV and NodRm-III were incubated with different enzymes and the relative rate of hydrolysis for each glycosidic bond was determined.
The rate of hydrolysis of the least stable bond was defined as 100%. The cleavage products, lipo-dimers (II), -trimers (III) and -tetramers (IV) are depicted below. For lysb1 and lysb2, the degradation of NodRm-III was rapid enough to contribute partly to the production of dimers from NodRm-V(S) and NodRm-IV. In these cases (marked by asterisks), the observed values for dimer production were overestimated, while those for the trimer formation were underestimated. Question marks indicate possible cleavage that was not tested.
Pz chitinase (class VI) shows weak similarity to class III chitinases and to bacterial exochitinases including ChiA of Serratia marcescens ( Heitz et al. 1994 ;Melchers et al. 1994 ). For comparison, we tested LCO degradation by ChiA. ChiA appeared to show similar specificity on LCOs than did Pz ( Table 1). NodRm-V(S) was hydrolyzed two orders of magnitude more rapidly than NodRm-IV and relatively little activity was observed with NodRm-IV(S) as substrate. However, analysis of the cleavage sites revealed that ChiA hydrolyzed the second glycosidic bond of the tetrameric LCOs whereas Pz hydrolyzed the third one of NodRm-IV ( Fig. 4). Therefore, Pz and ChiA are clearly distinguishable with LCOs as substrate. Interestingly, none of the tested plant chitinases was able to degrade the sulfated and O-acetylated NodRm-IV(Ac,S), whereas this molecule was a substrate for hen egg white lysozyme and ChiA of S. marcescens (data not shown).
The four different LCOs shown in Fig. 4 contain a total of eight accessible cleavage sites, three in NodRm-V(S), two each in NodRm-IV(S) and NodRm-IV, and one in NodRm-III. The hydrolysis at each site could be determined independently of each other. Additional LCOs (the non-sulfated pentamer NodRm-V and O-acetylated molecules) were tested with some of the chitinases. This increased the total number of cleavage sites tested and allowed us to obtain more complex ‘fingerprints’ (data not shown).
Determination of the reaction mechanism
The products of LCO degradation were separated into their anomeric forms ( 1-3). To determine which anomer is produced first, reactions were performed at high enzyme concentrations with the LCO that was most sensitive to the respective chitinase. This allowed for the incubation times to be kept short compared to the rate of mutarotation. Moreover, the products were not extracted with butanol but were immediately diluted in eluent and separated by HPLC. Depending on the stereochemistry of the reaction, a preferential production of either the β- or the α-anomer was observed. Whereas the reaction mechanism for chitinases class I from bean and class II from barley (inverting) and class III from cucumber (retaining) has been reported ( Hollis et al. 1997 ;Iseli et al. 1996 ), it remained to be shown for classes IV, V and VI. Figure 5 shows that the sugar beet chitinases CH4 and SP2 (class IV), and tobacco CBP20 (class V) inverted the anomeric configuration ( Fig. 5a–c), whereas it was retained with the class VI chitinase Pz of tobacco ( Fig. 5d). Inversion was also demonstrated for Chi30 from alfalfa and PR-P from tobacco ( Fig. 5e,f). This confirms the reaction mechanism expected for enzymes belonging to families 18 (class VI) and 19 (class I, II and IV) of the glycosyl hydrolases ( Henrissat & Bairoch 1993).
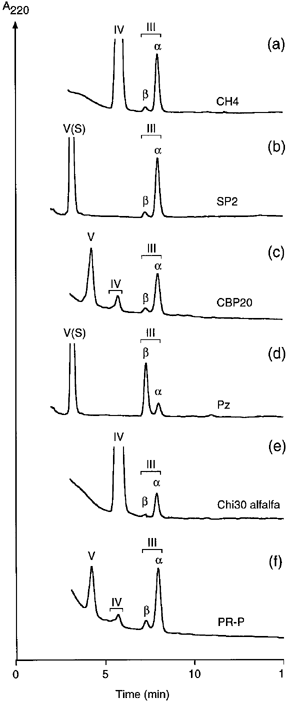
Stereochemistry of LCO hydrolysis.
Forty μm of NodRm-IV (a,e) or NodRm-V(S) (b,d) or 20 μm of NodRm-V (c,f) were incubated with (a) 0.2 μg sugar beet class IV chitinases CH4 for 3 min; (b) sugar beet class IV chitinase SP2 for 5 min; (c) 13 μg class V chitinase CBP20 for 6 min; (d) 0.7 μg tobacco class VI chitinase Pz for 7 min; (e) 0.5 μg alfalfa class I chitinase Chi30 for 4 min; and (f) 13 μg tobacco class II chitinase PR-P for 8 min. Reaction mixtures were diluted in eluent and separated on reverse phase HPLC. Preferential production of the β- or α-anomer indicates retention or inversion of the anomeric configuration, respectively. Hydrolysis of NodRm-V gave rise to minor amounts of tetrameric product NodRm-IV.
Discussion
By measuring the relative rate of degradation of different lipochitooligosaccharide molecules, and by analyzing the cleavage site preference and the reaction mechanism, it was possible to characterize different classes of plant chitinases. Related fingerprints were obtained for enzymes that had been classified previously according to their primary structure ( Meins et al. 1994 ;Neuhaus et al. 1996 ). Classes I, II and IV hydrolyzed NodRm-V(S) and NodRm-IV whereby the basic enzymes (class I, CH4 and CH3) preferred NodRm-IV as substrate over NodRm-V(S) and the acidic enzymes (SP2, Chi32 of carrot and PR-P) preferred NodRm-V(S) over NodRm-IV. In contrast to classes I and IV, the class II chitinase PR-P showed very low activity on LCOs despite its strong activity on colloidal chitin which was comparable to that of the class IV chitinases of sugar beet. The only available class VI enzyme, Pz of tobacco, differed from the group of class I, II and IV by their low activity on NodRm-IV compared to NodRm-V(S) as substrate ( Table 1). Moreover, the class VI enzyme showed very little activity on chitin as substrate. Consistent with this result, this enzyme was previously reported to have low chitinase activity in comparison to class I and II proteins but showed substantial activity on the oligosaccharidic substrate 4-methylumbelliferyl-chitotriose ( Heitz et al. 1994 ;Melchers et al. 1994 ). On the basis of cleavage specificity and reaction mechanism, the Pz enzyme was clearly distinguishable as belonging to a different group than classes I, II or III.
Differences in substrate specificity of various tobacco chitinases have previously been shown by testing chitin, chitin oligosaccharides and bacterial cell walls as substrate ( Brunner et al. 1998 ;Heitz et al. 1994 ;Melchers et al. 1994 ). Our data extend these results to a more general picture, i.e. enzymes from different plant species that are homologous according to their primary structure show similar fingerprints on LCOs as substrate. We are aware, however, that generalization of this rule would need a broader survey with a larger number of enzymes to be studied. Overall, our data suggest that the previous classification of plant chitinases ( Meins et al. 1994 ;Neuhaus et al. 1996 ) does not only reflect evolutionary relationships but that it is also functionally relevant. It is also clear that attribution of different chitinolytic enzymes to families 18 or 19 of glycosyl hydrolases does not reflect a strict similarity in their activity on LCOs. This is best illustrated by the different LCO-fingerprints observed for chitinases class III, class VI and ChiA of S. marcescens, all belonging to family 18 ( Henrissat 1991;Neuhaus et al. 1996 ).
We showed that LCOs are excellent substrates for the determination of the reaction mechanism of glycosyl hydrolases and we used this tool to determine the mechanism for those classes of plant chitinases that had not been analyzed previously. The inverting mechanism of class IV enzymes is consistent with their relationship to class I and II chitinases which were previously reported to invert the anomeric configuration ( Hollis et al. 1997 ;Iseli et al. 1996 ). Similarly, the retaining mechanism found for class VI is consistent with this enzyme belonging to family 18 of the glycosyl hydrolases. CBP20 chitinase (class V) showing the inverting mechanism has not been attributed to a particular family of glycosyl hydrolases yet.
As demonstrated in Fig. 4, three to four different LCOs were sufficient to discriminate between different plant chitinases and even isozymes within a given class (see class III chitinases). By comparing the activity of unknown samples with the fingerprints obtained for known enzymes, it is possible to make a prediction as to the type of enzyme contained in the sample. For more detailed analyses, additional LCOs can be included. For example, Chi32 from carrot, PR-P and CBP20, chitinases that were a little active on the LCOs shown in Fig. 4, hydrolyzed efficiently the non-sulfated pentameric NodRm-V (data not shown). Moreover, we demonstrated a strong influence of 6-O-acetylation at the non-reducing end on LCO cleavage ( 2, 3). Therefore, the use of 6-O-acetylated LCOs would increase the number of LCOs derived from S. meliloti to 10. The number of potential cleavage sites would be 22, not counting the first glycosidic bond from the non-reducing end (the hydrolysis of which has not been demonstrated yet). The 10 LCOs would allow to distinguish among a virtually unlimited number of theoretically possible combinations of cleavage specificity. On the basis of the high variation in substrate specificity, several new LCO-cleaving enzymes have been identified in plants ( Minic et al. 1998 ;Staehelin et al. 1995 ;Staehelin et al. 1996 ). LCO-fingerprinting might constitute an excellent tool for testing the effect of site-specific mutations introduced into various glycosyl hydrolases. Likewise, protein engineering aiming at the improvement of activities or the alteration of substrate specificity might profit from using the described approach.
The differences in substrate and cleavage specificity observed for the various plant chitinases may be interpreted with respect to their biological role. It seems plausible that localisation of the enzymes in different tissues, intra- or extracellularly, may necessitate the existence of many different types of chitinases in a single plant. Another explanation for the enzyme diversity would be variation in the natural substrates. The chitin component in the cell walls of different pathogenic fungi may be structurally diverse, with interconnections to other cell wall components and perhaps yet unidentified modifications. A single chitinase might not be able to degrade efficiently many different types of chitin derivatives and might require a synergistic action with other chitinases ( Brunner et al. 1998 ).
Alternatively, endogenous substrates for certain chitinases may exist in plants. GlcNAc disaccharides are ubiquitous in N-linked glycans ( Hart 1992). GlcNAc has been reported as an abundant component of secondary plant cell walls ( Benhamou & Asselin 1989). Moreover, lipophilic compounds that are sensitive to degradation by chitinases have been detected upon 14C in vivo labeling of plant tissues ( Spaink et al. 1993 ). Developmental abnormalities in transgenic plants producing the chitooligosaccharide-modifying enzymes NodA and NodB of S. meliloti have been taken as evidence for the existence of endogenous substrates in plants ( Schmidt et al. 1993 ; E. Kondorosi, unpublished results). These reports support the suggestion that chitinases may function in plant development by interacting with hitherto unknown endogenous signal molecules ( De Jong et al. 1993 ). Chi32 of carrot, the chitinase originally identified as a factor able to rescue a mutated carrot cell line defective in embryogenesis ( De Jong et al. 1992 ), showed a similar LCO-fingerprint as the acidic sugar beet chitinase SP2 ( Fig. 4). Whereas it has been reported that several different class IV and class I chitinases of carrot were active in rescuing the mutant, a class II enzyme from carrot and the basic class IV chitinase CH4 of sugar beet were shown to be inactive ( Kragh et al. 1996 ). Whether the chitinase SP2 was active in this assay was not reported. Direct evidence for the existence of LCO analogs in plants is missing. The method of LCO-fingerprinting might help to identify new glycosyl hydrolases which, in turn, could lead to the discovery of their endogenous substrates.
Experimental procedures
Enzymes and LCOs
The 16 plant chitinases used in this study are listed in Table 2. Representatives of classes I to VI have been tested. For comparison, two non-plant enzymes, the recombinant chitinase ChiA of S. marcescens and hen egg white lysozyme (cryst., 206 000 U mg–1, Serva), have been analyzed. Lipochitooligosaccharides were isolated from Sinorhizobium meliloti (formerly named Rhizobium meliloti) and derivated as described previously ( Schultze et al. 1992 ;Staehelin et al. 1994a, 1995 ).
| Enzyme | Organism | Class | Family of glycosyl hydrolase | Reference |
|---|---|---|---|---|
| Chi32 | Nicotiana Table 1 | 19 | ( Legrand et al. 1987 ) | |
| Chi30 | Medicago sativa | I | 19 | ( Staehelin et al. 1994a ) |
| Chi30 | Vicia sativa | I | 19 | ( Staehelin et al. 1994a ) |
| Chi30 | Phaseolus vulgaris | I | 19 | ( Staehelin et al. 1994b ) |
| CH4 | Beta vulgaris | IV | 19 | ( Mikkelsen et al. 1992 ) |
| CH3 | Beta vulgaris | IV | 19 | ( Mikkelsen et al. 1992 ) |
| SP2 | Beta vulgaris | IV | 19 | ( Nielsen et al. 1994 ) |
| Chi32 | Daucus carota | IV | 19 | ( De Jong et al. 1992 ) |
| PR-P | Nicotiana Table 2 | 19 | ( Legrand et al. 1987 ) | |
| lys28a | Nicotiana Table 2III | 18 | ( Brunner et al. 1998 ) | |
| AC | Cicer arietinum | III | 18 | ( Vogelsang & Barz 1993) |
| SE2 | Beta vulgaris | III | 18 | ( Nielsen et al. 1993 ) |
| lysb1 | Nicotiana Table 2III | 18 | ( Brunner et al. 1998 ) | |
| lysb2 | Nicotiana Table 2III | 18 | ( Brunner et al. 1998 ) | |
| CBP20 | Nicotiana Table 5 | unknown | ( Ponstein et al. 1994 ) | |
| Pz | Nicotiana Table 6 | 18 | ( Heitz et al. 1994 ) | |
| ChiA | Serratia marcescens | 18 | ( Jones et al. 1986 ) | |
| HEW-lysozyme | Chicken | 22 | Serva |
LCO degradation
Enzyme activity was tested by incubating LCOs at a concentration of 25 μm with different chitinases at 1 μg ml–1 in 20 m m sodium-acetate buffer, pH 5. Incubation times varied between 5 min to 24 h depending on the activity. For quantification, reaction times were adjusted to a maximum degradation of 25%. Reactions were performed in Eppendorf test tubes at volumes of either 50 or 100 μl. Very weak activities were quantified after increasing the enzyme concentrations up to 100 μg ml–1. Upon incubation with chitinases, non-degraded molecules and the products from the non-reducing end carrying the acyl chain were separated from the hydrophilic reducing end products by extraction with n-butanol, and analyzed by reverse phase HPLC as described by Staehelin et al. (1994a) . The specific analysis of only the lipophilic non-reducing end products permitted the unambiguous localisation of the cleavage sites. For the determination of the pH optimum the following buffer systems were used: pH 3–4, sodium-citrate; pH 4–5.5, sodium-acetate; pH 5.5–6.5, sodium-2-[N-morpholino]ethanesulfonate; pH 6.5–7, sodium-3-[N-morpholino]propanesulfonate. The ionic strength was adjusted with NaCl.
Chitinase activity
Hydrolysis of 3H-labelled colloidal chitin (kindly provided by Prof. Thomas Boller, University of Basel, Switzerland) was measured as described by Boller et al. (1983) .
Stereochemistry of LCO-degradation
For determination of the reaction mechanism, incubations were performed in a volume of 25 μl for a maximum of 8 min followed by the immediate dilution in mobile phase and fractionation by HPLC. The elution characteristics of α- and β-anomers of chitooligosaccharides and their relative proportion after mutarotation has been reported previously ( Armand et al. 1994 ).
Acknowledgements
We are grateful to Drs K.K. Nielsen (Danisco Biotechnology, Copenhagen), S.C. de Vries (University of Wageningen), R. Vogelsang and W. Barz (University of Münster), and J. Schmidt and M. John (Max-Planck-Institut für Züchtungsforschung, Cologne) for providing samples of purified enzymes. This work was supported in part by the Swiss National Science Foundation grant 83EU-044164 to C.S.