Constituents of the tapetosomes and elaioplasts inBrassica campestristapetum and their degradation and retention during microsporogenesis
Summary
In Brassica anthers during microsporogenesis, the tapetum cells contain two abundant lipid-rich organelles, the tapetosomes possessing oleosins and triacylglycerols (TAGs), and the elaioplasts having unique polypeptides and neutral esters. B. campestris , for its simplicity of possessing only the AA genome and one predominant oleosin of 45 kDa, was studied. In the developing anthers, the lipids and proteins of the tapetosomes and elaioplasts were concomitantly accumulated but selectively degraded or retained. Upon incubation of isolated tapetosomes in a pH-5 medium, the predominant 45 kDa oleosin underwent selective enzymatic proteolysis to a 37 kDa fragment, which was not further hydrolyzed upon prolonged incubation. The unreacted 45 kDa oleosin was retained in the organelles, whereas the 37 kDa fragment was released to the exterior. The fragment would become the predominant 37 kDa polypeptide in the pollen coat. Isolated tapetosomes did not undergo hydrolysis of the TAGs upon incubation in media of diverse pHs. An alkaline lipase in the soluble fraction of the anther extract was presumed to be the enzyme that would hydrolyze the tapetosome TAGs, which disappeared in the anthers during development. The tapetum elaioplasts contained several unique polypeptides of 31–36 kDa. The gene encoding a 32 kDa polypeptide was cloned, and its deduced amino acid sequence was homologous to those of two proteins known to be present on the surface of fibrils in chromoplasts. Upon incubation of isolated elaioplasts in media of diverse pHs, the organelle polypeptides were degraded completely and most rapidly at pH 5, whereas the neutral esters remained unchanged; these neutral esters would become the major lipid components of the pollen coat. The findings show that the constituents of the two major tapetum organelles underwent very different paths of degradation, or modification, and transfer to the pollen surface.
Introduction
During microsporogenesis in the anther, microspores mature in the locule to become pollen ( Goldberg et al. 1993 ;Taylor & Hepler 1997). They interact with the tapetum, which consists of one layer of metabolically active cells enclosing the locule. The tapetum serves several important roles ( Pacini & Franchi 1993). Throughout microsporogenesis, the tapetum transfers nutrients to, and regulates the composition of, the locule fluid. In addition, at an early stage of microsporogenesis, the tapetum secretes β-glucanase which hydrolyzes the β-glucan cell wall enclosing the microspore tetrads. The released solitary microspores continue to mature. Midway through microsporogenesis, the tapetum secretes exine precursors which are polymerized on the surface of the pollen inner wall (the intine) to form the outer wall (the exine). The exine has many bacules arranged into species-specific patterns, and the bacules generate cavities on the pollen surface. At a late stage of microsporogenesis, the tapetum cells lyze and discharge chemicals into the exine cavities and on the exine surface. These pollen surface chemicals are called pollen coat, tryphine or pollenkitt.
The pollen coat is the outermost layer of a pollen and will make the initial contact with the stigma surface during sexual reproduction. It may serve different functions, such as protecting the pollen from solar radiation, giving the pollen color, preventing the pollen from water loss, attracting insect pollinators, adhering the pollen together and to the pollinating insect bodies and the stigma surface, aiding hydration and thus the initial germination of the pollen, and exerting self-compatibility and incompatibility ( Pacini & Franchi 1993).
The constituents of the pollen coat materials are largely unknown. Those from Brassica have been studied more intensively in recent years ( Doughty et al. 1993 ;Murphy & Ross 1998;Ross & Murphy 1996;Ruiter et al. 1997 ;Wu et al. 1997 ). The pollen coat can be extracted with an organic solvent such as cyclohexane or diethyl ether without apparent damage to the pollen protoplast. The pollen coat contains neutral esters as the major lipid constituents, and its predominant proteins are related to oleosins, which are abundant structural proteins on the storage oil bodies in seeds ( Huang 1992). Findings similar to those in Brassica, although to a limited extent, have been obtained in Arabidopsis ( de Oliveira et al. 1993 ). The functions of the abundant neutral esters and oleosin fragments in the pollen coat are unknown. A mutant of Arabidopsis defective in long acyl lipid synthesis produces pollen without pollen coat; these pollen do not germinate on the stigma ( Preuss et al. 1993 ).
In Brassica, the pollen-coat neutral esters and oleosin fragments are produced in the tapetum cells. At the late stage of anther development immediately before lysis of the tapetum, the tapetum cells contain two dominant organelles. Both organelles are spherical, of about 3 μm in diameter, and contain abundant lipids. Recently, these two organelles have been isolated and their constituents studied. One of the organelles is a novel lipid particle, termed tapetosome ( Piffanelli & Murphy 1998;Wu et al. 1997 ), which contains TAGs situated among densely packed vesicles and does not have an enclosing membrane. The other organelle is the elaioplast, which is packed with globuli of neutral esters. Both organelles contain unique proteins. Whereas the proteins of the tapetosomes are oleosins, those of the elaioplasts have not been previously identified. After lysis of the tapetum cells, some of the constituents of the two organelles are degraded, whereas the other constituents remain unchanged or modified, and are released onto the surface of the maturing microspores. These latter constituents include fragmented oleosins of the tapetosomes and the neutral esters of the elaioplasts.
The mechanism of selective degradation and retention of the constituents of the two tapetum organelles in the anthers during microsporogenesis determines the constituents and functions of the pollen coat. This information has been lacking and is the subject of the current report. We also report characterizations of the tapetosome oleosins by genetic means and the elaioplast polypeptides via cloning.
Results
The multiple molecular species of oleosins in the tapetosomes of B. napus were partly due to the amphidiploidy of the species
Five oleosins of different molecular weights encoded by cloned genes were identified in the tapetosomes of B. napus tapetum ( Wu et al. 1997 ). This multiplicity might be due to the amphidiploidy of B. napus, which contains both the AA and CC genomes ( Song et al. 1990 ). To test this possibility, we examined the two-organelle fractions (mixture of tapetosomes and elaioplasts) isolated by flotation centrifugation ( Wu et al. 1997 ) from the extracts of florets of different species. These species included diploid B. campestris and B. rapa (syn. campestris, from a different source) (containing the AA genome), B. oleracea (containing the CC genome), B. nigra and B. fruticulosa (containing the BB genome), and B. geniculata (of an unknown genetic background). The proteins in the two-organelle fraction from each species were resolved into numerous polypeptides by SDS-PAGE ( Fig. 1). We focused our attention on the two most abundant oleosins, of 45 and 48 kDa, found in the B. napus tapetosomes. These two oleosins are encoded by cloned genes of very similar DNA sequences (95% identity in the coding sequences and 94% identity in the predicted amino acid sequences;Robert et al. 1994 ), and antibodies raised against either oleosin recognized both of them ( Wu et al. 1997 ). Immunoblotting analyses using antibodies raised against the 45 kDa protein from B. napus ( Fig. 1) revealed that the 45 kDa oleosin was present in the species containing the AA genome (B. campestris, B. rapa and B. napus), whereas the 48 kDa oleosin was present in the species containing the CC genome (B. napus and B. oleracea). The antibodies also recognized, although with less reactivity, one or more oleosins of 45–65 kDa in the species with the BB genome (B. nigra and B. fruticulosa) and in B. geniculata. The tapetosomes of B. napus possessed other identified ( Wu et al. 1997 ) and probably not yet identified oleosins of lesser amounts and lower molecular weights ( Fig. 1). The tapetosomes of other Brassica species apparently possessed these oleosins of similar molecular weights, but their identification in reference to those in B. napus could not be pinpointed.
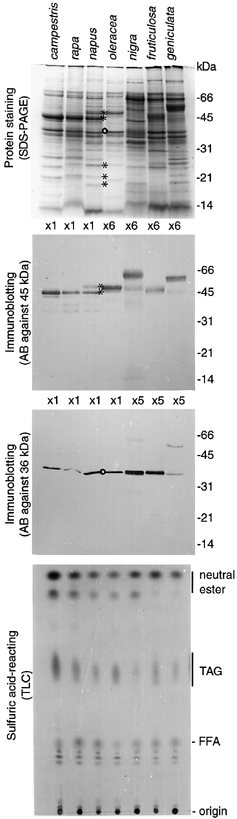
SDS-PAGE and immunoblotting of proteins and TLC of neutral lipids of the two-organelle (tapetosomes and elaioplasts) fractions isolated from diverse Brassica species.
The gel was stained for proteins or treated for immunoblotting using antibodies raised against the tapetosome 45 kDa oleosin or the elaioplast 36 kDa polypeptide from B. napus. In each of the four panels, the same proportional amounts of samples from the various Brassica species were used, with the exceptions being indicated as × 5 or × 6. Positions of markers for protein molecular weights and standard lipids are shown on the right. Asterisks indicate identified oleosins in B. napus, and (o) denotes the 36 kDa elaioplast polypeptide.
The elaioplasts isolated from B. napus were shown by SDS-PAGE to contain several polypeptides, of which the most abundant was 36 kDa ( Wu et al. 1997 ). The identity of this protein is unknown. Antibodies raised against this protein from B. napus recognized a protein of the same molecular weight in the two-organelle fractions from diverse Brassica species ( Fig. 1).
The two-organelle fraction isolated from diverse Brassica species contained similar neutral lipids, as revealed by TLC ( Fig. 1). They all possessed the tapetosome TAGs, and all had the elaioplast neutral esters, although some of them lacked the minor and less hydrophobic neutral ester.
The diploid B. campestris, which possessed the 45 kDa oleosin but not the 48 kDa oleosin as the major oleosin, was chosen for further studies because of its simplicity and, unless otherwise stated, is the species referred to hereafter.
During anther development, the tapetosome oleosins and TAGs, as well as the elaioplast proteins and neutral esters, accumulated concomitantly but underwent different subsequent changes
The temporal changes of the major 45 kDa oleosin and TAGs of the tapetosomes, and the most abundant 36 kDa polypeptide and neutral esters of the elaioplasts were examined. We divided the anthers into six developmental stages and analyzed the total anther extracts for protein constituents by SDS-PAGE ( Fig. 2). About 50% of the proteins in the mature anthers of stage 6 were already present in stage-1 anthers, and the patterns of proteins from all six stages were quite similar. As detected by immunoblotting, the 45 kDa oleosin accumulated concomitantly with the sporophytic TAGs and reached the highest level at stage 3. It then disappeared gradually and was replaced by a 37 kDa fragment. This 37 kDa fragment represented a large portion of the 45 kDa oleosin after removal of the N-terminal segment and would be transferred to the surface of the maturing microspores.
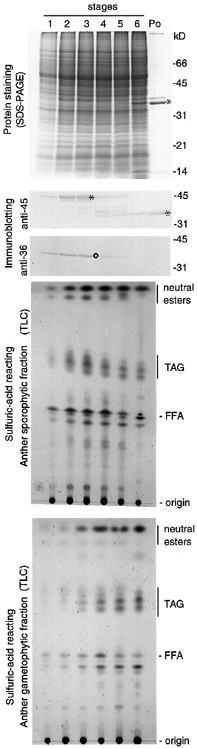
SDS-PAGE and immunoblotting of proteins and TLC of neutral lipids of the extracts of B. campestris anthers of different developmental stages.
Anthers of six different developmental stages and mature pollen were used. The gel was stained for proteins or treated for immunoblotting using antibodies raised against the tapetosome 45 kDa oleosin or the elaioplast 36 kDa polypeptide from B. napus. In the immunoblots, only the portions containing the visible reaction products are shown. For lipid analyses, the anthers were divided into the sporophytic fraction (extract of anthers including the microspore coat but omitting the microspore protoplasts) and the gametophytic fraction (microspore protoplasts). In each panel, samples of each developmental stage were derived from an equal number of anthers. Positions of markers for protein molecular weights and standard lipids are shown on the right. Asterisks indicate the tapetosome 45 kDa oleosin and its 37 kDa fragment and (o) denotes the elaioplast 36 kDa polypeptide.
To analyze the changes of anther lipids during development, we further separated the anthers into a sporophytic portion which contained all the anther sporophytic materials including the coat of the microspores, and a gametophytic portion which contained the protoplast materials of the microspores after the surface sporophytic coat had been removed. The tapetosome TAGs in the sporophytic portion reached a maximal level at stage 3 and decreased gradually to about one-fourth at stage 6 ( Fig. 2). These remaining TAGs were degraded further at late stage 6 and were not recovered in the pollen coat (data not shown).
The tapetum elaioplast major 36 kDa polypeptide and neutral esters accumulated during anther development in the same time pattern as that for the tapetosome constituents ( Fig. 2). Their quantities rose to a maximal level at stage 3. Thereafter, the 36 kDa polypeptide rapidly disappeared, whereas the neutral esters remained unchanged up to stage 6.
The temporal changes of the sporophytic lipids in the anthers were different from those of the gametophytic lipids inside the maturing microspores ( Fig. 2). At stage 3, the microspore gametophytic protoplasts started to accumulate a neutral ester (different from that of the pollen coat;Wu et al. 1997 ) and storage TAGs, and the highest levels were reached in the mature pollen.
Upon incubation of isolated tapetosomes in a pH 5 medium, the 45 kDa oleosin was enzymatically hydrolyzed to a 37 kDa fragment
During anther development, the tapetosome 45 kDa oleosin was converted to a 37 kDa fragment ( Fig. 2). We tested whether the oleosin was degraded inside the tapetosomes. Tapetosomes isolated from stage 3 anthers were incubated at 37°C in media of diverse pHs, and the proteins in the organelles before and after the incubation were analyzed by SDS-PAGE ( Fig. 3a). The major oleosin of 45 kDa in the isolated tapetosomes decreased, concomitant with an increase in a 37 kDa fragment. In media of pHs between 4 and 9, the proteolysis occurred maximally at pH 5. The 37 kDa fragment should have derived from the 45 kDa oleosin because there was no other protein in the tapetosomes greater than 37 kDa of a sufficient quantity that could have given rise to a 37 kDa fragment ( Fig. 3a). This interpretation was confirmed by the finding that the 37 kDa fragment, as well as the 45 kDa oleosin, was recognized by antibodies raised against the 45 kDa oleosin (data not shown). In addition, N-terminal sequencing of the 37 kDa fragment yielded a major sequence of LGIPESIKPSNIIPE, which indicates that the fragment was a proteolytic product of the 45 kDa oleosin after removal of the N-terminal 112 residues ( Robert et al. 1994 ). The other known oleosins of relatively minor quantities were also reduced in amount, and the reduction was highest at pHs between 5 and 6 ( Fig. 3a). Polypeptides of 14 kDa and < 10 kDa increased after the proteolysis and should represent fragments derived from some or all of the oleosins.
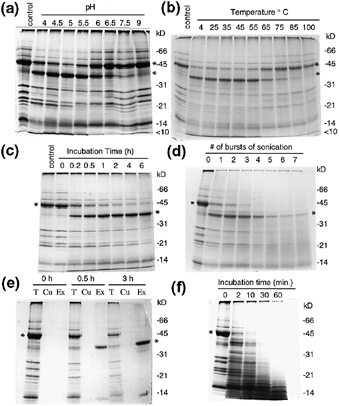
SDS-PAGE of proteins in isolated tapetosomes after treatments.
Positions of markers for protein molecular weights are shown on the right. Asterisks mark the 45 kDa oleosin and its 37 kDa fragment.
(a) Tapetosomes were incubated in media of different pH’s at 37°C for 30 min. Control sample was without incubation.
(b) Tapetosomes were treated at the indicated temperatures for 15 min and then subjected to self-proteolysis in a pH 5 medium at 37°C for 3 h. Control sample was without heat treatment and incubation.
(c) Tapetosomes were incubated in a pH 5 medium at 37°C for the indicated duration. Control sample was heat-treated at 80°C for 30 min to denature the protease and then incubated for 1 h.
(d) Tapetosomes in a pH 5 medium were sonicated repeatedly and then subjected to self-proteolysis. They were sonicated with 30 sec bursts in 4°C, and each burst was followed by an incubation at 37°C for 5 min. An aliquot was removed from the sample after each burst and incubation for SDS-PAGE. The numbers of the burst are indicated.
(e) Tapetosomes in a pH 5 medium before and after self-proteolysis were fractionated to produce an organelle fraction and an organelle-exterior fraction. Tapetosome fractions without or with self-proteolysis for the indicated duration were made to 0.8 m sucrose and centrifuged with an overlying layer of 0.4 m sucrose. The floated materials (tapetosomes, T), the overlying layer (cushion, Cu), and the remaining 0.8 M sucrose fraction (organelle exterior, Ex) were analyzed by SDS-PAGE. The overlying layer was used to prevent cross-contamination of the floated tapetosomes and the organelle interior fraction. Each of the three fractions loaded onto a gel lane was derived from the same amount of the initial tapetosomes.
(f) Tapetosomes were treated with external protease K. Tapetosomes in a pH 7.5 medium were treated with protease K for the indicated duration.
We examined whether the protease involved in the proteolysis of the 45 kDa oleosin in the tapetosomes was heat sensitive. Isolated tapetosomes in a pH 7.5 medium were treated at various temperatures for 15 min. After treatment, the tapetosomes were incubated in a pH 5 medium at 37°C, and the proteolysis of the 45 kDa oleosin to the 37 kDa fragment was monitored by SDS-PAGE. Figure 3(b) shows that the protease was inactivated at about 65°C and higher temperatures.
The 37 kDa fragment derived from the 45 kDa oleosin in isolated tapetosomes was not proteolyzed further unless the organelles were sonicated
A time course of the proteolysis of the 45 kDa oleosin in isolated tapetosomes in a pH 5 medium is shown in Fig. 3(c). The conversion of the 45 kDa oleosin to the 37 kDa fragment was complete after 1 h of incubation. The 37 kDa fragment was not subjected to further proteolysis, even after 6 h of incubation. Apparently, all the 45 kDa oleosin was quantitatively converted to the 37 kDa fragment because there was only a minimal reduction in the amount (stain) of the 37 kDa fragment compared to that of the original 45 kDa oleosin. During the incubation, the other oleosins of minor quantities were also reduced. This reduction was accompanied by an increase in polypeptides of about 14 kDa and < 10 kDa, which should represent fragments derived from the oleosins.
We subjected the isolated tapetosomes to sonication to examine whether a destruction of the suborganelle compartmentation would expose the 37 kDa fragment to the protease for further degradation. The tapetosomes in a pH 5 medium in a 1.5 ml vial on an ice bucket were subjected to short bursts of sonication. After each burst, the sample was incubated at 37°C for 5 min. After each sonication burst and incubation, an aliquot was taken for SDS-PAGE. As shown in Fig. 3(d), the 37 kDa fragment gradually disappeared after each burst of sonication and incubation. The findings indicate that the 37 kDa fragment and the protease(s) are separately compartmented within the tapetosomes.
During self-proteolysis in isolated tapetosomes, the produced 37 kDa fragment was released to the organelle exterior
We examined whether the 37 kDa fragment was retained in the organelles or released to the exterior after its production in isolated tapetosomes. The isolated organelles were incubated in a pH 5 medium for self proteolysis. At time intervals, the sample was separated into an organelle fraction and an exterior fraction, and the proteins in the fractions were analyzed by SDS-PAGE. Figure 3(e) shows that the undegraded 45 kDa oleosin was retained in the organelles, whereas the 37 kDa fragment was present in the organelle exterior.
When an external protease was added to the isolated tapetosomes in a pH 7.5 medium in which there would be minimal self-proteolysis, the 45 kDa oleosins and the 37 kDa fragment were rapidly proteolyzed to small peptides ( Fig. 3f). The result reveals that the 45 kDa oleosin in the organelles and the 37 kDa fragment released from the organelles were exposed to externally added protease.
The 37 kDa fragment produced in vitro in isolated tapetosomes was equivalent to the predominant 37 kDa protein in the pollen coat
It has been reported that the pollen coat of B. napus contains two groups of polypeptides of 32–40 kDa and less than 10 kDa ( Wu et al. 1997 ). Many of these polypeptides represent fragments of the tapetum oleosins. The pollen coat of B. campestris also contained these two groups of proteins, but the 32–40 kDa polypeptides were represented by one major polypeptide of 37 kDa ( Fig. 4). This 37 kDa polypeptide was slightly smaller than the 37 kDa fragment produced in vitro in isolated tapetosomes.
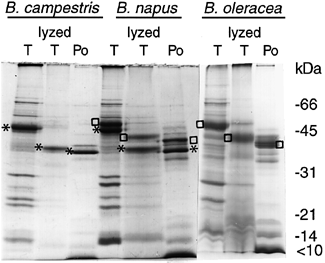
SDS-PAGE of proteins in isolated tapetosomes before and after self-proteolysis and in the pollen coat from B. campestris, B. napus and B. oleracea.
Tapetosomes (T), tapetosomes after proteolyzed in a pH 5 medium for 3 h (lyzed T), and pollen coat (Po) were subjected to SDS-PAGE. Positions of markers for protein molecular weights are shown on the right. Asterisks indicate the original 45 kDa oleosin and the fragments derived from it, and (o) denotes the 48 kDa oleosin and the fragments derived from it.
N-terminal sequences of these two 37 kDa polypeptides were identical, of LGIPESIKPSNIIPE. We infer that the predominant polypeptide of 37 kDa in the pollen coat was derived from the tapetosome 37 kDa fragment after proteolysis of the 45 kDa oleosin within the organelles. The slight difference between the observed molecular weights of the two fragments ( Fig. 4) could be due to the removal of an extra but minor component of the tapetosome 37 kDa fragment, such as several C-terminal residues or a non-amino-acid moiety, during its transfer to the pollen surface. N-terminal sequencing of the gel samples containing the tapetosome 37 kDa fragment and the pollen-coat 37 kDa polypeptide revealed in each of the two samples a second sequence of GIPES (with less certainty on the subsequent residues), of about one-quarter the major sequence of LGIPESIKPSNIIPE. The results suggest that (i) the protease exerted its major hydrolytic activity on the 45 kDa oleosin at –SLSL*LGIPESI– and a minor activity at –SLSLL*GIPSES–; (ii) the cleavage junction was not completely exposed (e.g. being associated with lipids) to the protease such that the enzyme produced the two different fragments; or (iii) the protease produced LGIPESI– fragment, which was subsequently trimmed to GIPESI– by the same or a different protease. Irrespective of the proteolytic mechanism, both oleosin fragments were preserved in the pollen coat.
We compared the proteolytic products of oleosins in isolated tapetosomes and the 32–40 kDa oleosin fragments in the pollen coat among B. campestris (AA genome), B. napus (AA and CC genomes), and B. oleracea (CC genome). As reported earlier ( Wu et al. 1997 ), B. napus tapetosomes had two major oleosins of 48 and 45 kDa, and its pollen coat had several polypeptides of 32–40 kDa ( Fig. 4). In the current study, the 48 and 45 kDa oleosins in isolated B. napus tapetosomes after incubation in a pH 5 medium were converted to two fragments of 40 and 37 kDa, respectively. The N-terminal sequence of the 40 kDa fragment was determined to be LGIPESIKPSNVIPE, indicative of this fragment being produced from the 48 kDa oleosin after removal of its N-terminus ( Robert et al. 1994 ). Similarly, the N-terminal sequence of the 37 kDa fragment was LGIPESIKPSNIIPE, illustrative of this fragment being derived from the 45 kDa oleosin after removal of its N-terminus. The N-terminal sequences of the two in vitro produced oleosin fragments of 40 and 37 kDa were identical to those of the two major polypeptides of 39 and 36 kDa in the pollen coat. In B. oleracea, the major 48 kDa oleosin in the isolated tapetosomes was converted to a 40 kDa fragment, which was slightly larger than the major 39 kDa fragment of the pollen coat ( Fig. 4). Thus, the results we have obtained in B. campestris can be applied to B. napus and B. oleracea.
Isolated tapetosomes incubated in media of diverse pHs did not undergo TAG lipolysis
When isolated tapetosomes were incubated in a pH 5 medium in which the 45 kDa oleosin was hydrolyzed to the 37 kDa fragment ( Fig. 3a), no apparent disappearance of TAGs was observed in this medium as well as in media of other pHs (data not shown). When the total sporophytic anther extract instead of isolated tapetosomes was incubated in media of diverse pHs, FFA was released, and the release was highest at alkaline pHs ( Fig. 5). The release was concomitant with a decrease in TAGs. When the total sporophytic anther extract was assayed for lipase activity on externally added TAGs, the lipase activity was highest at alkaline pHs ( Fig. 5). The location of the lipase in the total sporophytic anther extract was assessed after a subcellular fractionation. Most of the lipase activity was recovered in a supernatant fraction (prepared by centrifugation at 15 000 g for 2 h to remove pelleted materials and the floated tapetosomes and elaioplasts), and there was little activity in the isolated tapetosomes ( Fig. 5). Isolated tapetosomes did not undergo self-lipolysis of their TAGs, but when incubated with the supernatant, their TAGs were hydrolyzed; again, this lipolysis was most active at alkaline pHs. Together, the findings indicate that the tapetosome TAGs, unlike the oleosins, did not undergo degradation within the organelles. Rather, they were hydrolyzed by an alkaline lipase present somewhere else in the tapetum cells or in the locule fluid derived from other anther cells.
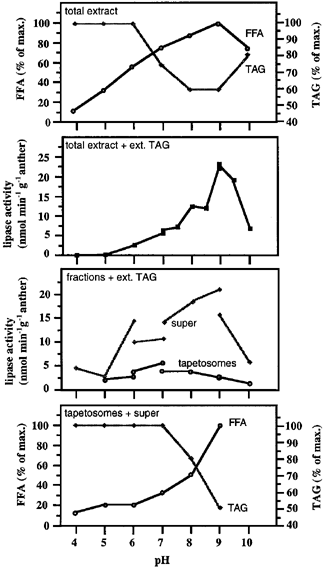
Self-lipolysis and lipase activity in various sporophytic anther preparations in media of different pHs.
The amounts of TAGs and FFA were monitored by TLC. Lipase activity was expressed as nmol FFA released (monitored by a colorimetric method) per min per g anthers.
First panel: The total sporophytic anther extract was incubated for 16 h.
Second panel: The total sporophytic anther extract was assayed for lipase activity with added external TAGs.
Third panel: Lipase activities in the supernatant fraction and the isolated tapetosomes were assayed with added external TAGs. The supernatant was prepared by centrifuging the total sporophytic anther extract at 15 000 g for 2 h to remove pelleted materials and the floated tapetosomes and elaioplasts.
Fourth panel: Isolated tapetosomes were mixed with the supernatant fraction (in amounts proportional to those derived from the same amount of anther extract) and incubated for 16 h.
Isolated elaioplasts contained several unique polypeptides, one of which shared high sequence homology with proteins associated with carotenoids in chromoplasts
Isolated elaioplasts of the tapetum contained several unique polypeptides of 31–36 kDa ( Wu et al. 1997 ;Fig. 6). N-terminal sequencing of these polypeptides produced the longest sequence with highly reliable residue identification from a 32 kDa polypeptide. The sequence was VAEK(Q)VAEEAIESAEETSRLKRVLAGSL(D)Y. Using 5′ primers encoding the underlined portion of the sequence and 3′ oligo-dT adapter, we performed PCR with floret cDNA and obtained a 0.9 kb cDNA fragment (termed Bcp32). Bcp32 had an ORF encoding a polypeptide of 243 residues ( Fig. 7, after accounting for those obtained from N-terminal sequencing) and a 162 bp 3′ non-coding region. The polypeptide had a predicted pI of 5.1 and 26 471 D. The predicted sequence immediately downstream of the N-terminal sequence for making the 5′ primers was identical to that obtained by N-terminal sequencing, and this identity confirmed the validity of the clone being that encoding the original 32 kDa polypeptide. A search of the gene banks for proteins homologous to BCP32 produced three full-length proteins. Two of the proteins, fibrillin of bell pepper ( Deruere et al. 1994 ) and CHRC of Cucumis corollas ( Vishnevetsky et al. 1996 ), were present on the surface of fibril structures in the chromoplasts, and the third protein from Arabidopsis had not been published (GenBank accession no. AL021712). An alignment of the sequences of BCP32 and the other three proteins is shown in Fig. 7. After accounting for their transit peptides, the mature pepper and Cucumis proteins were slightly larger than BCP32 by having about 25 extra residues at the N-termini. Otherwise, the three proteins and the Arabidopsis protein shared striking similarities in their sequences. These similarities, as mentioned earlier for the chromoplast proteins ( Deruere et al. 1994 ;Vishnevetsky et al. 1996 ), included an acidic pI, several consecutive aspartic/glutamic residues (on BCP32, nos 7, 14, 96), a cell adhesion motif RGD (no. 224), and no cysteine. In addition, we identified a sequence of four tandemly situated glutamic/aspartic residues each followed by two non-charged residues (no. 154), and two stretches of hydrophobic residues in β-strand that could be transmembranous or lipid-associated (16 residues from nos 71 and 12 residues from no. 116). In view of the high homology among the proteins shown in Fig. 7 and other proteins known for their incomplete sequences ( Pozueta-Romero et al. 1997 ), we infer that BCP32 and other related elaioplast proteins are present on the surface of the lipid globuli, thereby stabilizing the hydrophobic neutral esters inside the elaioplasts.
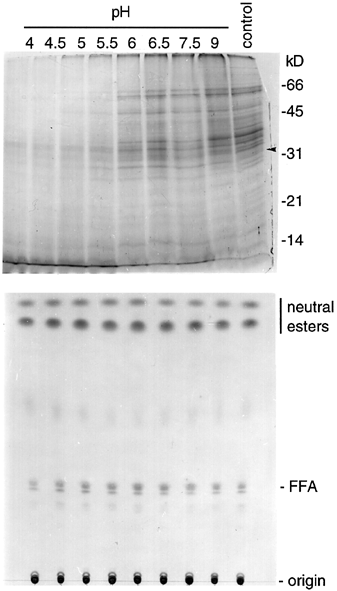
SDS-PAGE of proteins and TLC of neutral lipids in isolated elaioplasts after incubation in media of different pHs.
The organelles were incubated in media of different pHs for 3 h (for protein analyses) or 24 h (for lipid analysis). Control represents elaioplasts without the incubation. The arrow marks the 32 kDa polypeptide (BCP32) whose clone has been obtained. Positions of markers for protein molecular weights and standard lipids are shown on the right.
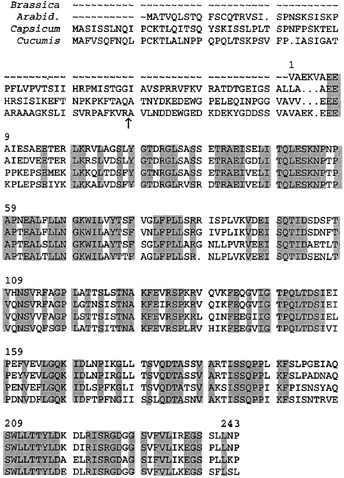
Alignment of the amino acid sequences of four full-length plastid proteins presumed to be associated with neutral lipids in the plastids.
The four proteins were from Brassica (BCP32) (GenBank accession number AF084554), Arabidopsis (GenBank accession number AL021712, unpublished), Capsicum (pepper fibrillin, Deruere et al. 1994 ), and Cucumis (CHRC, Vishnevetsky et al. 1996 ). Amino acid residues identical in all the four proteins are shadowed. Numbering of the residues starts with that first residue in BCP32. Dots denote potential gaps. Predicted cut of the transit peptides in Capsicum and Cucumis is indicated by an arrow.
The predicted molecular weight of BCP32 based on the cDNA sequence was 26 kDa, which was lower than the 32 kDa of the native polypeptide determined by SDS-PAGE. This inconsistency also occurred in the other two known plastid proteins of pepper (predicted to be 29 kDa for a native polypeptide of 32 kDa) and Cucumis (predicted to be 29 kDa for a native polypeptide of 35 kDa). It might be due to the polypeptides having special structures and thus deviating from linearity of the molecular weight determination by SDS-PAGE, or the mature polypeptide attaching to a non-protein moiety. In relation to the latter aspect, several potential sites for phosphorylation or myristoylation were identified in BCP32 (from a computer program prediction, data not shown).
Upon incubation of isolated elaioplasts in a pH 5 medium their polypeptides were hydrolyzed completely whereas the neutral esters remained unchanged
Elaioplasts isolated from stage 3 anthers were incubated at 37°C in media of diverse pHs, and the proteins in the organelles before and after the incubation were analyzed by SDS-PAGE ( Fig. 6). In media of pHs between 4 and 9, the disappearance of the 31–36 kDa and other polypeptides occurred maximally at pH 4.5–5. The polypeptides were reduced to less than 50% of the original amounts after a 1 h incubation (data not shown) and disappeared completely after a 3 h incubation ( Fig. 6). Contrary to the polypeptides, the elaioplast neutral esters remained unchanged after 24 h of incubation in media of diverse pHs ( Fig. 6).
Discussion
The elaioplasts of Brassica tapetum have no carotenoids and possess only very minimal amounts of internal membrane structures ( Wu et al. 1997 ). The organelles are packed with abundant spherical lipid globuli, which are mostly neutral esters (identified to be sterol esters, courtesy of Dr Robert Moreau, USDA, Wydmore, USA). We predict that BCP32 is present on the surface of the lipid globules to stabilize the hydrophobic lipids. BCP32 and all the known carotenoid-associated structural proteins in chromoplasts share striking similarities in their amino acid sequences and, possibly, length. All these proteins must have highly conserved and unique structures, which are yet to be defined, to interact with the hydrophobic lipids. Although the Brassica tapetum elaioplasts contain sterol esters rather than carotenoids and thus are different from the chromoplasts, sterol esters and carotenoids are derived from a common precursor, farnesyl diphosphate, in the terpene biosynthetic pathway. Some or all of the 31–36 kDa polypeptides in Brassica tapetum elaioplasts are probably closely related to BCP32 because the sizes of most of the known carotenoid-associated proteins are highly conserved, in the range of 30–35 kDa and because the Brassica elaioplasts contain predominantly globuli and minimal internal membranes. During Brassica anther development, proplastids in the tapetum cells gradually lose the internal membranous structures and accumulate lipid globuli ( Platt et al. 1998 ). It is likely that BCP32, similar to the unidentified 37 kDa polypeptide ( Fig. 2), accumulates concomitantly with the globuli and disappears completely at the late stage of anther development, as shown in the ease of their degradation in vitro in isolated elaioplasts. The sterol esters, originally enclosed by these proteins, are freed and deposited onto the pollen surface. Thus, the function of BCP32 and other related proteins is restricted to stabilizing the globules during the accumulation of the sterol esters and not to contributing to the protein constituents of the pollen coat.
Isolated tapetosomes contain an acidic protease that specifically hydrolyzes the major oleosin of 45 kDa into a 37 kDa fragment. The protease removes from the oleosin the central hydrophobic domain, which could have been associated with the TAGs or vesicular membranes in the tapetosomes. The central hydrophobic domain, by itself or still attached to the N-terminus, has about 5–7 kDa and may be degraded further or retained in the pollen coat as a component of the observed polypeptides of < 10 kDa. The current studies have focused on the abundant and predominant 45 kDa oleosin because of the ease of its detection. We do not know if the protease also hydrolyzes the other oleosins to specific fragments or completion. Isolated tapetosomes do not possess a lipase that hydrolyzes the TAGs. Their TAGs apparently are hydrolyzed by an alkaline lipase located outside the organelle. Thus, during microsporogenesis, the two major constituents of the tapetum tapetosomes, the oleosins and the TAGs, are subjected to different fates.
The fates of the other major tapetum organelles, the elaioplasts, during late microsporogenesis differ yet again. Isolated elaioplasts possess an acidic protease which hydrolyzes all the elaioplast polypeptides. Contrary to the proteins, the elaioplast neutral esters are not hydrolyzed but are transferred to the microspore surface.
During late microsporogenesis in Brassica, partial or complete hydrolysis of the proteins of both the tapetosomes and the elaioplasts occurs maximally at pH 5. It is unlikely that both organelles in the intact tapetum cells bear this low pH. It is more likely that the low pH environment is eventually reached when the tapetum cytoplasm is mixed with the contents of the abundant cell vacuoles ( Wang et al. 1997 ) before or during cell lysis. Alternatively, the lyzed tapetum cells release the organelles into the locule fluid, which may have a low pH, and in which the organelle constituents may undergo the observed modifications. The mechanism of hydrolyzing the organelle proteins in an acidic environment is different from that of organelle neutral lipids. The lipids in both isolated organelles in vitro do not breakdown in an acidic, neutral or alkaline medium. Rather, the tapetosome TAGs apparently are hydrolyzed by an alkaline lipase residing somewhere else in the tapetum cells or in the locule, whereas the elaioplast sterol esters are not hydrolyzed but, rather, preserved to be deposited onto the pollen surface.
Experimental procedures
Plant materials
Brassica napus L. var. Westar and B. campestris seeds were obtained from Calgene Inc. (Davis, CA, USA) and used to produce flowering plants in a greenhouse maintained at 26.5/18.5°C, 14/10 h day/night cycle. Flowering plants of B. rapa (syn. campestris), B. oleracea, B. nigra, B. fruticulosa, and B. geniculata (of an unknown genetic background) grown wild in Southern California were collected by Mr Andy Sanders, Museum Scientist, Botanic Garden, University of California, Riverside.
For developmental studies, B. campestris florets were divided into six developmental stages according to the following criteria (stages 1–4 were based on those used in Wang et al. 1997 ). At stage 1, the florets were 2 mm or less long and more than half of the microspores were in a tetrad condition. At stage 2, the florets were 2–3 mm long and all the microspores were solitary. At stage 3, the florets were 3–4 mm long and the tapetum cells were unlyzed and filled with organelles. At stage 4, the florets were 4–5 mm long and the tapetum cells had just lyzed. At stage 5, the florets were 5–6 mm long and the microspores were almost mature as pollen. At stage 6, the florets were 6 mm long, having the sepals already splitting longitudinally, exposing the petals, and the pollen matured. Mature pollen was collected from flowers that had opened on the same day.
Isolation of tapetosomes and elaioplasts from the sporophytic florets
The florets of stage 3 anthers (for B. campestris and B. napus) or mixed stages (the other species) were homogenized, and the homogenate (in 0.8 m sucrose) was used to prepare a mixture of the two organelles (tapetosomes and elaioplasts) in a one-step density gradient (with an overlying 0.4 M sucrose solution) or a tapetosome fraction and an elaioplast fraction in a three-step gradient (with overlying 0.4 M, 0.2 M and 0 M sucrose solutions) by centrifugation as described earlier ( Wu et al. 1997 ).
Preparation of surface and interior fractions from microspores and mature pollen
Microspores or mature pollen was washed with diethyl ether to remove the coat, and the leftover microspores/pollen was homogenized in chloroform methanol (2 : 1, v/v) to yield an interior fraction ( Wang et al. 1997 ). In some experiments, the coat fraction was added to the other sporophytic fraction (prepared according to Wang et al. 1997 ) to form the combined anther sporophytic fraction.
SDS-PAGE and blotting for immunoreactivity and microsequencing
All procedures followed those described previously ( Wu et al. 1997 ). Proteins were separated by 12.5% (w/v) SDS-PAGE and stained with Coomassie blue. For protein blot analyses, proteins separated on the gel were transferred to a nitrocellulose filter membrane and subjected to immunodetection using rabbit antibodies raised against purified 45 kDa oleosin or the 36 kDa elaioplast polypeptide from B. napus. For sequence analyses, proteins separated on the gel were transferred to a polyvinylidenedifluoride membrane and N-terminal sequenced.
Thin layer chromatography
Lipids extracted with diethyl ether or chloroform methanol (2 : 1, v/v) were applied to TLC plates (silica gel 60 A, Whatman Inc., Clifton, NJ, USA). The plates were developed in hexane:diethyl-ether:acetic-acid (80:20:2, v/v/v), and then charred with sulfuric acid.
In vitro lysis of proteins and neutral lipids in isolated organelles
To test the effects of pH on protein and lipid hydrolysis, isolated tapetosomes or elaioplasts were incubated in 0.2 m buffer at 37°C for 30 min. The buffer included succinate-NaOH, pH 4, 4.5, 5, 5.5, 6, and 6.5; HEPES-NaOH, pH 7.5; and CHES-NaOH, pH 9.
To test the effects of temperature on proteolysis, isolated tapetosomes in a pH 7.5 medium were treated at different temperatures (4, 25, 35, 45, 55, 65, 75, 85, 100°C) for 15 min. After treatment, the samples were incubated in 0.2 m succinate-NaOH, pH 5 at 37°C for 3 h.
To test the time required for proteolysis, isolated tapetosomes were incubated in 0.2 m succinate-NaOH, pH 5, at 37°C for various durations indicated in the Results section.
To test the effects of sonication on proteolysis, isolated tapetosomes were suspended in 0.2 m succinate-NaOH, pH 5. The sample in a tube placed on an ice bucket was sonicated with 30 sec bursts in a sonicator (Braun-Sonic, Allentown, PA, USA) at the highest setting. After each burst of sonication, the sample was incubated at 37°C for 5 min, and an aliquot was removed for SDS-PAGE analysis.
To test the effects of external protease on oleosin hydrolysis, isolated tapetosomes in 0.05 m HEPES-NaOH, pH 7.5 and 0.3 m sucrose were mixed with 4 volumes of 0.05 m HEPES-NaOH, pH 7.5. The mixture was centrifuged at 4°C for 20 min to pellet the tapetosomes. The pellet was resuspended in 50 m m Tris–HCl, pH 7.5, and 5 m m CaCl2, and 5 μg ml–1 proteinase K (Fisher Scientific Inc., Pittsburgh, PA, USA) was added. The mixture was incubated at 37°C for 10, 30 and 60 min, and then analyzed by SDS-PAGE.
To analyze the intra- or extra-organelle distribution of the oleosin and oleosin fragment before and after proteolysis, isolated tapetosomes were incubated in 0.2 m succinate-NaOH, pH 5 at 37°C for 30 or 60 min. After incubation, the samples were mixed with 3 volumes of 0.05 m HEPES-NaOH, pH 7.5 and 1.2 m sucrose. The mixture (50 μl) was placed in a 1.5 ml centrifuge tube. An overlying layer of 250 μl of 0.05 m HEPES-NaOH, pH 7.5 and 0.4 m sucrose was placed on top of the mixture. The tube was centrifuged at 12 000 g at 4°C for 20 min. After centrifugation, the floated materials (intact tapetosomes), the overlying layer, and the original volume containing 0.8 m sucrose (the organelle exterior) were collected and analyzed by SDS-PAGE.
Lipolytic reactions and lipase assays
To analyze the self-lipolysis of endogenous lipids in anther preparations, the reaction was performed in 500 μl containing 0.16 m buffer of different pHs at 34°C for 16 h. The buffer included succinate-NaOH, pH 4, 5, and 6; Tris–HCl, pH 7, 8, and 9; and CHES-NaOH, pH 10. The anther preparations included (i) the total sporophytic stage 3 anther extract; (ii) tapetosomes isolated from the extract; and (iii) the isolated tapetosomes plus the supernatant in amounts proportional to those derived from the same amount of anther extract. The supernatant was prepared by centrifuging the total extract at 15 000 g for 2 h to remove pelleted materials and the floated tapetosomes and elaioplasts. The reaction was stopped by adding 4 ml chloroform/methanol (2/1, v/v), 2 ml 0.1 m K-phosphate, pH 2.5, and 0.8 ml 0.1 m H3PO4. After centrifugation, the chloroform/methanol layer was retained and 1 ml of 0.1 N HCl was added. After centrifugation, the chloroform/methanol layer was retained and dried under a stream of nitrogen. The residual materials were re-dissolved in 20 μl chloroform and subjected to TLC as described above. The amounts of lipids (TAGs and FFA) were estimated by visual comparison of the samples with those of a serial dilution of known amounts of trioleins and oleic acid.
Lipase ( EC3.1.1.3) activities in the total sporophytic anther extract, the supernatant, and the isolated tapetosomes were measured by a colorimetric assay with trilinolein as the substrate ( Wang & Huang 1987). The reaction mixture of 400 μl contained 0.16 m buffer of different pHs, 5 m m emulsified trilinolein (from a stock solution of 50 m m trilinolein in 5% gum acacia), 5 m m dithiothreitol, and the anther preparation. The buffer included succinate-NaOH, pH 4, 5, and 6; imidazole-HCl, pH 6 and 7; Tris–HCl, pH 7, 7.5, 8, 8.5 and 9; and CHES-NaOH, pH 9 and 10. The reaction was carried out at 34°C in a shaker-incubator. Aliquots of the reaction mixture were sampled at time intervals for up to 10 h. The amounts of fatty acids released were determined using 1,5-diphenyl carbazide.
RNA extraction, RT–PCR and DNA sequencing
Total RNA was isolated from stage 2 B. campestris florets as described by Wessler (1994). Poly(A) RNAs were prepared from total RNAs using oligo dT cellulose by the procedure described in the manufacturer’s instructions (Rapid mRNA Purification Kit, AMRESCO, Solon, OH, USA). Poly(A) RNAs were used as templates for synthesis of cDNA. They were denatured at 65°C and cooled. First-strand cDNA was synthesized at 42°C for 2 h in 20 m m Tris–HCl, pH 8.4, 50 m m KCl, 2.5 m m MgCl2, 10 m m DTT, 1 m m dNTPs, 0.5 μl (40 units) of RNAsin, 0.5 μl of (dT)17-adaptor (5′-GACTCGAGTCGACATCGA(T)17–3′) (1 μg μl–1), 200 units of MMLV (Moloney Murine Leukemia Virus)-reverse transcriptase (Promega, Madison, WI, USA). The RNA templates were removed with RNase H. The first-strand cDNAs were used for PCR amplification.
Three degenerate primers were designed for PCR based on the N-terminal amino acid sequence of BCP31, VAEK(Q)VAEEAIESAEETSRLKRVLAGSL(D)Y. Primer no. 301 (5′-GTNGCNGARMARGTNGC-3′) was based on VAEK(Q)VA. Primers 302 (5′-GARGARGCNATHGARTCNGC-3′) and 303 (5′-GARGARGCNATHGARAGYGC-3′) were based on EEAIESA. The 3′-end primer was (dT)17-adaptor (5′-GACTCGAGTCGACATCGA(T)17–3′). PCR amplification was performed using primer no. 301, the (dT)17-adaptor, and the floret cDNA. The fragments generated were subjected to second and third rounds of amplification using primers 302 and 303, respectively. The resulting 0.9 kb fragment was subcloned into the vector pBSK and sequenced by DNA Sequencing Core Laboratory, University of Florida, USA.
Acknowledgment
Supported by USDA grant no. 98353016037.