Overexpression of a stress-inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance
Summary
In plants, oxidative stress is one of the major causes of damage as a result of various environmental stresses. Oxidative stress is primarily because of the excessive accumulation of reactive oxygen species (ROS). The amplification of ROS damage is further stimulated by the accumulation of toxic degradation products, i.e. aldehydes, arising from reactions of ROS with lipids and proteins. Previously, the isolation of dehydration-inducible genes encoding aldehyde dehydrogenases (ALDHs) was reported from the desiccation-tolerant plant Craterostigma plantagineum and Arabidopsis thaliana. ALDHs belong to a family of NAD(P)+-dependent enzymes with a broad substrate specificity that catalyze the oxidation of various toxic aldehydes to carboxylic acids. Analysis of transcript accumulation revealed that Ath-ALDH3 is induced in response to NaCl, heavy metals (Cu2+ and Cd2+), and chemicals that induce oxidative stress (methyl viologen (MV) and H2O2). To investigate the physiological role and possible involvement of ALDHs in stress protection, we generated transgenic Arabidopsis plants overexpressing Ath-ALDH3. Transgenic lines show improved tolerance when exposed to dehydration, NaCl, heavy metals (Cu2+ and Cd2+), MV, and H2O2. Tolerance of transgenic plants is correlated with decreased accumulation of lipid peroxidation-derived reactive aldehydes (as measured by malondialdehyde) compared to wild-type plants. Increased activity of Ath-ALDH3 appears to constitute a detoxification mechanism that limits aldehyde accumulation and oxidative stress, thus revealing a novel pathway of detoxification in plants. We suggest that Ath-ALDH3 could be used to obtain plants with tolerance to diverse environmental stresses.
Introduction
It is well established that many environmental stressors such as drought, salinity, temperature or heavy metals cause a rapid and excessive accumulation of reactive oxygen species (ROS) in plant cells. Therefore, oxidative stress is thought to be one of the major causes of cellular damage and cell death (Allen, 1995; Bartels, 2001; Mittler, 2002; Ramanjulu and Bartels, 2002). Oxidative stress can be divided into two phases. During the first phase, ROS directly react with proteins, amino acids, and nucleic acids, and cause peroxidation of membrane lipids (Halliwell and Gutteridge, 1989). The second phase is characterized by a lipid peroxidation chain reaction, resulting in chemically reactive cleavage products including alkanes, alkenes, aldehydes, ketones, and hydroxy acids (Esterbauer et al., 1991; Witz, 1989). Given their potentially toxic nature, cellular strategies have evolved to detoxify both ROS and toxic products. ROS accumulation is largely counteracted by intrinsic antioxidant systems that include enzymatic scavengers such as superoxide dismutase, ascorbate peroxidase, glutathione peroxidase, glutathione-S-transferase, and catalase (Mittler, 2002). In addition, a variety of low-molecular-mass molecules like ascorbate, tocopherol, carotenoids, and glutathione are important scavengers of ROS (Allen, 1995). Despite the deleterious effects of ROS, recent studies indicate that ROS at low concentrations act as signaling molecules (Mittler, 2002). For instance, H2O2 is involved in abscisic acid-mediated stomatal closure (Pei et al., 2000) or in plant–pathogen interactions (Bowler and Fluhr, 2000). Therefore, a fine tuning of ROS levels is required to regulate the balance between essential and deleterious levels of ROS.
Aldehydes comprise a major portion of the lipid peroxidation products, and are toxic because of their chemical reactivity. In mammals, there is increasing evidence that aldehydes generated during the process of lipid peroxidation are involved in pathophysiological effects associated with oxidative stress (Esterbauer et al., 1991). Increased aldehyde dehydrogenase (ALDH) activities represent one defense strategy in the detoxification of aldehydes (Yoshida et al., 1998).
Aldehyde dehydrogenases constitute a diverse protein family found in various organisms. Based on amino-acid-sequence comparison and substrate specificity, ALDHs can be grouped into two main phylogenetic groups with at least 13 subfamilies (Perozich et al., 1999). ALDHs catalyze the oxidation of aldehydes to their corresponding carboxylic acids and require NAD or NADP as a co-factor (Perozich et al., 1999; Yoshida et al., 1998). Some ALDHs are highly substrate-specific, while others accept a broad range of substrates (Yoshida et al., 1998). ALDHs have been considered as general detoxifying enzymes, which eliminate toxic biogenic and xenobiotic aldehydes (Yoshida et al., 1998). A physiological function of class 3 ALDHs in animals is the oxidation of lipid aldehyde substrates, indicating a role in detoxification (Lindahl and Petersen, 1991). Recent studies have demonstrated that expression of class 3 ALDHs is an important determinant of resistance to toxicity mediated by intermediate-chain-length aldehydes that are produced during lipid peroxidation (Townsend et al., 2001).
In plants, not many enzymatic pathways that reduce aldehyde accumulation are known. Recently, an aldose/aldehyde reductase (MsALR) was reported from soybean, which seems to be involved in the reduction of aldehydes (Oberschall et al., 2000), and a recombinant Arabidopsis alkenal, α,β-hydrogenase (Ath-ALH), was shown to reduce 2-alkenals (Mano et al., 2002), but it is likely that other enzymes also contribute to the detoxification of aldehydes. A well-studied ALDH is betaine aldehyde dehydrogenase (BADH), which is induced in response to osmotic stress and catalyzes the synthesis of the osmolyte glycine betaine using betaine aldehyde as substrate (Chen and Murata, 2002). Other plant ALDHs have been correlated with stress responses based on transcript accumulation, but their direct function is often unknown (Chen et al., 2002; Guerrero et al., 1990; Kirch et al., 2001; Stroeher et al., 1995). Plant turgor-responsive ALDHs are expressed in response to water stress (Guerrero et al., 1990; Stroeher et al., 1995) and show homology to antiquitin, which is able to oxidize aldehydes (Tang et al., 2002). The rice class 1/2 aldehyde dehydrogenase ALDH2a was shown to be upregulated by submergence (Nakazono et al., 2000). The steady-state transcript level of ALDH21A1 in Tortula ruralis was increased in response to dehydration, NaCl, ABA, and UV light (Chen et al., 2002). Cp-ALDH and Ath-ALDH3 from Craterostigma plantagineum and Arabidopsis thaliana, respectively, are responding to a variety of stress treatments (Kirch et al., 2001; this study). Two ALDH genes from Arabidopsis were shown to be upregulated by dehydration, high salinity, and cold in a microarray study using 7000 full-length cDNAs (Seki et al., 2002). Two ALDHs from barley were also shown to be upregulated by drought stress (Ozturk et al., 2002).
Enhancing antioxidant defense seems to be one of the approaches to reduce the level of oxidative stress. Previous attempts support the effectiveness of such a strategy to improve tolerance to environmental stresses (Foyer et al., 1994; McKersie et al., 1996; Oberschall et al., 2000; Roxas et al., 1997; Van Camp et al., 1996). Here, we report on the effect of the Arabidopsis aldehyde dehydrogenase Ath-ALDH3 in transgenic plants with regard to stress tolerance. Our results suggest that overexpression of this ALDH improves stress tolerance most likely by scavenging toxic aldehydes and thus reducing lipid peroxidation.
Results
Ath-ALDH3 is induced by salinity, heavy metals, methyl viologen, and H2O2
It was previously shown that Ath-ALDH3 is induced by dehydration and ABA (Kirch et al., 2001). To further extend this analysis, Ath-ALDH3 expression was studied under other environmental stress conditions. When Ath-ALDH3 was analyzed in response to exposure to 250 mm NaCl, the mRNA signal increased after 1 h and continued to increase to a maximum steady-state level after 8 h (Figure 1a).
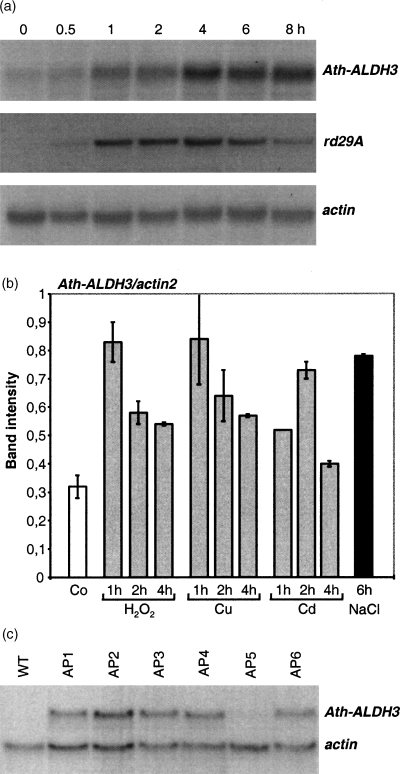
Transcript accumulation of Ath-ALDH3 in response to NaCl.
(a) Time course of Ath-ALDH3 expression in response to NaCl. RNA gel blot loaded with 30 µg total RNA isolated from wild-type plants and treated with 250 mm NaCl for the times indicated. The membrane was hybridized with the Ath-ALDH3 cDNA. The same membrane was used for control hybridizations with the osmotic stress-related Ath-rd29A (Yamaguchi-Shinozaki and Shinozaki, 1993) and with a constitutively expressed actin fragment.
(b) Expression of Ath-ALDH3 under oxidative stress conditions using RT-PCR. RT-PCR specific for Ath-ALDH3 mRNA was carried out on total RNA (4 µg) extracted from untreated (Co) plants, plants treated with NaCl for 6 h, and from plants treated with H2O2 (1 mm), Cu2+ (100 µm), and Cd2+ (250 µm) for 1, 2, and 4 h. Relative amounts of Ath-ALDH3 are shown in relation to the amount of actin2 transcripts, which was amplified in parallel. The graph represents the mean values of at least three independent experiments with the exception of the 1-h Cd2+ treatment. For details of quantification, see Experimental procedures.
(c) Analysis of transgene expression in Arabidopsis plants transformed with the Ath-ALDH3 gene. RNA gel blot loaded with 30 µg total RNA extracted from wild-type and transgenic Arabidopsis plants (AP1–AP6). The membrane was hybridized with the Ath-ALDH3 cDNA. Equal loading of RNA was confirmed by re-probing the filter with an actin cDNA fragment.
RT-PCR analysis of Ath-ALDH3 amplified higher amounts of the 463-bp ALDH gene-specific DNA fragment from RNA isolated from tissue treated with H2O2 and heavy metals (Cu2+ and Cd2+) than the wild type (Figure 1b).
Ath-ALDH3 expression analysis in transgenic plants
Transgenic Arabidopsis lines were obtained that ectopically express Ath-ALDH3 under the transcriptional control of the CaMV 35S promoter. Expression of the transgene was analyzed in six independent lines (AP1–AP6). The transgenic lines showed varying expression levels of Ath-ALDH3 (Figure 1c). The AP5 line was not different from the wild type with respect to Ath-ALDH3 expression. The other lines showed an increased expression with the highest levels in AP2, AP3, and AP6; therefore, AP3 and AP6 lines were selected for further studies. All lines carried one or two copies of the transgene: AP3 carried one and AP6 two copies of the transgene.
Dehydration stress
The drought response of the AP3 and AP6 lines was examined in adult plants. Twelve-day-old seedlings grown on MS agar plates were transferred to a tray and grown for another 3 weeks. When the soil was allowed to dry by withholding water, significantly more wild-type plants showed wilting symptoms compared to transgenic plants (Figure 2a). Also, the number of plants that recovered after re-watering was quantified (Figure 2a). These results indicate that transgenic plants displayed improved tolerance to dehydration.
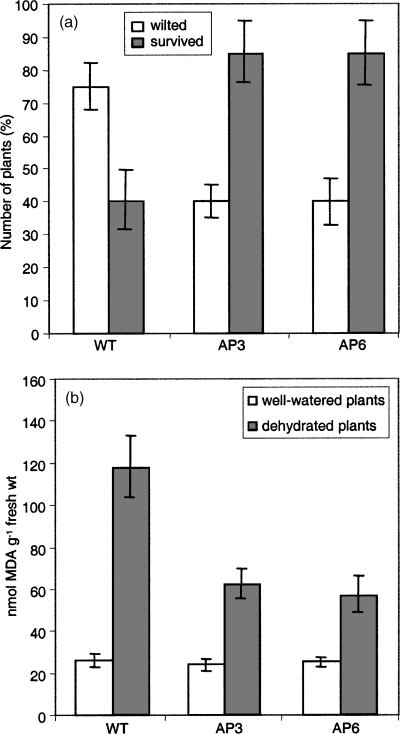
Arabidopsis plants exposed to dehydration stress.
(a) Percentage of plants scored as wilted on day 12 after dehydration and of plants scored as surviving after re-watering (plants were scored on the 4th day after re-watering).
(b) Lipid peroxidation as measured by MDA levels in transgenic and wild-type Arabidopsis plants 12 days after dehydration and in well-watered plants (MDA determination is described in detail in Experimental procedures).
The level of oxidative stress as a consequence of dehydration was determined by measuring malondialdehyde (MDA) in plants, which were not watered for 12 days (Figure 2b). MDA levels were elevated both in wild-type and transgenic plants, but the degree of lipid peroxidation was significantly lower in transgenic plants than in stressed wild-type plants.
NaCl stress
The response to NaCl was evaluated in 12-day-old in vitro-grown seedlings and in soil-grown rosette plants. Seeds were germinated on MS agar plates supplemented with different NaCl concentrations (0, 100, and 150 mm NaCl). No differences were observed between wild-type and transgenic plants with respect to germination frequency. Differences became apparent during seedling development: transgenic seedlings grew faster than the wild type on 100 and 150 mm NaCl (Figure 3a). More seedlings of the AP3 and AP6 lines than wild-type seedlings developed the first true pair of leaves under salt stress (Figure 3b).
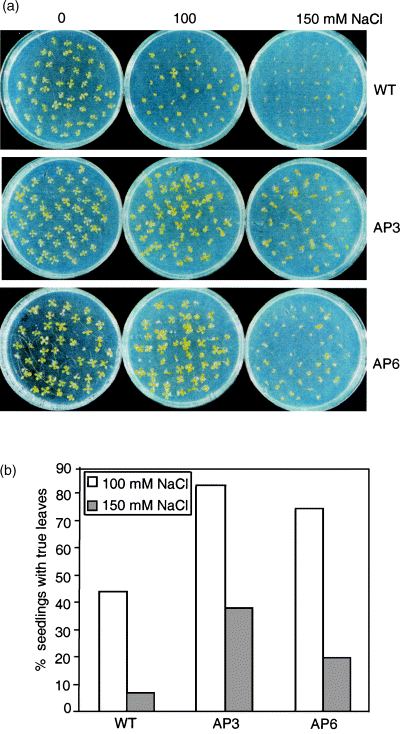
Germination and seedling growth of AP3 and AP6 transgenic lines and the wild type exposed to NaCl.
(a) Seeds were germinated on MS agar plates containing the indicated concentrations of NaCl. The photographs show 12-day-old seedlings.
(b) True leaves (second pair of leaves) were counted from 12-day-old seedlings grown on 100 and 150 mm NaCl. The data are expressed in per cent of 150 seeds germinated from each line, and represent the mean of three independent experiments.
To analyze the salt response in adult plants (4 weeks old), 12-day-old seedlings were transferred to pots and allowed to grow for two more weeks. Then, the plants were irrigated every second day with 0–400 mm NaCl solutions for 2 weeks. The phenotypic effects of the salt treatments after 7 and 15 days are shown in Figure 4(a). Wild-type plants developed chlorotic symptoms earlier than the AP3 and AP6 transgenic lines. Some of the rosette leaves of transgenic plants remained green at higher concentrations of NaCl, whereas the leaves of wild-type plants turned yellow and started to die after 15 days of treatment. NaCl treatment caused elevated levels of MDA both in wild-type and transgenic plants, but lipid peroxidation was lower in transgenic plants (Figure 4b). The Na+ content was analyzed in rosette leaves of plants after 7 days of exposure to NaCl. Na+ levels did not differ significantly in wild-type and transgenic plants without NaCl stress. Na+ accumulated in wild-type and transgenic plants in response to NaCl treatment, but wild-type plants contained significantly (P ≤ 0.05) less Na+ than the transgenic lines (Figure 4c).
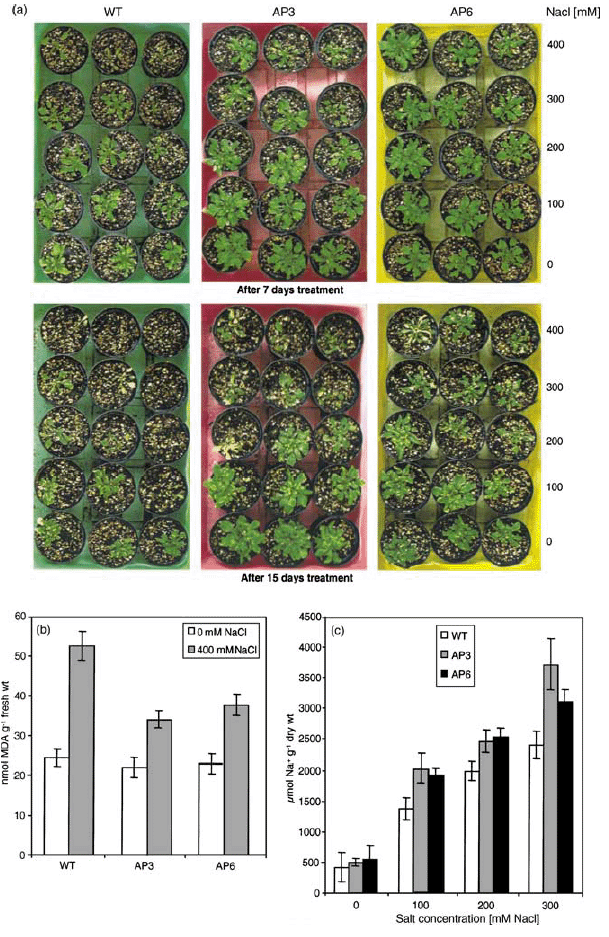
Response of transgenic and wild-type plants to NaCl treatment.
Salinity stress was imposed on 4-week-old, soil-grown plants, which were irrigated with the indicated NaCl solutions every second day for up to 15 days.
(a) Phenotype of plants after 7 and 15 days of treatment.
(b) Lipid peroxidation expressed as MDA contents in leaves. Samples were collected from AP3 and AP6 transgenic lines and wild-type plants treated with 400 mm NaCl for 7 days. Data represent the mean ± SD of three independent experiments.
(c) Na+ accumulation in leaves of AP3 and AP6 transgenic lines and wild-type plants after 7 days of NaCl treatment. The Na+ content was determined as described in Experimental procedures. Data are the mean ± SD of three independent experiments.
Response to Cu2+ and Cd2+ treatment
Heavy metals can induce oxidative stress in plants either by direct transfer of electrons to oxygen and ROS, or by inhibiting metabolic reactions (e.g. Dietz et al., 1999). Therefore, wild-type and transgenic plants were evaluated for their tolerance to Cu2+ and Cd2+. Cu2+ toxicity is mainly because of its participation in redox cycles producing hydroxyl radicals (Stochs and Bagchi, 1995). Cd2+ is a non-redox metal unable to participate in Fenton-type reactions.
Seeds were sown on MS agar plates containing different concentrations of Cu2+ (0, 10, 100, 125, 150, and 175 µm). When seedlings were monitored 18 days after germination, no obvious differences were observed in wild-type and transgenic seedlings on medium with up to 100 µm Cu2+, but higher Cu2+ concentrations adversely affected seedling development of both wild-type and transgenic lines. Cu2+ concentrations of 175 µm resulted in chlorosis and retardation of seedling development in wild-type seedlings. The transgenic seedlings showed less severe symptoms and continued to grow, although at a slower rate (Figure 5a). Addition of 150 µm Cu2+ to the medium led to a reduction of FW (Figure 5b). The physiological performance of the transgenic seedlings was superior to that of the wild type and transgenics accumulated a higher biomass than wild-type seedlings. Total chlorophyll levels decreased as a consequence of the Cu2+ treatment both in wild-type and transgenic lines, but the transgenic lines retained more chlorophyll than the wild type (Figure 5c). The same trend was observed when MDA levels were determined. Lipid peroxidation was higher in wild-type seedlings than in transgenic seedlings grown in the presence of 150 µm Cu2+ (Figure 5d).
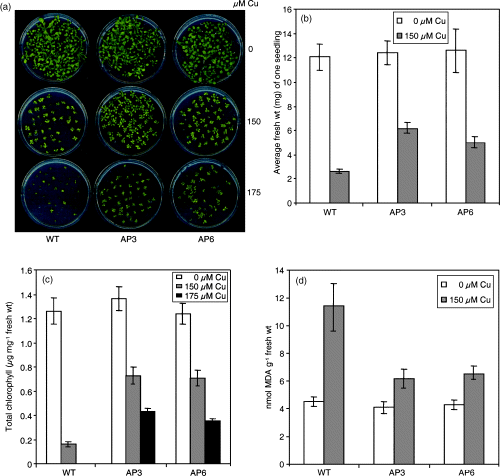
Response of transgenic and wild-type plants to Cu2+.
(a) Germination ability and seedling development of AP3 and AP6 transgenic lines and the wild type exposed to 0, 150, or 175 µm Cu2+ for 18 days.
(b) Average FW of seedlings grown on MS agar plates containing 0 or 150 µm Cu2+ for 18 days. (For each data point, 30 seedlings were collected and weighed. The results are presented as average FW of one seedling). Data are the mean ± SD of three independent experiments.
(c) Chlorophyll content of seedlings grown on MS agar plates in the absence or presence of Cu2+ for 18 days. Data are the mean ± SD of three independent experiments.
(d) Lipid peroxidation expressed as MDA content in seedlings of AP3 and AP6 transgenic lines and the wild type after 18 days of exposure to 150 µm Cu2+. Data are the mean ± SD of three independent experiments.
Increasing concentrations of Cd2+ (0, 200, 250, and 300 µm) adversely affected seedling development of wild-type and transgenic lines (Figure 6a). The extent of growth inhibition was evaluated by determining the FW (Figure 6b). Transgenic seedlings had a higher FW and longer roots than the wild type when grown on Cd2+-containing medium (Figure 6b,c). Transgenic seedlings exhibited a lower degree of oxidative stress compared to wild-type plants grown in the presence of 300 µm Cd2+ (Figure 6d). Chlorophyll levels were not affected by Cd2+ both in wild-type and transgenic plants (data not shown).
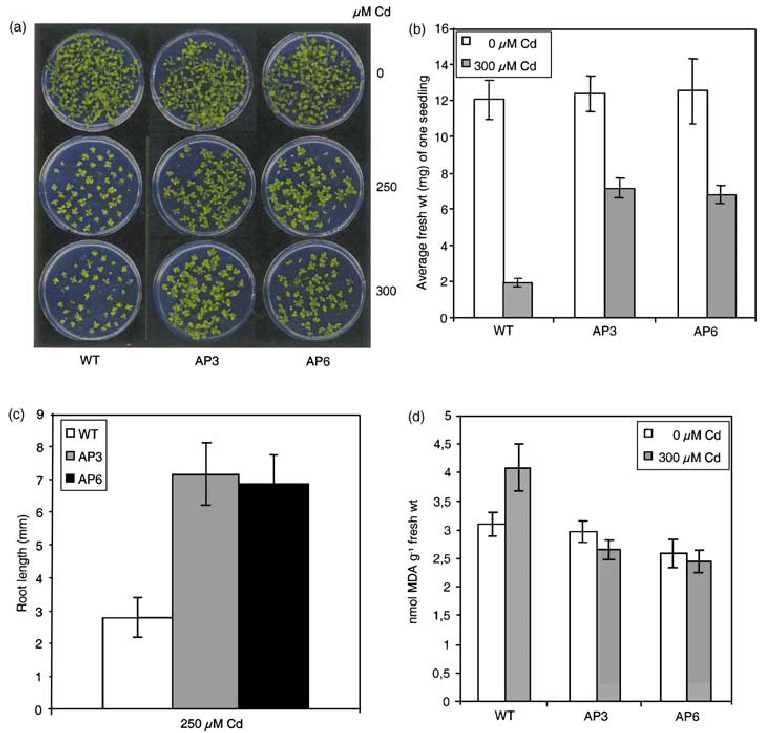
Response of AP3 and AP6 transgenic lines and the wild type to Cd2+ treatment.
(a) Germination and seedling development of AP3 and AP6 transgenic lines and the wild type exposed to 0, 250, and 300 µm Cd2+. The control (0 µm Cd2+) was the same as in 6, 5(a). Seeds were germinated on MS agar plates containing the indicated concentrations of Cd2+. The photographs were taken 18 days after germination.
(b) Average FW of seedlings grown on MS agar plates containing 0 and 300 µm Cd2+ for 18 days. (For each data point, 30 seedlings were collected and weighed. The results are presented as average FW per individual seedling). Data are the mean ± SD of three independent experiments.
(c) Root length of seedlings of AP3 and AP6 transgenic lines and the wild type exposed to 250 µm Cd2+ for 18 days. Root length was calculated as the mean ± SD of 30 seedlings for each line.
(d) Lipid peroxidation expressed as nanomoles MDA in seedlings of AP3 and AP6 transgenic lines and the wild type after 18 days of growth on medium containing 300 µm Cd2+. Data are the mean ± SD of three independent experiments.
Paraquat (methyl viologen) treatment
Methyl viologen (MV) or paraquat can bind to thylakoid membranes of the chloroplast and transfer the electrons to O2 in a chain reaction causing continuous formation of superoxide radicals in the presence of light (Asada, 1996). When seeds were germinated on half-strength MS liquid medium containing different concentrations of MV, germination was impaired in the wild type to a larger extent than in the AP3 and AP6 lines. Wild-type seedlings showed a concentration-dependent loss of chlorophyll compared to transgenic lines, which were able to germinate and retain more chlorophyll. This observation was supported by FW measurements, which was higher for the MV-exposed AP3 and AP6 seedlings than for the wild type (Figure 7a). MV tolerance was further tested by exposing leaf discs (0 and 1 µm MV) in MS solution (one-eighth) for 96 h under low-light conditions. The damage caused by the paraquat treatment was reflected by the degree of bleaching. Leaf discs of wild-type plants were almost completely bleached after 96 h of exposure, whereas the transgenic leaf discs retained some chlorophyll, and the promotion of senescence was retarded. The effects of paraquat were quantified by determining the chlorophyll contents of the leaf discs, which yielded higher chlorophyll levels in the paraquat-treated leaves of transgenic lines than those in the wild type (Figure 7b).
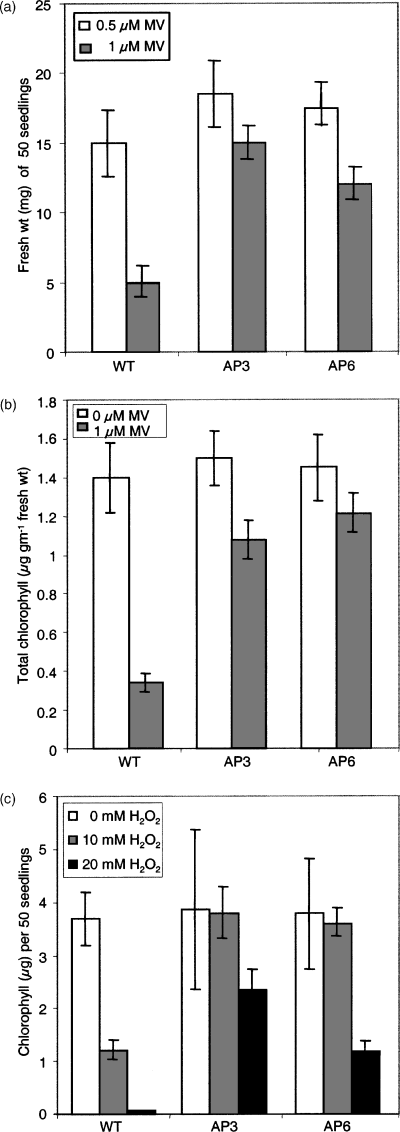
Phenotype of AP3 and AP6 transgenic lines and the wild type in response to MV and H2O2 treatment.
(a) Fresh weight of 50 seedlings grown in the presence of the indicated concentrations of paraquat for 6 days. The results are presented as the average FW of 50 seedlings for each data point. Data are the mean ± SD of three independent experiments.
(b) Chlorophyll content of MV-treated leaf discs of AP3 and AP6 transgenic lines and wild-type plants floated on 0 and 1 µm paraquat prepared in MS (one-eighth) solution and incubated for 96 h under continuous light at 22°C.
(c) Response of AP3 and AP6 transgenic lines and the wild type to H2O2. Chlorophyll content of 50 seedlings grown in the absence or presence of H2O2 for 4 days.
H2O2 tolerance during germination and early seedling development
Seeds were germinated on filter paper pre-wetted with water containing different concentrations of H2O2. 20 mm H2O2 completely inhibited the germination of wild-type seeds, whereas transgenic lines were able to develop, although at a slower rate, compared to untreated plants, which was reflected in higher chlorophyll levels (Figure 7c).
Correlation between expression levels of Ath-ALDH3 in transgenic plants and stress tolerance
From the above studies, it was evident that transgenic plants expressing Ath-ALDH3 were more tolerant than wild-type plants under dehydration, NaCl, Cu2+, Cd2+, and oxidative stress conditions. Here, we only report data on the high-expressing lines AP3 and AP6; nevertheless, the experiments were conducted with all six transgenic lines (Figure 1c). These studies allowed to conclude that the degree of tolerance is correlated to ALDH expression levels. In line AP5 in which the Ath-ALDH3 RNA levels are indistinguishable from that of the wild type, the tolerance is similar to the wild type. In AP1, in which Ath-ALDH3 is expressed weakly, a higher tolerance was observed than in AP5. AP4 has an intermediate transgene expression level and showed a modest tolerance. The highest expressors AP2, AP3, and AP6 are the most tolerant lines. Thus, the level of Ath-ALDH3 expression correlates with stress tolerance, indicating that overexpression of Ath-ALDH3 is able to increment abiotic stress tolerance.
Discussion
In an earlier study, we reported the isolation of a dehydration-inducible Cp-ALDH from C. plantagineum and of an Arabidopsis homolog, Ath-ALDH3, belonging to class 3 ALDHs (Kirch et al., 2001). Here, we show that the Ath-ALDH3 transcript accumulated rapidly (within 1–2 h) in response to a range of stress treatments whose common component is oxidative stress. This led to the hypothesis that this ALDH may have a role in oxidative stress defense.
Overexpression of an aldehyde dehydrogenase leads to stress-tolerant transgenic plants
One of the consequences of stress-induced cellular accumulation of ROS is an increase in lipid peroxidation, which is the consequence of the production of free radical reactions primarily involving membrane polyunsaturated fatty acids (Halliwell and Gutteridge, 1989). The improved tolerance of the Ath-ALDH3-overexpressing transgenic lines can be explained by diminished damage caused by different stressors. Improved stress tolerance was present in seedlings and adult plants, indicating that the stress tolerance is present throughout the vegetative growth period. It is an intriguing question whether aldehyde molecules are detoxified, reducing the level of oxidative molecules. To test this, we determined MDA levels, a secondary end product of the oxidation of polyunsaturated fatty acids, as indicator for the degree of oxidative stress. Lower lipid peroxidation occurred in transgenic plants than in wild-type plants under all different stress treatments tested. This suggests that Ath-ALDH3 has a potential role in detoxification of reactive aldehydes derived from lipid peroxidation.
Heavy metals are known to promote ROS accumulation in plants. Specifically, Cu2+ and Fe3+, being involved in Fenton-type reactions, have a potential to generate hydroxyl radicals. Consistent with this, our experiments revealed different degrees of tolerance to Cu2+ and Cd2+. Cu2+ exerted severe toxic effects on seedling development at much lower concentrations than Cd2+. The primary toxic lesions of Cu2+ and Cd2+ are unknown. Cu2+ might directly catalyze hydroxyl radical formation, resulting in lipid peroxidation. Cd2+ is redox-inactive, but it is also an effective inducer of lipid peroxidation, although indirectly (Sandalio et al., 2001). Thus, the protective effect observed is likely to be a result of better protection against lipid peroxidation, which is consistent with the determined MDA levels.
A clue for the action of the Ath-ALDH3 gene may be derived from the exposure of plants to NaCl. Transgenic plants accumulated higher levels of Na+ and yet showed less stress damage than the wild type. Enhanced accumulation of Na+ in transgenic plants is likely to cause an increased toxic burden on these plants affecting vital processes. This leads us to speculate that the higher Na+ levels in transgenics may indirectly reflect membrane integrity allowing Na+ uptake and therefore accumulation, whereas the membrane system in the wild types is more damaged leading to attenuation of Na+ uptake. The relative fitness of the transgenic plants, despite being loaded with Na+ may be related to the constitutive defense mechanism by overproducing Ath-ALDH3.
Diverse members of oxido-reductases are associated with oxidative stress tolerance
The observation that broad stress tolerance was obtained by overexpressing an enzyme (Ath-ALDH3) is comparable to the overexpression of MsALR from alfalfa in tobacco. Transgenic tobacco plants revealed improved tolerance to drought, salt, or heavy metals, and reduced damage when exposed to oxidative stress (Hideg et al., 2003; Oberschall et al., 2000). The analysis of Arabidopsis (Ath-ALDH3) and tobacco plants (MsALR transgene) suggests that the increased tolerance may, in both cases, be a result of a lower level of aldehydes, which, in turn, decreases lipid peroxidation. Although the alfalfa MsALR and the Arabidopsis ALDH are not related on the protein sequence level, they appear to share functional characteristics, which could explain the properties of the transgenic plants. Recently, another enzyme (2-alkenal α,β-hydrogenase; Ath-ALH) that is capable of aldehyde detoxification (2-alkenals to n-alkanals) by hydrogenation of the α,β-unsaturated bond has been reported (Mano et al., 2002). All these enzymes belong to the class of oxido-reductases but to different subclasses. Although these enzymes use aldehydes as substrates, their reaction pathways are different. The in vivo substrate is not known for any of these enzymes, but in vitro experiments suggested some substrate preference. The MsALR and Ath-ALH proteins reacted with 4-hydroxynon-2-enal, a lipid aldehyde (Mano et al., 2002; Oberschall et al., 2000), and the functional homolog of Ath-ALDH3 from C. plantagineum reacts best with its saturated counterpart nonanal (Kirch et al., 2001). Another difference between these enzymes is their cellular compartmentation. The alfalfa MsALR seems to be cytoplasmic or partially localized in mitochondria (Oberschall et al., 2000), whereas the Arabidopsis ALDH is targeted to the chloroplast (Kirch et al., 2001) and the Arabidopsis alkenal hydrogenase is localized either in cytosol or in peroxisomes (Mano et al., 2002).
Detoxification of aldehydes and ROS
In plants, the highly energetic reactions of photosynthesis and abundant oxygen supply make the chloroplast a particularly rich source of ROS, especially under stress conditions (Asada, 1996). When CO2 fixation is limited by environmental stress conditions, excess PSI reduction and increased ROS production can occur. Efficient removal of ROS from chloroplasts is critical, as H2O2 concentrations as low as 10 µm can inhibit photosynthesis by 50% as a result of oxidation of the thiol-modulated enzymes in the photosynthetic carbon reduction cycle (Kaiser, 1979). ROS accumulation in chloroplasts subsequently leads to accumulation of aldehydes that can amplify the degree of oxidative stress. Compared with ROS, lipid peroxidation-derived aldehydes are generally stable; they can diffuse and attack targets away from their original site of production. Like ROS detoxification, detoxification of aldehydes in chloroplasts may also be critical for photosynthesis. The importance of efficient antioxidizing systems for survival under extreme environmental conditions is convincingly illustrated by Kranner et al. (2002), who correlated the survival of the resurrection plant Myrothamnus flabellifolia with the status of the antioxidants. The plant lost its desiccation tolerance when the antioxidant pathways were broken down.
Recently, more enzymes have been identified as potential detoxifiers of products derived from lipid peroxidation. 2-cysteine peroxiredoxin has been implicated in detoxifying the hydroperoxides and thus protecting the chloroplast from oxidative stress (Dietz et al., 2002; König et al., 2002). Peroxiredoxins reduce a broad range of alkyl hydroperoxide substrates including short- and long-chain hydroperoxides, phospholipid peroxides, and cholesterol peroxides (reviewed in Dietz et al., 2002). Another example for a novel gene with a function in oxidative stress defense is AtSXL3, encoding an Arabidopsis methionine sulfoxide reductase. It is likely to play an important role in antioxidant processes in cells, converting free and protein-bound Met(O) residues to Met (Rodrigo et al., 2002). ROS can oxidize methionine leading to the formation of methionine sulfoxide [Met(O)], resulting in loss of protein function. Arabidopsis AtSXL3 transcript levels are upregulated by dehydration and H2O2 treatment. Knock-out mutant plants contained higher Met(O) compared to wild-type and revertant plants, when exposed to H2O2 (Rodrigo et al., 2002).
Conclusions and outlook
Up to now, different transgenic strategies have been employed to improve tolerance to environmental stresses: (i) Overexpressing transcription factors, which turn on pathways synthesizing protective proteins (Jaglo-Ottosen et al., 1998; Liu et al., 1998); (ii) increasing the production of compatible solutes (Chen and Murata, 2002); although tolerance was obtained by engineering osmolyte accumulation, the capacity to synthesize particular solutes is under metabolic constraints and may therefore be metabolically limited; and (iii) decreasing deleterious effects of oxidative stress by overexpressing enzymes involved in detoxification. Genes involved in antioxidant defense were overexpressed and some protection under defined conditions was obtained, but it was not sufficient to provide stable protection (Bowler et al., 1991; Gupta et al., 1993; McKersie et al., 1996; Roxas et al., 1997; Van Camp et al., 1996). Increased tolerance observed in response to high levels of osmolytes such as mannitol or glycine betaine might also be because of antioxidant properties (ROS scavenging) of these osmolytes (Chen and Murata, 2002; Shen et al., 1997). Overexpression of β-carotene hydroxylase, an enzyme in the zeaxanthin biosynthetic pathway, led to reduced lipid peroxidation under high light stress (Davison et al., 2002). Scavenging of reactive molecules, which accumulated as a result of stress conditions, was obtained by overexpressing aldose/aldehyde reductase (Hideg et al., 2003; Oberschall et al., 2000). Now, we have presented another example by overexpressing an aldehyde dehydrogenase, which leads to decreased lipid peroxidation under diverse stress conditions. The fact that we showed that different levels of tolerance were observed in response to different stressors (only a small effect in response to heavy metals) reflects the complexity of the oxidative-stress pathways and responses. Analyses of transgenic plants suggest that, in both cases, stress tolerance is obtained by reducing reactive aldehydes to non-reactive molecules. It will be interesting to test whether the combined overexpression of the differentially compartmentalized aldose reductase and aldehyde dehydrogenase will lead to further tolerance or whether there are metabolic restrictions.
Experimental procedures
Plant material and growth conditions
Arabidopsis thaliana ecotype C-24 was used in this study. Wild-type seeds were sown in plastic pots containing a 3 : 1 mixture of potting soil and vermiculite. In experiments that included transgenic plants (T2 or T3), seeds were surface-sterilized and germinated on MS agar plates containing 50 mg l−1 kanamycin. Twelve-day-old in vitro-grown seedlings were then transferred to trays containing soil. The plants were grown under white light of approximately 7000–8000 lux at 22°C with a day/night cycle of 16/8 h.
Arabidopsis transformation
A 1760-bp SmaI/SspI Ath-ALDH3 cDNA fragment with 40 bp of 5′-untranslated leader and 60 bp of 3′-untranslated trailer was subcloned into the plant gene expression vector pRTL2, containing a double CaMV 35S promoter, the tobacco etch virus (TEV) 5′-untranslated leader sequence and the CaMV polyadenylation signal (Carrington et al., 1991), forming pRTL2-Ath-ALDH3 sense. The expression cassette was inserted into the SmaI site of the binary vector pBIN19 (Bevan, 1984). The CaMV 35S promoter-Ath-ALDH3 cDNA sense construct was stably integrated into A. thaliana C-24 by Agrobacterium tumefaciens (LBA4404/PAL4404) (Hoekema et al., 1983)-mediated transformation using a floral dip method (Clough and Bent, 1998). Transformants (T1) were selected on MS agar plates containing 50 mg l−1 kanamycin.
Stress treatments for in vitro-grown plants
For NaCl, Cu2+ (as CuSO4), or Cd2+ (as CdCl2) treatment, seeds were surface-sterilized and sown on agar plates containing MS medium supplemented with the indicated salt concentrations. The seedlings were grown with a day/night cycle of 16/8 h at 22°C with a light intensity of approximately 2500 lux.
For the seed germination assay in the presence of paraquat (methyl viologen, Sigma, St Louis, USA), seeds were placed in Petri dishes (3.5 cm diameter) containing 2 ml of half-strength liquid MS medium (pH 5.7) supplemented with paraquat (0, 0.5, 1, 5, 10, or 20 µm). Plates were kept for 6 days at 22°C with a day/night cycle of 16/8 h.
For H2O2 treatment, seeds were placed on filter paper pre-wetted with deionized water containing different concentrations of H2O2. Plates were kept for 4 days at 22°C in the presence of 2500-lux white light.
Stress treatment of soil-grown plants
Twelve-day-old seedlings from the wild type, AP3, and AP6 were randomly planted in a tray containing soil. Dehydration stress was imposed by withholding watering after the plants had been grown for 3 weeks. Re-watering was done on day 12 after dehydration stress.
For salinity stress, 4-week-old plants were irrigated with NaCl solutions (0, 100, 200, 300, and 400 mm) every second day for up to 15 days.
Uprooted plants were transferred to medium (one-eighth MS medium) containing MV (10 µm), H2O2 (1 mm), Cu2+ (100 µm) and Cd2+ (250 µm). Plant material was frozen immediately after the treatment for further analysis.
RNA blot analysis and hybridization probes
Total RNA was isolated according to De Vries et al. (1988). RNA was fractionated on 1.2% agarose gels containing 2.2 m formaldehyde and transferred onto nylon membranes. The filters were hybridized in 5× SSC, 0.1 m PIPES (pH 6.8), 1× Denhardt's solution, 0.1% (w/v) SDS, 50% (v/v) formamide at 42°C. Following hybridization, the filters were washed in 2× SSC, 0.1% (w/v) SDS at 65°C, and autoradiographed. A 870-bp FbaI/EcoRV fragment of the Ath-ALDH3 cDNA (Kirch et al., 2001), a full-length cDNA insert of the rd29A gene (Yamaguchi-Shinozaki and Shinozaki, 1993), or a 650-bp actin cDNA fragment was used as hybridization probes. Probes were labeled with 32P by random oligonucleotide labeling.
Gene-specific RT-PCR
First-strand cDNA was synthesized in 20 µl using Superscript II RNase H– reverse transcriptase (Invitrogen GmbH, Karlsruhe, Germany) and 4 µg total RNA. Two microliters of this assay was used in a 50-µl PCR reaction which contained 5 µl of 10× PCR buffer (200 mm Tris–HCl (pH 8.4), 500 mm KCl), 1.5 µl of 50 mm MgCl2, 1 µl of 10 mm dNTPs, 1 µl each of gene-specific primers (20 pmol µl−1), and 2.5 units of Taq polymerase. The reaction (94°C, 30 sec; 55°C, 45 sec; 72°C, 60 sec) was run for 30 cycles without a final extension. To monitor that equal amounts of cDNA were synthesized, a cDNA fragment of the constitutively expressed actin2 (Accession number U37281) gene was amplified simultaneously in 30 cycles. The primer sequences and predicted amplicon sizes were (forward 5′-CTACTGGATGTGCCTGAAGCATC and reverse 5′-CATGAGTCTTTAGAGAACCCAAAG (463 bp)) for Ath-ALDH3 and ((forward 5′-GCGGATCCATGGCTGAGGCTGATGATATTCAACC and reverse 5′-CGTCTAGACCATGGAACATTTTCTGTGAACGATTCC (1151 bp)) for actin2. The band intensity of the PCR products and the relative expression level of Ath-ALDH3 were quantified by AIDA Image Analyzer version 2.11 (Raytest GmbH, Straubenstadt, Germany).
Determination of Na+ concentrations
Dried plant material (50–100 mg per sample) was mineralized, and sodium concentrations were measured by atomic absorption spectrophotometry (Perkin Elmer AAS 1100, Perkin Elmer Inc., Boston, USA), according to Ernst and Nelissen (2000).
Leaf disc senescence assay
Fully expanded leaves were detached from 4-week-old plants and rinsed briefly with deionized water. Leaf discs of 0.5 cm diameter were excised and floated on paraquat (0, 0.5, 1, 5, 10, and 20 µm) prepared in MS (one-eighth) solution. The treatment was carried out in continuous white light at 22°C for 96 h.
Determination of chlorophyll content
Leaf chlorophyll content was determined spectrophotometrically after extraction of fresh plant material with 80% acetone, as described by Arnon (1949). Absorption of the extracts was measured at 663 and 645 nm.
Lipid peroxidation assay
The thiobarbituric acid (TBA) test, which determines MDA as an end-product, was used to analyze lipid peroxidation (Heath and Packer, 1968; Loreto and Velikova, 2001). Briefly, 0.2 g plant material was homogenized in 4 ml of 0.1% (w/v) TCA solution on ice. The suspension was rinsed into a centrifuge tube with an additional 1 ml of TCA. The homogenate was centrifuged at 10 000 g for 5 min, and the supernatant was collected. One milliliter of 20% (w/v) TCA containing 0.5% (w/v) TBA was added to a 0.5-ml aliquot of the supernatant. The mixture was kept in a boiling water bath for 30 min and then quickly cooled in an ice bath. After centrifugation at 10 000 g for 10 min, the absorbance of the supernatant was measured at 532 and 600 nm. The absorbance at 600 nm was subtracted from the absorbance at 532 nm, and the MDA concentration was calculated using its extinction coefficient 155 mm−1 cm−1 (Heath and Packer, 1968). No significant readings were obtained without addition of the reactive TBA.
Acknowledgements
We would like to thank H. Geithmann for photographic work, B. Eilts for help with transformation, B. Walldorf for technical support, Stefanie Kelbert and Annelie Heuft for constructing the transgenic plants, S. Kotchoni for contributing to some stress experiments, H. Nelissen for measuring Na+ concentrations, and Dr K. Shinozaki for the rd29A clone. S.R. is a recipient of a postdoctoral fellowship from the Alexander von Humboldt foundation.