Metabolic engineering of p-hydroxybenzylglucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor
Summary
Glucosinolates are natural products in cruciferous plants, including Arabidopsis thaliana. CYP79A1 is the cytochrome P450 catalysing the conversion of tyrosine to p-hydroxyphenylacetaldoxime in the biosynthesis of the cyanogenic glucoside dhurrin in sorghum. Both glucosinolates and cyanogenic glucosides have oximes as intermediates. Expression of CYP79A1 in A. thaliana results in the production of high levels of the tyrosine-derived glucosinolate p-hydroxybenzylglucosinolate, which is not a natural constituent of A. thaliana. This provides further evidence that the enzymes have low substrate specificity with respect to the side chain. The ability of the cyanogenic CYP79A1 to integrate itself into the glucosinolate pathway has important implications for an evolutionary relationship between cyanogenic glucosides and glucosinolates, and for the possibility of genetic engineering of novel glucosinolates.
Introduction
Plants respond to environmental challenges and to attacks from herbivores and microbial pathogens by adjusting and defending themselves rather than escaping. Natural products play an important role in these responses, particularly in the chemical warfare between plants and pests. From a human perspective, many natural plant products are high-value compounds with specific biological activities or desirable properties. The wide use of natural plant products in medicine and the food industry, for example, has made them important targets for metabolic engineering ( Dixon et al. 1996 ; Nessler 1994). Glucosinolates, also called mustard oil glucosides, are natural plant products found primarily in the order Capparales, which includes agriculturally important crop plants such as oilseed rape and Brassica cabbages, and the model plant Arabidopsis thaliana. Hydrolysis of glucosinolates by endogenous β-thioglucosidases (myrosinases) generates a complex array of products, e.g. isothiocyanates, nitriles and thiocyanates ( Halkier 1999). Product formation depends on the structure of the side chain, and on external factors such as pH and the presence of Fe2+ ions and epithiospecifier proteins ( Chew 1988). The degradation products have a wide range of biological activities and uses, for example as insect repellents and attractants, anticarcinogens, flavour compounds and bioherbicides.
The more than 100 different glucosinolates identified to date ( Sørensen 1990) are derived from relatively few protein amino acids and their chain-elongated homologues ( Chew 1988; Halkier 1999). Arabidopsis thaliana, for example, synthesizes 23 different glucosinolates, derived entirely from tryptophan and chain-elongated homologues of methionine and phenylalanine ( Hogge et al. 1988 ). The high chemical diversity is obtained by secondary side-chain modifications such as hydroxylations and desaturations. The conversion of precursor amino acid to the corresponding oxime is a key step in the biosynthetic pathway of glucosinolates. In Sinapis alba, Carica papaya and Tropaeolum majus, this step has been shown biochemically to be catalysed by cytochrome P450-dependent monooxygenases ( Bennett et al. 1996 ; Du & Halkier 1996; Du et al. 1995 ).
Cyanogenic glucosides are a related group of amino acid-derived natural products that also have oximes as intermediates ( Møller & Seigler 1999). Studies of the biosynthetic pathway of the tyrosine-derived cyanogenic glucoside dhurrin in Sorghum bicolor has shown that tyrosine is converted to p-hydroxyphenylacetaldoxime by the multifunctional cytochrome P450 CYP79A1, which catalyses two consecutive N-hydroxylation reactions, followed by a dehydration and a decarboxylation reaction ( Sibbesen et al. 1995 ). The oxime is then converted by another cytochrome P450, CYP71E1, to the aglycone p-hydroxymandelonitrile ( Bak et al. 1998a ; Kahn et al. 1997 ) which is subsequently glucosylated by a soluble UDPG-glucosyltransferase to produce dhurrin ( Jones et al. 1999 ; Kahn et al. 1997 ).
Cytochrome P450 monooxygenases are heme-thiolate proteins that receive reducing equivalents from NADPH via NADPH-cytochrome P450-oxidoreductase ( Halkier 1996). The many different cytochromes P450 are typically served by a single NADPH-cytochrome P450-reductase. In A. thaliana two reductase genes have been identified ( Urban et al. 1997 ). Both the cytochromes P450 and the reductases are targeted to the endoplasmic reticulum. Based on available sequence information for A. thaliana, approximately 300 cytochromes P450 are expected in this species ( http://drnelson.utmem.edu/biblioD.html#79A). To date, seven homologues of CYP79A1 have been identified in A. thaliana. In addition, CYP79 homologues have been identified in Brassica napus, S. alba, C. papaya and T. majus ( Bak et al. 1998b ), which represent distantly related families within the order Capparales ( Rodman et al. 1998 ). The biochemical function of these CYP79 homologues is not known, but the amino acid sequence identity to the sorghum CYP79A1 is between 53 and 63%, which makes the CYP79s form a strong phylogenetic group ( Bak et al. 1998b ). The presence of highly conserved CYP79 homologues in the Capparales suggests that the enzymes involved in the conversion of amino acids to oximes in the biosynthesis of glucosinolates and cyanogenic glucosides are evolutionarily conserved ( Bak et al. 1998b ).
The wide range of biological effects of glucosinolates and their degradation products has made tissue-specific control of the level of individual glucosinolates desirable. The biological function of the individual glucosinolates is not well understood. The design of transgenic plants with altered glucosinolate profiles provides an important tool to elucidate the biological functions of these compounds. The present report describes the transformation of A. thaliana with sorghum CYP79A1, which results in the production of high levels of p-hydroxybenzylglucosinolate (p-OHBG) not previously found in A. thaliana. The ability of CYP79A1 to integrate itself into the glucosinolate pathway has numerous implications for the evolution of cyanogenic glucoside and glucosinolate biosynthesis, as well as for the possibility of genetic engineering of novel glucosinolate chemistries.
Results
Expression of sorghum CYP79A1 in A. thaliana
The plasmid pPZP111.79A1 was introduced into A. thaliana by Agrobacterium-mediated transformation, and transgenic plants were selected on kanamycin. Microsomes were prepared from a selected transgenic A. thaliana line in the presence of high amounts of ascorbate in order to prevent hydrolysis of glucosinolates by myrosinases, as glucosinolate degradation products would otherwise inactivate enzymatic activity ( Du et al. 1995 ). In the presence of NADPH, radiolabelled tyrosine was converted by the microsomes to p-hydroxyphenylacetaldoxime ( Fig. 1). No p-hydroxyphenylacetaldoxime was produced by microsomes isolated from control plants. This shows that CYP79A1 is functionally expressed and correctly targeted to the endoplasmatic reticulum. In addition, this shows that endogenous Arabidopsis NADPH-cytochrome P450-oxidoreductase is capable of donating electrons to sorghum CYP79A1 in planta.
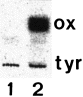
Production of p-hydroxyphenylacetaldoxime in microsomes from transgenic A. thaliana expressing CYP79A1.
1, wild type; 2, transgenic CYP79A1 plants. After incubation of microsomes with radiolabelled tyrosine for 30 min, the reaction mixture was extracted with ethyl acetate and the ethyl acetate phase analysed by thin-layer chromatography. ox, p-hydroxyphenylacetaldoxime.
Transgenic A. thaliana produces p-hydroxybenzyl-glucosinolate
The modification of tyrosine metabolism in transgenic A. thaliana plants expressing CYP79A1 was analysed in vivo by administration of radiolabelled tyrosine to detached leaves. Methanol extracts of the leaves contained large amounts of a tyrosine-derived metabolite that co-migrated with p-OHBG on TLC ( Fig. 2). Radiolabelled p-OHBG in extracts from transgenic A. thaliana migrated slightly differently from the p-OHBG standard on TLC plates ( Fig. 2). The migration of p-OHBG depends on the nature of the counter-ion to the sulfate group of the glucosinolate. The counter-ion to p-OHBG in the transgenic Arabidopsis plants is unknown. When excess amounts of the tetramethyl ammonium salt of p-OHBG (standard) were mixed with radiolabelled p-OHBG from the plant extract, exchange of counter-ions resulted in co-migration of labelled and standard p-OHBG on TLC plates ( Fig. 2). Unambiguous identification of p-OHBG was obtained by GC–MS ( Fig. 3d,e). The GC profile of silylated extracts of CYP79A1 plants ( Fig. 3b) showed a major peak at retention time 25.7 min. The retention time and mass spectrum of the new major metabolite and the p-OHBG standard were identical ( Fig. 3). The ion at m/z 778 corresponded to [M + H]+ for silylated desulfo-p-OHBG, and fragmentation ions that relate to the side chain of p-OHBG were in accordance with the previously published mass spectrum of p-OHBG ( Christensen et al. 1982 ). The peak in the GC profile at 21.7 min originates from sucrose and was found in both CYP79A1 and control plants.
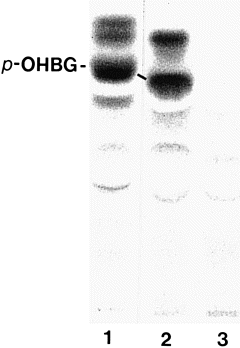
Thin-layer chromatogram of p-OHBG production in leaves of transgenic A. thaliana expressing sorghum CYP79A1.
Detached leaves were incubated with 250 nCi tyrosine for 16 h, followed by solvent extractions as described under Experimental procedures. 1, 2, transgenic CYP79A1 plants; 3, wild type. An excess of the tetramethyl ammonium salt of p-OHBG was added to extracts 2 and 3 before TLC analysis. This resulted in the exchange of the p-OHBG counter-ions enabling co-migration of p-OHBG from A. thaliana with the p-OHBG standard.
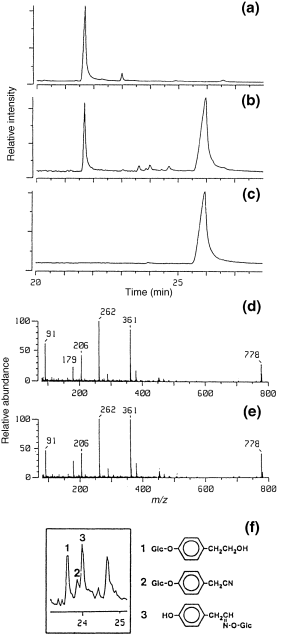
Formation of p-OHBG in transgenic A. thaliana as demonstrated by chemical ionization GC–MS.
(a,b) GC profiles of silylated extracts from wild-type and transgenic A. thaliana expressing sorghum CYP79 A1. The silylated derivatives of endogenous sucrose and p-OHBG have a retention time of 21.7 and 25.9 min, respectively. Compounds 1, 2 and 3 are new, minor metabolites (see f). (c) GC profile of the p-OHGB standard; (d) mass spectrum of peak at 25.9 min; (e) mass spectrum of the p-OHBG standard; (f) expansion of part of GC profile of (b) showing three new, minor metabolites. Compounds 1 and 2 were identified as silylated phenolic glucosides of p-hydroxyphenylethanol and p-hydroxyphenylacetonitrile, respectively. Both compounds were glucosylated at the para position. Compound 3 was p-hydroxyphenylacetaldoxime glucosylated at the oxime function.
Characterization of additional minor metabolites of p-hydroxyphenylacetaldoxime in transgenic A. thaliana
As observed by TLC and GC–MS analysis, p-OHBG was not the only new metabolite in the methanol extracts from the A. thaliana plants expressing CYP79A1, which catalyses the production of p-hydroxyphenylacetaldoxime ( 2, 3). Three minor metabolites were observed in the GC profile ( Fig. 3b,f). Compounds 1, 2 and 3 were identified by comparison of TLC and GC–MS data from untreated extracts and from extracts treated with different glucosidases. Treatment of the extracts with β-glucosidase generated p-hydroxyphenylacetonitrile and p-hydroxyphenylethanol, as evidenced by co-migration with authentic standards in TLC and GC–MS analysis (data not shown). Treatment of the extract with Viscozyme L, a multi-enzyme complex containing a wide range of carbohydrases, released p-hydroxyphenylacetaldoxime in addition to p-hydroxyphenylethanol and p-hydroxyphenylacetonitrile. GC–MS analyses performed in both chemical ionization (CI) and electron impact (EI) mode of methanol extracts before and after treatment with β-glucosidase and Viscozyme L resulted in the identification of compounds 1 and 2 as p-glucoxyphenylethanol and p-glucoxyphenylacetonitrile, respectively, i.e. two compounds that are glucosylated at the para position. The identity of compound 2 as p-glucoxyphenylacetonitrile was confirmed with the retention time and ionization fragmentation pattern of an authentic standard. Compound 3 could be hydrolysed only with Viscozyme L, and was identified as p-hydroxyphenylacetaldoxime glucosylated at the oxime function ( Fig. 3f).
Quantification of p-OHBG in transgenic A. thaliana
At pH < 7, hydrolysis of p-OHBG and indole glucosinolates by endogenous myrosinases releases an unstable isothiocyanate that spontaneously disintegrates into stoichiometric amounts of the corresponding alcohol and SCN– ( Chew 1988; Kawakishi et al. 1967 ). The latter can be quantified colorimetrically ( Epstein 1947). Eighteen independent A. thaliana lines were obtained with a p-OHBG leaf content ranging from 0.2 to 4.7 nmol mg−1 FW (0.06–1.4% of DW) ( Fig. 4). The contribution from endogenous indole glucosinolates to SCN– is indicated in the wild-type control plant. T1 plants with high levels of p-OHBG were smaller and more compact, were delayed in flowering, and had markedly reduced seed production.
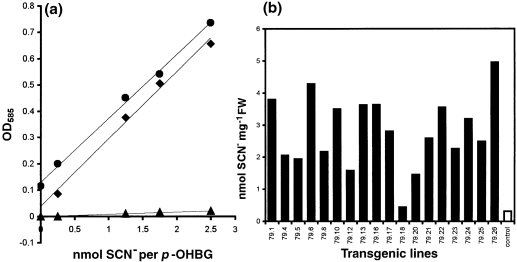
The production of p-OHBG in individual lines of transgenic A. thaliana expressing CYP79A1.
(a) Standard curves of SCN−1 (♦), p-OHBG (▴), and p-OHBG after incubation with an A. thaliana extract (●). (b) p-OHBG was quantified colorimetrically as SCN−1. Two independent leaves were analysed per line. Control plants transformed with the pPZP111 vector show the background level of SCN−1 derived from endogenous indole glucosinolates.
Line 79.1 contained a high level of p-OHBG ( Fig. 4). At the same time this line produced a significant amount of seeds, and so it was brought to homozygosity and used for enzymatic, TLC, GC–MS and HPLC analyses. The level of p-OHBG produced in this line was stable over subsequent generations. Based on kanamycin segregation analysis of T1 plants, this line contains a single insertion.
p-OHBG production does not affect the levels of endogenous glucosinolates
Analysis of the individual glucosinolates, by HPLC analysis of desulfo-glucosinolates revealed that the overall level of glucosinolates in the 79.1 line was increased fourfold compared to control plants grown under the same conditions ( Fig. 5). p-OHBG accounted for approximately 75% of the total level of glucosinolates in the 79.1 line, whereas control plants did not contain this glucosinolate. The production of large amounts of p-OHBG did not alter the level of the major endogenous glucosinolates (B.L. Petersen, unpublished results). As the indole glucosinolate level was not affected, the contribution of SCN– in the transgenic lines ( Fig. 4) reflects the amount of p-OHBG introduced by the expression of CYP79A1 in A. thaliana.
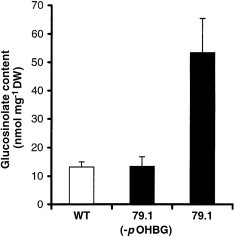
Analysis of glucosinolate content in leaves of wild type and the transgenic 79.1 line.
The columns designated WT and 79.1 (–pOHBG) represent the glucosinolates endogenous to wild-type Arabidopsis (Columbia ecotype). The column designated 79.1 represents the total content of glucosinolates in the transgenic line 79.1, including the novel glucosinolate p-OHBG. The glucosinolate was quantified by HPLC analysis.
Discussion
In the present report we demonstrate that expression of the ‘cyanogenic’ CYP79A1 from sorghum in A. thaliana results in production of high levels of the tyrosine-derived glucosinolate p-OHBG, which is not known to accumulate in wild-type A. thaliana plants. The CYP79A1 enzyme converts tyrosine to p-hydroxyphenylacetaldoxime, which is very efficiently channelled into the biosynthetic pathway of glucosinolates. This is in agreement with previous biochemical data, which showed that the UDPG-thiohydroximate glucosyl transferase and PAPS-desulfo-glucosinolate transferase enzymes in the glucosinolate pathway have a low specificity for the side chain of the oxime (for review see Halkier 1999). In an earlier study, administration of p-nitrobenzaldoxime to a cell suspension culture of Brassica juncea resulted in synthesis of the corresponding artificial glucosinolate ( Grootwassink et al. 1990 ). Our data provide further evidence for the low substrate specificity for the postoxime enzymes.
Introduction of CYP79A1 into A. thaliana resulted in the production of high levels of p-OHBG without alteration of the level and profile of the major endogenous glucosinolates. The fourfold increase in total level of glucosinolates in the selected line 79.1 shows that the postoxime enzymes have the capacity to produce substantially more glucosinolates which, combined with the low substrate specificity, shows significant flexibility in the glucosinolate biosynthetic pathway. By the expression of CYP79A1 we have obtained levels of p-OHBG in leaves of A. thaliana that are comparable to the levels of p-OHBG in the leaves of S. alba, which has p-OHBG as its major glucosinolate. The transgenic A. thaliana lines accumulate up to 4.7 nmol p-OHBG mg−1 FW, compared to S. alba which contains 2 nmol p-OHBG mg−1 FW in young leaves ( Bodnaryk 1994).
Conjugation of glucose to nucleophilic xenobiotics is a general detoxification mechanism in higher plants ( Coleman et al. 1996 ). The minor amounts of the p-hydroxyphenylacetaldoxime that were not channelled into the production of p-OHBG were detoxified and stored as phenolic glucosides of p-hydroxyphenylacetonitrile and p-hydroxyphenylethanol, and as p-hydroxyphenylacetaldoxime glucosylated at the functional oxime group. Formation of p-hydroxyphenylacetonitrile can be accounted for by dehydration of p-hydroxyphenylacetaldoxime. It has previously been shown in tracer studies that unphysiologically high levels of p-hydroxyphenylacetaldoxime are converted into p-hydroxyphenylethanol, probably by a transoximation step involving an α-keto acid, followed by reduction of the aldehyde to alcohol ( Kindl & Schiefer 1971).
The p-hydroxyphenylacetaldoxime produced in situ in the transgenic CYP79A1 plants is efficiently channelled into the glucosinolate pathway, leading to the production of large amounts of p-OHBG compared to the small amounts of compounds derived from detoxification of p-hydroxyphenylacetaldoxime ( Fig. 6). This shows a very tight coupling between the ‘cyanogenic’ CYP79A1 enzyme and the oxime-metabolizing enzyme in the glucosinolate pathway. The ability of CYP79A1 to integrate itself into the biosynthetic pathway of glucosinolates supports the hypothesis that evolutionarily related CYP79 homologues catalyse the conversion of amino acid to oxime in the biosynthetic pathway of glucosinolates and cyanogenic glucosides ( Bak et al. 1998b ), and that biosynthesis of glucosinolates is based on a cyanogenic predisposition with the oxime as a metabolic branchpoint ( Rodman et al. 1998 ). The nature of the oxime-metabolizing enzyme(s) in the glucosinolate pathway is unknown. The tight coupling between sorghum CYP79A1 and the oxime-metabolizing enzyme in the glucosinolate pathway suggests that the latter has evolved from the oxime-metabolizing enzyme CYP71E1 in the cyanogenic pathway. Currently just over 60% of the A. thaliana genome has been sequenced, and an obvious candidate for a CYP71E1 homologue has not yet been identified.

Schematic view of the genetically engineered biosynthetic pathway of p-OHBG in transgenic A. thaliana.
In A. thaliana glucosinolates are derived from tryptophan and chain-elongated homologues of methionine and phenylalanine. Oximes are common intermediates for glucosinolates and cyanogenic glucosides. When CYP79A1, which catalyses the conversion of tyrosine to p-hydroxy- phenylacetaldoxime in the biosynthesis of the cyanogenic glucoside dhurrin, is expressed in A. thaliana, p-hydroxyphenylacetaldoxime is channelled into the pre-existing glucosinolate pathway to produce p-OHBG.
Today’s crop plants have obtained their qualitative and agronomic traits by classical breeding. With molecular techniques it is possible to genetically engineer crop plants with modified agronomic characteristics, for example an altered composition of natural products, in order to improve disease resistance or nutritional/industrial value. In principle any biosynthetic pathway can be manipulated; in practice there are several variables that affect the outcome of a metabolic engineering approach. The variables are due to incomplete understanding of the metabolic networks, and include unidentified feedback controls and targeting of the transgene (and its product) to the appropriate cell or organ. There are few reports on successful metabolic engineering in transgenic plants ( Chavadej et al. 1994 ; Hain et al. 1993 ; Holton & Tanaka 1994; Nawrath et al. 1994 ; Siebert et al. 1992 ; Yun et al. 1992 ). Common to these successful studies on metabolic engineering is that the introduced transgenes encode for enzymes that catalyse the final step in the biosynthetic pathway, resulting in the desired product. The present study differs from the above examples in that sorghum CYP79A1, when introduced into A. thaliana, catalyses the first committed step in the biosynthetic pathway of p-OHBG, i.e. the introduced p-hydroxyphenylacetaldoxime feeds into a ’foreign’ biosynthetic pathway rather than forming an end product.
Cyanogenic glucosides are derived from five protein amino acids (valine, leucine, isoleucine, phenylalanine, tyrosine) and one non-protein amino acid (cyclopentenylglycine) (for review see Møller & Seigler 1999). Glucosinolates are derived from alanine, valine, leucine, isoleucine, tyrosine, phenylalanine and tryptophan ( Halkier 1999). The glucosinolates in the cruciferous plants (Brassicaceae) are derived from only tryptophan and chain-elongated homologues of methionine and phenylalanine. In cyanogenic plants the involvement of cytochrome P450 in oxime production has been shown for both aliphatic and aromatic amino acids ( Møller & Seigler 1999). In glucosinolate-producing plants, CYP79 homologues are thought to catalyse the conversion of amino acid to oxime, as several CYP79 homologues have been identified in distantly related families in the order Capparales ( Bak et al. 1998b ). Production of p-OHBG in transgenic A. thaliana expressing sorghum CYP79A1 demonstrates that it is possible to use genetic engineering for production of Brassica crops with new glucosinolate profiles.
The availability of glucosinolate-producing plants with altered glucosinolate profiles is important for studies of the biological role of individual glucosinolates with respect to pest/insect interactions and nutritional value, and for generation of crop plants with improved disease resistance and/or nutritional value. In another genetic engineering approach for the alteration of glucosinolate profiles, the level of indole glucosinolates were down-regulated in transgenic rape (B. napus) by redirecting tryptophan away from the indole glucosinolate pathway by introducing tryptophan decarboxylase ( Chavadej et al. 1994 ). This resulted in reduction of the indole glucosinolates in the rape seeds to only 3% of wild-type values ( Chavadej et al. 1994 ). An alternative strategy is to modify glucosinolate profiles genetically. Considerable variation in glucosinolate profiles can be found in wild and cultivated forms of the progenitors, e.g. B. napus. This variation can be used in crosses to introduce novel alleles at relevant loci ( Mithen & Toroser 1995). Following this approach, a broccoli plant has been developed that is enriched with the anticarcinogenic 4-methylsulphinylbutylglucosinolate ( Faulkner et al. 1998 ).
In the strategy described here, CYP79A1 was used for alteration of the glucosinolate profile in A. thaliana. CYP79 homologues with different substrate specificities from other glucosinolate-and cyanogenic glucoside-producing plants can be used to introduce novel glucosinolates with a desired biological function in Brassica crop plants. In principle, this approach can produce a new glucosinolate derived from cyclopentenylglycine. In addition, cyanogenic plants may be able to make cyanogenic glucosides derived from tryptophan. In a similar approach, introduction of the oxime-metabolizing cytochrome P450 from cyanogenic plants into glucosinolate-producing plants may channel the many oximes produced in A. thaliana into hydroxynitriles which may become glucosylated into new cyanogenic glucosides.
Experimental procedures
Transgenic plants
A vector construct for introduction of CYP79A1 into Arabidopsis thaliana was designed as follows. CYP79A1 was excised from pBluescript by EcoRI ( Koch et al. 1995 ) and introduced into pRT101 ( Töpfer et al. 1987 ) to introduce a 35S promoter and a CaMV polyadenylation site generating pRT101.79A1. Prior to introduction of CYP79A1, part of the pRT101 polylinker was removed by digestion with SacI and XbaI, blunt-ended with Klenow and religated. CYP79A1, including the introduced 35S promoter and polyadenylation site, was excised from pRT101.79A1 with SphI, blunt-ended and ligated into the blunt-ended and phosphorylated EcoRI site of pPZP111 ( Hajdukiewicz et al. 1994 ). The construct designated pPZP111.79A1 was introduced into Agrobacterium tumefaciens C58C1/pGV3850 by electroporation. Arabidopsis thaliana L. cv. ecotype Columbia was transformed using the vacuum infiltration method. Seeds were germinated on MS medium containing 2% sucrose, 50 mg l−1 kanamycin sulphate and 0.8% agar for 2 weeks, and transgenic plants were selected. Seedlings were transplanted to peat and grown in a controlled environment in an Arabidopsis chamber (Percival AR-60L, Boone, Iowa, USA) with a 12 h photoperiod at a photosynthetic flux of 100–120 mol photons m−2 s−1, 20°C, and 70% relative humidity.
Biosynthetic activity as determined using microsomal enzyme assays
Approximately 0.3 g of mature leaves of homozygotic A. thaliana line 79.1 was homogenized with 300 mg polyvinylpolypyrrolidine and 10 ml isolation buffer A [250 m m Tricine, 250 m m sucrose, 100 m m ascorbic acid, 50 m m NaHSO3, 2 m m dithiotreitol, 2 m m EDTA, 1 m m phenylmethylsulfonyl fluoride, 5 mg bovine serum albumin ml−1, pH 8.2] using a pre-chilled mortar and pestle. The isolation buffer was degassed and flushed with argon three times before use. The homogenate was spun for 10 min at 15 000 g, and the resulting supernatant was spun for 30 min at 100 000 g. The pellet was washed in isolation buffer B (50 m m NaCl, 100 m m Tricine, 250 m m sucrose, 2 m m dithiotreitol, 2 m m EDTA, 1 m m phenylmethylsulfonyl fluoride, pH 8.2) and spun again, and the pellet resuspended in 100 μl buffer B. 37 μl of microsomes were incubated with 25 ng NADPH and 500 nCi L-[U-14C]tyrosine in a total volume of 50 μl at 30°C for 30 min. After incubation the reaction mixtures were extracted with ethyl acetate, and the ethyl acetate extracts analysed by TLC (Silica gel 60 F254, Merck). TLC plates were developed in ethyl acetate/toluene (1 : 5) and radioactive bands were visualized using a STORM 840 phosphor-imager (Molecular Dynamics).
Identification of p-OHBG
250 nCi L-[U-14C]tyrosine were administered to excised leaves of transgenic A. thaliana line 79.1. After incubation overnight at room temperature in a closed test tube within a growth chamber with 12 : 12 h light periods, the leaves were extracted with 1 ml 85% boiling methanol for 2 min. The extracts were concentrated in vacuo to a 50 μl aqueous solution that was clarified by extraction with ethyl acetate. An aliquot of the resulting water phase was applied to TLC plates, which were developed in isopropanol–ethyl acetate–water (7 : 1 : 2). Radioactive bands were visualized as described above. For GC–MS analysis, five to 10 fresh leaves were extracted with 35 ml 85% boiling methanol for 2 min, filtered and concentrated in vacuo to a small aqueous volume that was cleared by extraction with ethyl acetate, and finally lyophilized to dryness.
Quantification of p-OHBG
Detached leaves of transgenic A. thaliana expressing sorghum CYP79A1 were weighed, quickly frozen in 200 μl 50 m m MES pH 6.5, thawed, and incubated at 30°C for 2 h. The freezing and thawing steps serve to degrade the subcellular structure in order to render the glucosinolates accessible to degradative myrosinases. Upon degradation at pH 6.5, p-OHBG releases stoichiometric amounts of SCN−1 ( Chew 1988; Kawakishi et al. 1967 ), which was quantified colorimetrically using a cyanide detection assay ( Epstein 1947). The reaction was stopped by addition of 40 μl 6 N NaOH, and SCN– was determined spectrophotometrically as described for CN– ( Halkier & Møller 1991). p-OHBG was quantified based on NaSCN standard curves, which were identical to standard curves based on myrosinase-treated p-OHBG.
Identification of p-hydroxyphenylacetaldoxime metabolites
Detached leaves of transgenic A. thaliana expressing sorghum CYP79A1 were incubated with 500 nCi L-[U-14C]tyrosine. After 12 h incubation, the leaves were extracted with 35 ml 85% boiling methanol for 2 min. The extract was filtered, lyophilized to dryness and the dry matter resuspended in 300 μl water. After clarification by extraction with ethyl acetate, 50 μl of the extract was digested with 0.1 mg emulsin or 1 μl Viscozyme L (Novo Nordisk A/S) in a total volume of 200 μl 50 m m MES pH 6.5 at 30°C for 1 h. After incubation, the aglucones were extracted into ethyl acetate and analysed by TLC as described above. Aliquots of the ethyl acetate extracts were lyophilized and analysed by GC–MS.
GC–MS analysis
GC–MS analyses were performed on a system consisting of an HP5890 Series II gas chromatograph directly coupled to a Jeol JMS-AX505 W mass spectrometer. An SGE column was used (BPX5, 25 × 0.25 mm, 0.25 μm film thickness, head pressure 100 kPa, splitless injection). The ion source temperature was 200°C in EI as well as in CI mode. In EI mode, 70 eV ionization energy was used. Underivatized aglycones were identified by GC-MS (El mode) using library searching. Carbohydrate-containing extracts were silylated before GC–MS analysis by heating samples to 100°C for 1 h with a 1 : 1 mixture of bis-trimethylsilyl-trifluoro acetamide pyridine containing 1% trimethylchlorosilane. The oven temperature program used was as follows: 80°C for 2 min, 80–200°C at 20°C min−1, 200–300°C at 5°C min−1, 300°C for 10°min.
HPLC analysis
Extraction of glucosinolates from leaves of wild-type and homozygous 79.1 plants was carried out essentially as described by Hogge et al. (1988) . Leaves (1–2 g) were freeze-dried and 100 mg of this material was extracted three times in boiling 70% MeOH. At the start of each extraction 50 nmol benzyl glucosinolate (Sigma) was added as internal standard. The glucosinolates were bound to a DEAE ion-exchange column, subjected to sulfatase treatment, and eluted as desulpho-glucosinolates. HPLC analysis of desulpho-glucosinolates for HPLC was performed on a Shimadzu LC-10Atvp equipped with an LC-ABZ 59142 C18 column (25 cm × 4.6 mm, 5 mm) (Supelco) at a flow rate of 1 ml min−1. The following gradient was applied: (i) de-ionized H2O, 2 min; (ii) a linear gradient from 0 to 60% MeOH, 48 min; (iii) a linear gradient from 60 to 100% MeOH, 3 min; (iv) 100% MeOH, 3 min.
Acknowledgements
We thank Novo Nordisk A/S for the kind gift of Viscozyme L. Desulpho-glucosinolate standards were provided as a generous gift from Dr Niels Agerbirk, Department of Chemistry, The Royal Agricultural University, Denmark. Karina Peitersen is thanked for technical assistance with the manuscript. The work was partially supported by the Center for Plant Biotechnology and the Danish National Research Foundation.