CD14-Mediated Induction of Interleukin-8 and Monocyte Chemoattractant Protein-1 by a Heat-Resistant Constituent of Porphyromonas gingivalis in Endothelial Cells
Abstract
Viable and inactivated Porphyromonas gingivalis dose-dependently induced interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) secretion in human umbilical vein endothelial cells (HUVECs). The inactivated P. gingivalis, in comparison with viable bacteria, tended to enhance the production of both chemokines more strongly. The production of MCP-1 protein began increasing immediately after stimulation by P. gingivalis, and there was a nearly linear increase from 0 to 8 h of incubation, whereas IL-8 production showed a linear increase between 4 and 12 h of incubation. The IL-8 and MCP-1 mRNA expressions in HUVECs as determined by reverse transcriptase-polymerase chain reaction (RT-PCR) or Quantikine mRNA colorimetric quantification kits were found to be enhanced by P. gingivalis. Furthermore, the time courses of IL-8 and MCP-1 mRNA expressions were in accordance with those of protein production. Addition of polymyxin B or boiling did not weaken the stimulatory effect of P. gingivalis, which inhibited the effect of Escherichia coli lipopolysaccharide (E. coli LPS) and tumour necrosis factor-α (TNF-α), respectively. In contrast, the induction of IL-8 and MCP-1 by P. gingivalis was significantly reduced by anti-CD14 antibody. Our results suggest that some heat-stable component of P. gingivalis, including LPS, may be responsible for the induction of IL-8 and MCP-1 in HUVECs by a CD14-dependent mechanism. These effects might be involved in the accumulation and activation of neutrophils and monocytes at an early stage of the periodontal pathogenesis.
Introduction
Periodontitis is a group of diseases resulting from infection by oral micro-organisms, which induces a wide variety of inflammatory reactions in the periodontal tissue of the affected teeth [1–3]. Porphyromonas gingivalis is thought to be one of the important pathogens in adult periodontitis. Many reports have shown that infection with P. gingivalis provokes an inflammatory and immunological response in the process of the disease [4, 5]. The infiltration of leucocytes into the inflammatory site is an important process following infection by micro-organisms. Leucocytes adhere to the endothelial cells and then migrate into the inflammatory site by means of chemotactic factors, and chemokine expression contributes to the presence of different leucocyte populations. Interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) are important pro-inflammatory chemokines involved in the initiation and maintenance of immune and inflammatory reactions [6]. IL-8 exhibits multiple effects on neutrophils, including induction of shape change, release of lysosomal enzymes, generation of bioactive lipids and increase in the expression of adhesion molecules [7–9]. MCP-1 also has multiple functions against monocytes/macrophages, such as inducing intracellular calcium influx and adhesion molecule expression and producing tissue factor and pro-inflammatory cytokines [10–12]. Neutrophils are the first line of immune cells recruited to infected sites; and monocytes secrete large amounts of chemokines when appropriately stimulated. Therefore, it is considered that IL-8 and MCP-1 play an important role at the inflammatory site by regulating the recruitment of specific leucocytes. Accumulated studies have shown that P. gingivalis influences IL-8 and MCP-1 release in monocytes, gingival fibroblasts and epithelial cells [13–15]. However, little is known regarding endothelial cells of blood vessels in terms of IL-8 and MCP-1 expressions following infection with P. gingivalis. Furthermore, recent studies reported identifying P. gingivalis in atherosclerotic vascular tissue by polymerase chain reaction (PCR), and discussed the possible role of P. gingivalis in the development and progression of atherosclerosis and coronary heart disease [16, 17]. Therefore, studies aimed at the inflammatory activation of endothelial cells by P. gingivalis may provide a better understanding of the role of this organism after infection, which may resemble that of other microbial pathogens [18–20]. In the present study, we employed human umbilical vein endothelial cells (HUVECs) to investigate the effect of P. gingivalis on IL-8 and MCP-1 protein release and gene expression.
Materials and methods
Bacteria culture P. gingivalis strains W83 and ATCC 33277 were kindly provided by Dr Hidenori Kaminishi (Fukuoka Dental College, Fukuoka, Japan). Bacteria were grown in brain heart infusion broth supplemented with 0.5% of yeast-extract, hemin (5 mg/l) and vitamin K3 (1 mg/l). Incubation was carried out in an AnaeroPack® anaerobic culture system (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan) at 35 °C for 48 h. The bacterial cells were washed twice with sterile phosphate buffered saline (PBS) by centrifugation at 7000 × g for 10 min, and suspended in PBS at a concentration of 109 CFU/ml. The concentration of bacteria was calculated by a predetermined conversion factor that related the optical density to the number of bacteria, and confirmed retroactively by counting colonies formed on sheep blood agar plates.
Endothelial cell cultures Primary HUVECs were obtained from human umbilical veins by 0.1% of collagenase treatment as described previously by others [21]. The cells were cultured in M199 medium (Nikken Biomedical Laboratory Co., Kyoto, Japan) supplemented with 30 µg/ml of endothelial cell growth supplement (ECGS) (Becton Dickinson, Bedford, MA, USA) and 20% heat-inactivated foetal calf serum (FCS). HUVECs were maintained at 37 °C in a 5% CO2 atmosphere in all experiments. The endothelial origin of the cells was confirmed by an acetylated low-density lipoprotein uptake assay (Biomedical Technologies Inc., Stoughton, MA, USA) as described previously [21–23]. The HUVECs from passage 3 to passage 5 were used in the experiments.
Cocultivation of HUVECs with viable or inactivated P. gingivalis HUVECs were cultured in collagen-coated 12-well plates until a confluent monolayer had been achieved. Prior to the cocultivation, 109 CFU/ml of P. gingivalis suspensions were inactivated by UV irradiation (10 W, at a distance of 10 cm) for 5 min, or by 3 min of sonication (Bioruptor UC-100D, Olympus, Tokyo, Japan) for five cycles. The validity of the treatment was confirmed by the absence of growth on sheep blood agar plates. Serially diluted viable or inactivated P. gingivalis at a concentration of 105–108 CFU/ml was cocultivated with HUVECs in M199 supplemented with 20% FCS (without ECGS). About 1 µg/ml of Escherichia coli lipopolysaccharide (E. coli LPS, serotype O111: B4, Sigma, St. Louis, MO, USA) was used as a positive stimulant. The supernatants were collected after 20 h of cocultivation and were subjected to assay for IL-8 and MCP-1 protein. To investigate the kinetic effect of P. gingivalis, we used UV-treated P. gingivalis at a density of 108 CFU/ml to stimulate HUVECs for various times. The supernatants were collected at 4, 8, 12, 16, and 20 h after cocultivation. The protein amounts of IL-8 and MCP-1 released by HUVECs in the supernatant samples were determined later.
Treatments of P. gingivalis and HUVECs with various inhibitors To investigate the involvement of LPS in the secretion of IL-8 and MCP-1, polymyxin B sulphate (Sigma, St. Louis, MO, USA) was used. About 107 CFU/ml of UV-treated P. gingivalis or 1 µg/ml of LPS was treated with polymyxin B (37 °C, 60 min) at concentrations of 125, 250, 500 and 1000 U/ml before stimulating HUVECs. Furthermore, to examine the effect of boiled P. gingivalis on IL-8 and MCP-1 secretion in HUVECs, P. gingivalis suspended in PBS at a density of 108 CFU/ml was boiled for 15 min and then diluted to 107 CFU/ml in M199 with 20% FCS. About 2000 U/ml of recombinant human tumour necrosis factor-α (TNF-α, Genzyme, Boston, MA, USA) and 10 µg/ml of LPS were treated by the same method as for P. gingivalis, and then 10-fold diluted with 20% FCS M199 medium and also used as working solutions. Anti-CD14 monoclonal antibody MY4 (Beckman Coulter Inc., Fullerton, CA, USA) was used for the inhibition of CD14 binding. HUVECs were pretreated at 37 °C for 30 min with various amounts of MY4 prior to incubation with medium only, LPS (0.1 µg/ml), UV-irradiated P. gingivalis (107 CFU/ml), or TNF-α (200 U/ml). After these treatments of P. gingivalis or HUVECs, HUVECs were stimulated by various stimulators for 20 h, and the concentrations of IL-8 and MCP-1 in the supernatants were determined.
Determination of IL-8 and MCP-1 production The concentrations of IL-8 and MCP-1 in the samples were measured using enzyme-linked immunosorbent assay (ELISA) kits IL-8 (Endogen, Inc., USA) and MCP-1 (BioSource International, Inc., USA) according to the manufacturers' instructions. In brief, the media of samples were collected after cocultivation for the appropriate time. After centrifugation, the supernatants were diluted to 20 times with the diluents provided with the ELISA kits, and the protein amounts of IL-8 and MCP-1 secreted by HUVECs were assayed with the kits. Detection of IL-8 and MCP-1 mRNA expressions in HUVECs was done by reverse transcriptase-polymerase chain reaction (RT-PCR). HUVECs were cultured in collagen-coated 60 mm dishes with M199 medium supplemented with 20% FCS to reach confluent monolayers. UV-treated P. gingivalis at a density of 107 or 108 CFU/ml was added to HUVECs and cocultured for 4 h. Subsequently, total RNA was isolated from the cells in each dish using TRIZOL reagent (GibcoBRL, Rockville, MD, USA) according to the manufacturer's instructions. The isolated RNA was treated with RNase-free DNase prior to use for transcript analysis. The complementary DNA (cDNA) was synthesized from RNA using a cDNA Cycle Kit (Invitrogen, Carlsbad, CA, USA), which uses avian myeloblastosis virus (AMV) reverse transcriptase. The cDNA was amplified with Taq DNA polymerase (Tkara, Otsu, Japan) and specific primers in a thermal cycler (PC-700, ASTEC, Fukuoka, Japan). The PCR conditions were as follows: initial denaturation for 5 min at 94 °C; denaturation for 1 min at 95 °C; annealing for 1 min at 58 °C; extension for 1 min at 72 °C for 30 cycles; and a 10 min elongation step at 72 °C. Primer sequences were the following: IL-8, forward 5′-CTTGGCAGCCTTCCTGATTT-3′, reverse 5′-CTCAGCCCTCTTCAAAAACT-3′; MCP-1, forward 5′-ACTGAAGCTCGTACTCTC-3′, reverse 5′-CTTGGGTTGTGGAGTGAG-3′; glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene, forward 5′-ACCACCTGGTGCTCAGTGTA-3′, reverse 5′-ACCATCTTCCAGGAGCGAGA-3′[24]. PCR products were electrophoresed on 4% agarose gel and visualized by ethidium bromide staining.
Quantifying mRNA of IL-8 and MCP-1 Confluent HUVECs in 60 mm dishes were stimulated with 107 or 108 CFU/ml of P. gingivalis for 1, 3 and 5 h separately. Total RNA was extracted from the cells in each dish using TRIZOL reagent according to the manufacturer's instructions. The concentrations of IL-8 and MCP-1 mRNA of the samples were determined with Quantikine mRNA colorimetric quantification kits (R&D Systems Inc. USA). In brief, 2 µg of the total RNA sample in 150 µl diluent was hybridized with 50 µl of IL-8 or MCP-1 gene-specific biotin-labelled capture oligonucleotide probes and digoxigenin-labelled detection probes in a 96-well microplate in duplicate in a 65 °C water bath for 1 h. About 150 µl of hybridization solution was then transferred to a streptavidin-coated microplate and incubated for 60 min at room temperature on a shaker. After washing, 200 µl of antidigoxigenin conjugate was added to each well and incubated for 60 min. After the unbound conjugate solution had been washed away, 50 µl of substrate solution was added to every well. After another 60 min of incubation, 50 µl of amplifier solution was added and colour was developed for 30 min in proportion to the amount of gene-specific IL-8 or MCP-1 in the original samples. The absorbance at 490 nm with correction of 690 nm was determined following the addition of 50 µl of stop solution. The concentration of IL-8 or MCP-1 mRNA was calculated by interpolation of a standard calibration curve.
Data analysis All experiments were performed in duplicate or triplicate, and the results were analysed using the StatView statistical program (Abacus Concepts, Inc., Berkeley, CA, USA).
Results
Viable or treated P. gingivalis induced the release of IL-8 and MCP-1 in HUVECs
As shown in Fig. 1, live P. gingivalis induced the production of IL-8 and MCP-1 protein in HUVECs in a bacterial dose-dependent manner, although the effect was not as strong as that of 1 µg/ml of E. coli LPS. To determine whether viable P. gingivalis was required for the induction of IL-8 and MCP-1 release from HUVECs, experiments were carried out using inactivated P. gingivalis. As a result, treatment with UV irradiation did not abolish the stimulatory effect of the bacteria on the production of IL-8 and MCP-1. Moreover, even bacterial cell fragments obtained by sonication had the ability to induce both chemokines. The inactivated P. gingivalis, in comparison with the viable bacteria, tended to enhance the production of both chemokines more strongly.
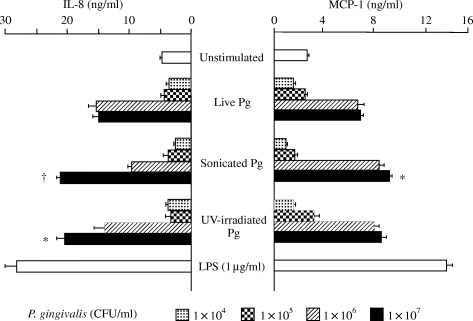
Effect of Porphyromonas gingivalis (Pg) on the production of interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) in human umbilical vein endothelial cells (HUVECs). HUVECs were cocultivated with live or inactivated P. gingivalis for 20 h, and the respective concentrations of IL-8 and MCP-1 in the culture supernatants were determined by enzyme-linked immunosorbent assay. *P < 0.05 and †P < 0.01 compared with live bacteria at the same concentration. P-values were calculated by Student's t-test.
Time-dependent induction of IL-8 and MCP-1 in HUVECs
Time-courses of IL-8 and MCP-1 release in HUVECs following exposure to UV-treated P. gingivalis were investigated (Fig. 2). The production of MCP-1 protein began increasing immediately after incubation, and there was a nearly linear increase from 0 to 8 h of incubation. In contrast, the IL-8 curve shows a linear increase between 4 and 12 h of incubation. The amount of IL-8 protein reached a maximum at 12 h, whereas that of MCP-1 reached a maximum more rapidly at 8 h.
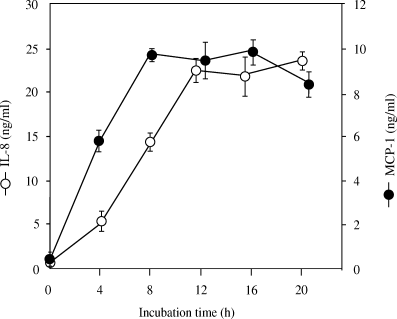
Time courses of interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) production in human umbilical vein endothelial cells (HUVECs) stimulated by Porphyromonas gingivalis. HUVECs were cocultivated with UV-inactivated P. gingivalis (equivalent to 108 CFU/ml), and the culture supernatants were assayed for IL-8 and MCP-1 protein by enzyme-linked immunosorbent assay.
Induction of IL-8 and MCP-1 mRNA expressions in HUVECs stimulated by P. gingivalis
Our RT-PCR results showed that UV-treated P. gingivalis induced the expression of IL-8 and MCP-1 mRNA in HUVECs (Fig. 3). There was a constitutive transcription of IL-8 and MCP-1 even in unstimulated HUVECs. However, the mRNA levels of IL-8 and MCP-1 were further enhanced in the cells stimulated with P. gingivalis for 4 h. Compared with 107 CFU/ml of P. gingivalis, 108 CFU/ml of P. gingivalis induced stronger mRNA transcriptions of IL-8 and MCP-1.
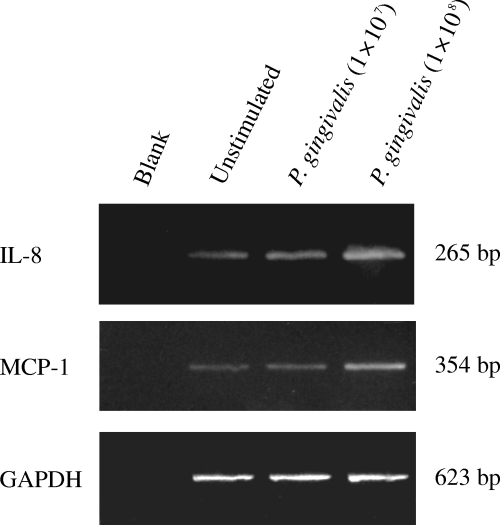
The expression of interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) mRNA in human umbilical vein endothelial cells (HUVECs) stimulated by Porphyromonas gingivalis (reverse transcriptase-polymerase chain reaction (RT-PCR). HUVECs were cocultivated with UV-inactivated P. gingivalis (equivalent to 107 or 108 CFU/ml) for 4 h. Total RNA was extracted from the HUVECs and processed for RT-PCR. Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as housekeeping gene. Blank: no complementary DNA (cDNA) was added to the PCR reaction.
Quantification of IL-8 and MCP-1 mRNA
We quantified the levels of IL-8 and MCP-1 mRNA in HUVECs stimulated by P. gingivalis. The expression levels of IL-8 and MCP-1 mRNA in HUVECs after 4 h of cocultivation with 107 or 108 CFU/ml P. gingivalis were significantly enhanced, in keeping with the results of RT-PCR (data not shown). Additionally, the respective time courses of IL-8 and MCP-1 mRNA expressions in HUVECs were investigated. We obtained differential time courses of IL-8 and MCP-1 (Fig. 4). The expression of MCP-1 mRNA increased immediately after stimulation and peaked at 3 h, whereas the mRNA expression of IL-8 peaked at 5 h or later.
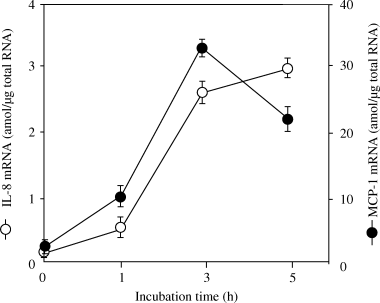
Time courses of the mRNA expression of interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) in human umbilical vein endothelial cells (HUVECs) stimulated by Porphyromonas gingivalis. Total RNA was extracted from the HUVECs, which had been stimulated by 108 CFU/ml of UV-inactivated P. gingivalis for the indicated time, then IL-8 and MCP-1 mRNA levels were determined with a Quantikine mRNA coloriometric quantification kit.
Boiling did not abolish the effect of P. gingivalis on the protein release of IL-8 and MCP-1 by HUVECs
To investigate the involvement of some heat-sensitive molecules, such as a protein in the outer membrane of the bacterium, P. gingivalis was boiled (100 °C, 15 min) before being used to stimulate HUVECs. Boiling did not inhibit the stimulatory activity of P. gingivalis and LPS (Fig. 5). On the other hand, it completely abolished the effect of TNF-α, a heat-sensitive protein.
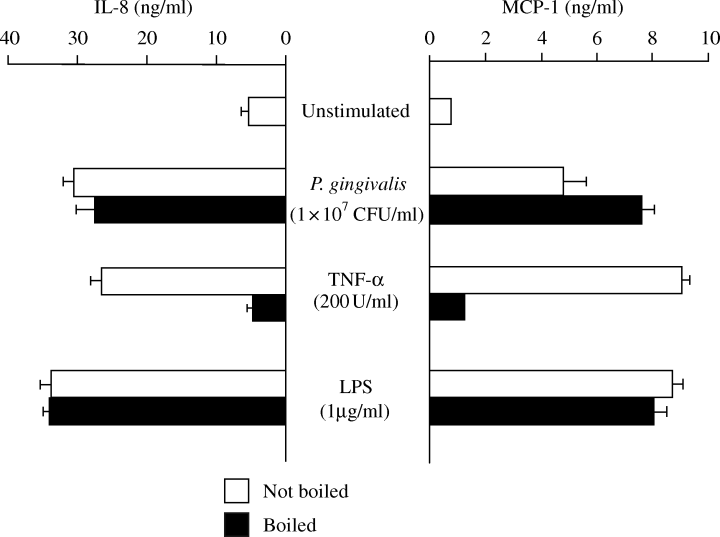
Effect of boiling of Porphyromonas gingivalis on the production of interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1). Human umbilical vein endothelial cells (HUVECs) were incubated with the various conditioned media for 20 h, and the levels of IL-8 and MCP-1 in culture supernatants were determined.
Polymyxin B did not inhibit the effect of P. gingivalis on protein-release of IL-8 and MCP-1 in HUVECs
In order to examine the participation of LPS in enhancing IL-8 and MCP-1 production, UV-inactivated P. gingivalis was treated with polymyxin B before being used to stimulate HUVECs. The polymyxin B treatment strongly inhibited the induction of IL-8 and MCP-1 production by E. coli LPS (Fig. 6). In contrast, polymyxin B had no influence on the stimulatory activity of P. gingivalis, even at high concentrations.
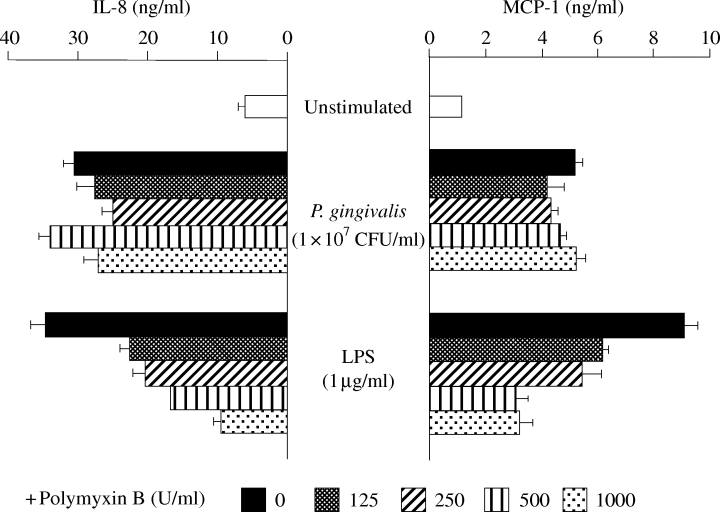
Effect of treatment of Porphyromonas gingivalis with polymyxin B on the production of interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1). The conditioned medium, including UV-inactivated P. gingivalis or Escherichia coli LPS, was treated with polymyxin B (125–1000 U/ml) before being used to stimulate human umbilical vein endothelial cells (HUVECs). The concentrations of IL-8 and MCP-1 in the supernatants after 20 h of stimulation were determined.
P. gingivalis-induced chemokine induction is CD14-mediated
Finally, we examined whether the chemokine-inducing effect of P. gingivalis was mediated by the CD14 pathway. In contrast to the lack of effect of polymyxin B on stimulatory activity, anti-CD14 antibody (MY4) was found to significantly inhibit the production of IL-8 (Fig. 7) and MCP-1 (data not shown).
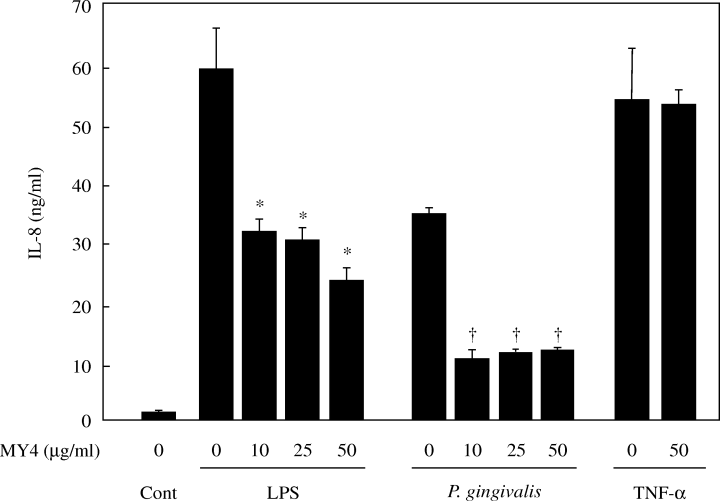
Effect of anti-CD14 antibody (MY4) on IL-8 production by human umbilical vein endothelial cells (HUVECs) stimulated with Porphyromonas gingivalis. HUVECs were pretreated at 37 °C for 30 min with various amounts of MY4 prior to incubation with medium only (Cont), LPS (0.1 µg/ml), UV-irradiated P. gingivalis (107 CFU/ml), or tumour necrosis factor-α (200 U/ml). After incubation for 20 h, the concentrations of IL-8 in the supernatants were determined by enzyme-linked immunosorbent assay. *P < 0.05 and †P < 0.005 compared with the samples treated by the respective stimulator without MY4. P-values were calculated by Student's t-test.
Discussion
The present study has demonstrated that HUVECs increased the IL-8 and MCP-1 expressions at both the protein and the gene level following association with P. gingivalis. This micro-organism has a very strong effect on the induction of both chemokines, comparable with the effect of 1 µg/ml of E. coli LPS. These findings raise the possibility that IL-8 and MCP-1 secreted in endothelial cells may contribute to the recruitment of neutrophils and monocytes from the circulating pool into extravascular tissues in periodontal disease. Indeed, localization of IL-8 and MCP-1 in inflamed gingival tissues has been demonstrated in adult periodontal disease [25–27]. In addition, IL-8 and MCP-1 may be involved in the alveolar bone loss observed in adult periodontal disease. Gamonal et al. reported that periodontal therapy reduced the cell number in the infiltrate and the levels of IL-8 and RANTES (regulated on activation normal T cell expressed and secreted), suggesting a relationship between these chemokines and periodontal status [28]. Therefore, we speculate that P. gingivalis triggered the transmigration of neutrophils and monocytes from blood vessels into periodontal tissue and amplified the inflammatory and immunological reaction by these cells, producing a higher amount of cytokines.
The association of IL-8 and MCP-1 with the pathogenesis of P. gingivalis infection has been reported previously. Jiang et al. demonstrated that peripheral blood mononuclear cells increased the IL-8 and MCP-1 gene expression and protein release after P. gingivalis infection [29]. Steffen and Hanazawa et al. obtained results from gingival fibroblasts similar to those in our study [25, 30]. However, it was found that in the epithelial cells, IL-8 at the protein level was decreased and up or downregulated at the mRNA level following P. gingivalis challenge [31, 32]. The differential response could be related to the function of epithelial cells, which serve to keep the barrier intact. We consider that the increased IL-8 and MCP-1 are a synergistic effect of various cells in periodontal tissue, and the endothelial cells of blood vessels may play a crucial role as they are involved closely in the accumulation and activation of neutrophils and monocytes at an early stage of the inflammatory process.
Our kinetic studies revealed an early increase of gene expression at 1 h and of protein secretion at 4 h of both IL-8 and MCP-1 in HUVECs after P. gingivalis infection. These findings suggest that the induction of both chemokines is a direct result of P. gingivalis and not of the indirect stimulation by inflammatory mediators released by infected endothelial cells via an autocrine mechanism. In addition, the early upregulation of IL-8 and MCP-1 following stimulation by this micro-organism could contribute to the recruitment and retention of neutrophils as a first defence mechanism in periodontal disease. The early response to pathogens may induce a serial amplification of inflammatory and immunological reactions; therefore, IL-8 and MCP-1 secreted by endothelial cells might play a key role in the pathogenesis of periodontal disease.
P. gingivalis has been shown to possess several virulent factors including fimbriae, capsule, protease, LPS and outer membrane structure, peptidoglycans and so on. These factors have been demonstrated to facilitate the destruction of periodontal tissue [33]. Just prior to the completion of the present report, Nassar et al. reported that P. gingivalis fimbriae stimulate a chemokine response in endothelial cells and that live P. gingivalis inhibits the effect by gingipain-mediated mechanisms [34]. Indeed, in our results, the induction of IL-8 and MCP-1 by live bacteria was relatively weak compared with that by inactivated bacteria. However, the stimulatory effect, even that by live bacteria, observed in the present study was considerable. This difference may be owing to differences in experimental procedures, especially with regard to the viability of bacteria. The role of gingipain secreted by P. gingivalis in modulating chemokines may require further investigations. In our study, a P. gingivalis-stimulatory effect on chemokines was observed, even with inactivated or disrupted bacterial cells, suggesting that the stimulatory factor may be some bacterial component(s) rather than soluble factors secreted from live P. gingivalis. The stimulatory effect could not be modified, even by boiling, which was also the case with E. coli LPS. This result implied the participation of P. gingivalis LPS in the stimulatory effect. However, a sufficient dose of polymyxin B, which strongly inhibited the effect of LPS, did not exert any influence on the stimulatory activity of P. gingivalis. It has been demonstrated that polymyxin B inhibits the effect of LPS by binding the lipid A part of the LPS molecule [35], and it could be proposed that lipid moiety is involved in the LPS–endothelial interaction. The chemical structure of lipid A in P. gingivalis LPS has been shown to be different from that of E. coli[36]. Furthermore, unusual properties of P. gingivalis LPS have been demonstrated in terms of endotoxic potency, immunological activity and its signalling pathway [37–40]. To our knowledge, the response of P. gingivalis LPS or lipid A to polymyxin B remains unsettled [39–41]. Therefore, at present, we cannot completely exclude the possibility that P. gingivalis LPS participates in the stimulatory effect. The anti-CD14 antibody experiments suggest that the CD14 pathway may play a crucial role in the mechanism of P. gingivalis-mediated chemokine induction. Previous studies have also demonstrated that human gingival fibroblasts were activated by P. gingivalis through a CD14-dependent mechanism [14, 42]. In our study, membrane CD14 expressed on endothelial cells [43] and/or soluble CD14 in culture medium were considered to be involved in the stimulatory effect of P. gingivalis. Interestingly, it has been reported that serum levels of sCD14 in patients with periodontitis were significantly higher than those in healthy individuals, and that these levels decreased after treatment [44]. Taken together, these findings allow us to speculate that the stimulatory molecules of P. gingivalis are not ordinary heat-sensitive proteins, but rather are heat-resistant molecules including LPS, polysaccharides, lipids and heat-stable proteins. Further experimental work, using P. gingivalis LPS and neutralizing antibodies against Toll-like receptor-2 and -4, are currently underway to clarify the mechanism of P. gingivalis-mediated chemokine induction.
In conclusion, we have shown that P. gingivalis can induce the expression of IL-8 and MCP-1 on endothelial cells. Our observations also suggest that a heat-resistant bacterial constituent may be responsible for the effects observed on endothelial cells, through a CD14-dependent mechanism after stimulation by P. gingivalis. P. gingivalis-mediated upregulation of chemokines could be an important event in the pathogenesis of P. gingivalis-associated infections.
Acknowledgment
This study was supported in part by a grant-in-aid for Scientific Research (C) from the Ministry of Education, Science, Sports and Culture, Japan (No. 12670259 and 13670278), and by a grant from Yakult Honsha Co., Ltd., Tokyo, Japan.




