Macrophages from High-Risk HLA-DQB1*0201/*0302 Type 1 Diabetes Mellitus Patients are Hypersensitive to Lipopolysaccharide Stimulation
Abstract
Levels of nonantigen-induced pro-inflammatory cytokines and prostaglandin in macrophages isolated from human leucocyte antigen (HLA)-matched type 1 diabetes mellitus patients, first-degree relatives and healthy controls were determined. We hypothesize that monocytes isolated from patients are sensitized or preactivated and therefore, have an altered response to in vitro stimulus compared with control groups as measured by levels of pro- and anti-inflammatory mediators. In this study, peripheral blood monocytes were differentiated to macrophages with macrophage-colony stimulating factor (M-CSF) to determine lipopolysaccharide (LPS)-stimulated tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-12 and prostaglandin E-2 (PGE-2) secretion from hetero- or homozygous HLA DQB1*0201 and *0302 type 1 diabetes mellitus patients, first-degree relatives and homozygous HLA DQB1*0602 healthy controls. LPS-stimulated secretion of TNF-α, IL-1β and IL-6 was immediate and markedly higher in the HLA-DQB1*0201/*0302 type 1 diabetes patients compared with all other groups including HLA-matched healthy first-degree relatives. In DQB1*0201/*0302 diabetes patients PGE-2 secretion was delayed but increased by LPS stimulation compared with HLA-matched healthy relatives. IL-12 was not detected at any condition. These data suggest that macrophages from DQB1*0201/*0302 type 1 diabetes patients are sensitized to secrete both cytokines and PGE-2 following nonantigenic stimulation. Sensitized macrophages may be important to high-risk DQB1*0201/*0302-associated type 1 diabetes.
Introduction
The underlying pathology of insulin-dependent type 1 diabetes mellitus is a specific destruction of pancreatic islet β cells by the immune system. At the time of clinical diagnosis the islets of Langerhans are often infiltrated by both macrophages and dendritic cells as well as T and B cells [1–3]. It has been suggested that macrophage infiltration precedes lymphocyte infiltration, and that peripheral blood macrophages have increased levels of tumour necrosis factor (TNF)-α and prostaglandin E-2 (PGE-2) [4–8]. It is speculated that macrophage dysfunction leading to predisposition to autoimmunity is related to the strong susceptibility between type 1 diabetes and human leucocyte antigen (HLA) class II alleles HLA-DQB1*0201 and *0302, or both [9–11]. Thus, antigen-presenting cells (APC) could result that either differ in their ability to phagocyte, process and present antigens in a tolerogenic manner, or are diabetogenic because of cytokine overproduction [11, 12].
Only few studies have focused on the functional participation of APC despite evidence indicating that, e.g., macrophages or macrophage-secreted cytokines are important to initial stages of type 1 diabetes both in humans and in the spontaneous animal models of the disease [3, 13–15]. Indeed, islet inflammation has been demonstrated to be pleomorphic in nature with macrophages being among the first cell types to invade the islets in accordance with detection of TNF-α, interleukin (IL)-1, IL-6, IL-12 and interferon (IFN)-α[1, 9, 16–22]. Both in vitro and in vivo TNF-α, IL-1β and IFN-γ secreted by activated macrophages have been shown to be lethal to rodent islets and β cells, as well as inhibiting human β-cell function [22–24]. Besides pro- and anti-inflammatory cytokines, macrophages also secrete chemotactic proteins and free oxygen radicals that have been detected during islet inflammation [5–7, 14, 16, 18, 21, 25]. An altered secretion of PGE-2 has also been demonstrated [8, 26–28]. This finding is interesting as PGE-2 negatively modulates glucose-induced insulin secretion from isolated islets [29, 30].
The aim of the present study was to differentiate monocytes with macrophage-colony stimulating factor (M-CSF) to macrophages isolated from hetero- or homozygous HLA DQB1*0201 and *0302 type 1 diabetes patients or first-degree relatives and homozygous HLA DQB1*0602 healthy controls. We analysed baseline secretion of TNF-α, IL-1β, IL-6, IL-12 as well as PGE-2, and lipopolysaccharide (LPS)-inducible secretion to test whether type 1 diabetes is associated with altered macrophage function.
Research design and methods
Subjects Subjects donating peripheral blood were volunteers of the Clinical Core of the Diabetes Endocrinology Research Center (DERC), University of Washington, Seattle, USA (Table 1). After informed consent, each volunteer initially donated 10 ml blood for extraction of DNA by proteinase K digestion and phenol/chloroform protein extraction. HLA was typed by a high-resolution polymerase chain reaction (PCR) methodology at the Puget Sound Blood Center, Seattle, USA. Of the 141 HLA-typed subjects, a total of 26 had an HLA-type of interest for our study, i.e. hetero- or homozygous for DQB1*0201 or *0302 (associated with an increased risk for type 1 diabetes mellitus), or homozygous for DQB1*0602 (associated with a minimal risk). Twenty-three subjects participated in the study with a median age of 29 years, a range of 9–53 years, and a female/male ratio of 1 : 3. All participants were screened for GAD65 and IA-2 antibodies. Several 50 ml peripheral blood draws were obtained into EDTA-containing vacutainers from the 23 subjects included in our study. The study was approved by the Institutional Review Board of the University of Washington.
| Subject | Age (year) | Duration (year) | Sex | GAD65 Ab | IA-2 Ab | |
|---|---|---|---|---|---|---|
| Patients | ||||||
| 0201/0302 | D5 | 24 | 4 | M | – | + |
| D77 | 11 | 1 | M | – | + | |
| D81 | 13 | 2 | F | + | + | |
| 0201/0201 | D75 | 43 | 1 | F | + | – |
| D85 | 33 | 1 | F | – | – | |
| D89 | 14 | 0.5 | M | – | – | |
| 0302/0302 | D24 | 10 | 3 | M | – | + |
| D72 | 27 | 2 | F | – | + | |
| D90 | 12 | 0.5 | F | + | + | |
| Relatives | ||||||
| 0201/0302 | R14 | 44 | NA | M | – | – |
| R23 | 46 | NA | F | – | – | |
| R59 | 26 | NA | F | – | – | |
| R79 | 9 | NA | M | – | – | |
| LH15 | 53 | NA | F | – | – | |
| LH13 | 25 | NA | M | – | – | |
| 0201/0201 | R45 | 37 | NA | F | + | – |
| 0302/0302 | R21 | 34 | NA | F | – | – |
| R44 | 37 | NA | F | – | – | |
| R67 | 24 | NA | M | – | – | |
| LH14 | 52 | NA | M | – | – | |
| LH12 | 31 | NA | M | – | – | |
| Controls | ||||||
| 0602/0602 | N0 | 31 | NA | F | – | – |
| N7 | 36 | NA | F | – | – | |
- * Abbreviations: Ab: antibody; NA: not applicable; F: female; M: male; +: antibody positive; –: antibody negative.
GAD65 and IA-2 antibody assay GAD65Ab were detected by a previously described radioimmunoassay (RIA) [31] with recent modifications [32]. Recombinant 35S-GAD65 was produced by in vitro coupled transcription/translation with SP6 RNA polymerase and nuclease treated rabbit reticulocyte lysate (Promega, Madison, WI, USA) as previously described [31]. The in vitro translated 35S-GAD65 was stored at −80 °C and used within 2 weeks. IA-2 antibodies were measured as described [33].
LPS isolation and purification To obtain high-quality LPS that closely resembles the endotoxin infections we are exposed to in vivo, we used a Mg2+-based methodology which results in LPS containing both shorter and longer O-antigen side chains. The LPS used in this study was isolated from virulent Salmonella Typhimurium (gift from Dr Sam I Miller, University of Washington, Strain 14028s from the American Type Tissue Culture, Rockville, MD, USA) as described [34]. The LPS was analysed for purity by isolating the lipid A moiety and inspecting by Delayed Extraction Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry (DE MALDI-TOF MS) using Voyager-Elite software (PE Biosystems, Foster City, CA, USA) [35]. A control external mass calibrant LPS was purchased from Ribi ImmunoChem Research, Inc. (Hamilton, MT, USA).
Isolation of monocytes Monocytes were purified as described [36] and precultured in 0 or 50 ng/ml rhM-CSF (Sigma Chemical Company, St Louis, MO, USA) and 1% foetal calf serum (FCS) (endotoxin concentration = 0.06 ng/ml; Summit Biotechnology, Ft. Collins, CO, USA) for 2 days to generate nonesterase active and pan-major histocompatibility complex (MHC) class II and CD14 positive macrophages (data not shown). At this time point (0 h) macrophages were stimulated with 0, 0.001, 0.01, 0.1, 1 or 10 µg/ml LPS. Culture supernatants were harvested at 0, 6, 18, 24 and 48 h post-LPS stimulation. A total of 23 donors have been used in these experiments (12 relatives: 1 DQB1*0201/0201, 6 DQB1*0201/0302 and 5 DQB1*0302/0302; 9 diabetes patients: 3 DQB1*0201/0201, 3 DQB1*0201/0302 and 3 DQB1*0302/0302; and 2 healthy DQB1*0602/0602 control subjects). Initially, monocytes were stimulated with or without M-CSF and LPS on day 0 and supernatants were harvested on day 1 and 2.
Stability of recombinant cytokines The stability of recombinant human TNF-α, IL-1β and IL-6 purchased from Endogen and Sigma was compared in parallel in three separate experiments with or without 1 × 106 cells/well (isolated from a DQB1*0602/*0602 healthy individual), 50 ng/ml M-CSF and 1 µg/ml LPS. The used concentrations of 1000 pg/ml TNF-α, 200 pg/ml IL-1β and 10 000 pg/ml IL-6 reflected the levels typically found in the supernatants of the main study. Supernatants were harvested at 0, 6, 18, 24, 48, 144 and 216 h and measured for concentration of cytokine as described.
Cytokine and PGE-2 analysis Commercially available enzyme-linked immunosorbent assay (ELISA) kits (Endogen, Woburn, MA, USA) were used to determine TNF-α, IL-1β, IL-6 and IL-12 p70 in culture supernatants isolated from diabetes patients, relatives and healthy controls with sensitivity levels of 10 pg/ml, less than 3 pg/ml and 7 pg/ml and 5 pg/ml, respectively. PGE-2 was determined by an ELISA kit (31 pg/ml sensitivity level; Cayman Chemical, Ann Arbor, MI, USA). All supernatants were tested in triplicate.
Statistical analysis Data are expressed as mean ± SEM. Statistical comparisons were made using paired, two-tailed Student's t-test.
Results
Stability of cytokines
Recombinant human TNF-α, IL-1β and IL-6 from two different vendors were compared to determine the stability of the cytokines over time (Fig. 1). The half-life of TNF-α, IL-1β and IL-6, respectively, was determined to be 22 h, 68 h and more than 216 h (maximal time-span analysed). Approximately the same half-life was found during culture of monocytes stimulated with M-CSF and LPS (Table 2). Neither monocytes, M-CSF nor LPS had any effect on cytokine concentration and stability (data not shown) indicating that IL-6 but not TNF-α and IL-1β is stable in the culture medium.
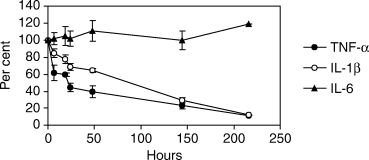
The stability of recombinant human tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 purchased from Endogen, Woburn (MA, USA) and Sigma (St Louis, MO, USA) was compared in parallel in three separate experiments with or without 1 × 106 cells/well (isolated from a DQB1*0602/*0602 healthy subject), 50 ng/ml macrophage-colony stimulating factor (M-CSF) and 1 µg/ml lipopolysaccharide (LPS) (Mean ± SEM). The concentration of TNF-α, IL-1β and IL-6 was 1000 pg/ml, 200 pg/ml and 10 000 pg/ml, respectively, reflecting the respective cytokine levels typically found in the supernatants of the main study. Supernatants were harvested at 0, 6, 18, 24, 48, 144 and 216 h and measured by enzyme-linked immunosorbent assay (ELISA) for concentration of cytokine (pg/ml). The figure shows data obtained using cytokines purchased from Sigma.
| Hours | TNF-α | IL-1β | IL-6 |
|---|---|---|---|
| 0 | 2 ± 2 | 10 ± 4 | 196 ± 66 |
| 6 | 232 ± 52 | 10 ± 4 | 596 ± 145 |
| 18 | 121 ± 23 | 31 ± 12 | 1674 ± 462 |
| 24 | 96 ± 17 | 53 ± 19 | 1915 ± 575 |
| 48 | 42 ± 13 | 5 ± 3 | 3707 ± 1897 |
- * Cytokine secretion determined in culture supernatants harvested from macrophage-colony stimulating factor (M-CSF) generated human macrophages after 6, 18, 24 or 48h LPS-stimulation (Mean pg/ml±SEM). Monocytes were obtained from homozygous human leucocyte antigen (HLA) DQB1*0201 (n = 3) and *0302 (n = 3) type 1 diabetes patients and first-degree relatives (n = 1 and n = 5, respectively), heterozygous HLA DQB1*0201/*0302 patients (n = 3) and first-degree relative (n = 6), and homozygous HLA DQB1*0602 healthy controls (n = 2).
LPS-induced macrophage cytokine secretion
Peripheral blood monocytes isolated from HLA-matched type 1 diabetic patients, first-degree relatives and healthy control subjects were stimulated in vitro with LPS and with or without M-CSF to assess endotoxin-induced cytokine secretion. Initial response to LPS measured by TNF-α, IL-1β and IL-6 after 6 h of stimulation demonstrated that HLA DQB1*0201/*0302 diabetes patients are significantly hypersensitive to this endotoxin (Fig. 2A–C). No difference in cytokine secretion was found between the six homozygous diabetes patients, the six heterozygous and six homozygous relatives, or the two DQB1*0602/*0602 healthy controls (data not shown) tested. At 6 h and maximal LPS stimulation (0.01–1 µg/ml), the concentration of TNF-α, IL-1β and IL-6 in the heterozygous patient group was 646 ± 229 (P < 0.01), 127 ± 61 (P < 0.05) and 1459 ± 375 (P < 0.001) compared with 154 ± 50 (P < 0.01), 13 ± 3 (P < 0.001) and 378 ± 53 (P < 0.001) in all other groups, respectively. By 24 h the homozygous diabetes patients, relatives and controls (data not shown) showed a production of IL-6 similar to the DQB1*0201/*0302 patients with all IL-6 concentrations increasing steadily or remaining constant over time (Fig. 3C). In addition, the levels of TNF-α and IL-1β were comparable at 24 h in culture when patients, relatives and controls (data not shown) showed similar cytokine levels (Fig. 3A and B). M-CSF did not affect the cytokine secretion, and IL-12 was not detectable at any time and culture condition (data not shown).
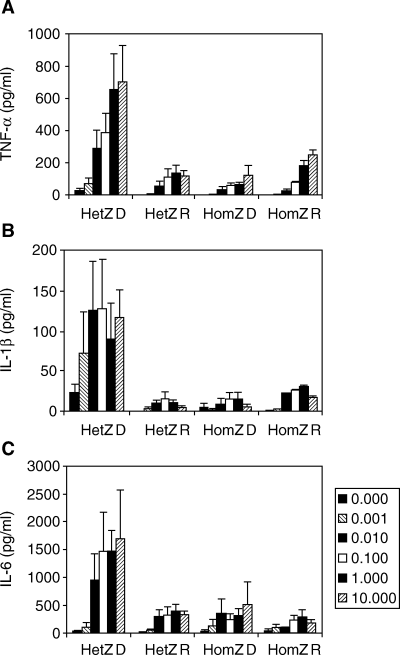
Cytokine secretion determined in culture supernatants harvested from macrophage-colony stimulating factor (M-CSF) generated human macrophages after 6 h lipopolysaccharide (LPS)-stimulation (Mean ± SEM). Monocytes were obtained from heterozygous human leucocyte antigen (HLA) DQB1*0201/*0302 patients (n = 3) and first-degree relatives (n = 6), and six homozygous HLA DQB1*0201 (n = 3) and *0302 (n = 3) type 1 diabetes patients and six first-degree relatives (n = 1 and n = 5, respectively). All cells were stimulated with 0, 0.001, 0.01, 0.1, 1 or 10 µg/ml purified LPS. (A) Secretion of tumour necrosis factor (TNF)-α (pg/ml). (B) Secretion of IL-1β (pg/ml). (C) Secretion of IL-6 (pg/ml). Abbreviations used: HetZ = HLA DQB1*0201/*0302; HomZ = HLA DQB1*0201/*0201 and HLA DQB1*0302/*0302; D = diabetic patient; R = relative.
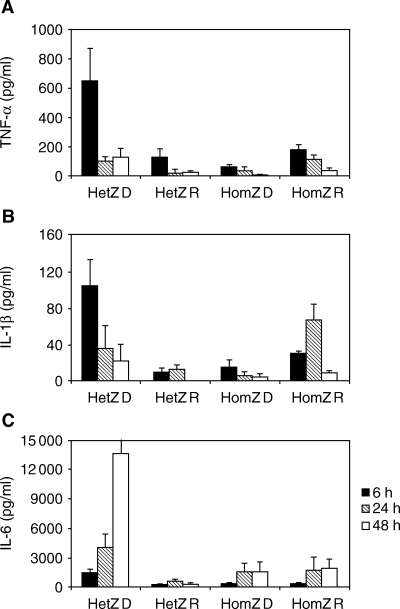
Cytokine secretion determined in culture supernatants harvested from macrophage-colony stimulating factor (M-CSF) generated human macrophages after 6, 24 or 48 h lipopolysaccharide (LPS)-stimulation (Mean ± SEM). Monocytes were obtained from heterozygous human leucocyte antigen (HLA) DQB1*0201/*0302 patients (n = 3) and first-degree relatives (n = 6), and six homozygous HLA DQB1*0201 (n = 3) and *0302 (n = 3) type 1 diabetes patients and six first-degree relatives (n = 1 and n = 5, respectively). Cells were stimulated with 1 µg/ml purified LPS. (A) Secretion of tumour necrosis factor (TNF)-α (pg/ml). (B) Secretion of interleukin IL-1β (pg/ml). (C) Secretion of IL-6 (pg/ml). Abbreviations used: HetZ = HLA DQB1*0201/*0302; HomZ = HLA DQB1*0201/*0201 and HLA DQB1*0302/*0302; D = diabetic patient; R = relative.
Maximum stimulation (Vmax) of TNF-α, IL-1β and IL-6 secretion at 6, 18 and 24 h was obtained with 3.6 ± 5.2 (n = 18), 2.5 ± 4.0 (n = 18) and 1.5 ± 1.9 (n = 16) µg/ml LPS suggesting that the secretion of IL-6 is more easily provoked (Table 3). In most supernatants measured, a bell-shaped dose–response relationship was found with the maximal responses obtained using 0.01–1 µg/ml LPS whereas 10 µg/ml LPS resulted in a lower cytokine response.
| LPS concentration (µg/ml) | |||
|---|---|---|---|
| Hours | TNF-α | IL-1β | IL-6 |
| 6 | 3.02 ± 4.75 | 1.03 ± 1.23 | 1.62 ± 2.08 |
| 18 | 3.88 ± 6.91 | 1.58 ± 2.20 | 1.20 ± 1.38 |
| 24 | 3.96 ± 6.62 | 5.01 ± 8.84 | 1.84 ± 2.33 |
- Concentration of LPS necessary to induce maximal cytokine secretion determined in culture supernatants harvested from macrophage-colony stimulating factor (M-CSF) generated human macrophages after 6, 18 and 24h (Mean±SEM). Monocytes were obtained from homozygous human leucocyte antigen (HLA) DQB1*0201 (n = 3) and *0302 (n = 3) type 1 diabetes patients and first-degree relatives (n = 1 and n = 5, respectively), heterozygous HLA DQB1*0201/*0302 patients (n = 3) and first-degree relative (n = 6), and homozygous HLA DQB1*0602 healthy controls (n = 2).
The baseline secretion of TNF-α, IL-1β and IL-6 for all individuals after a 2-day preculture period with M-CSF in the absence of LPS was 2 ± 2, 10 ± 4 and 196 ± 66 pg/ml (P < 0.01; Table 2). All patients had zero or very low basal cytokine secretion compared with the relatives. All relatives with high-baseline secretion of cytokines were older than 24 years of age whereas no correlation was found to HLA-type, gender or GAD65 and IA-2 antibody positivity (Table 1).
A positive correlation between TNF-α, IL-1β and IL-6 was observed which is consistent with the view that TNF-α exerts effects on both the cellular and the humoral immune response. However, the interpretations of these correlations are complicated by the difference in half-life of the cytokines (Fig. 1).
LPS-induced macrophage PGE-2 secretion
The major finding obtained by analysing the supernatants for the presence of TNF-α, IL-1β and IL-6 was the fact that DQB1*0201/*0302 diabetes patients were hypersensitive to LPS-stimulus (2, 3). These data prompted us to analyse both the three DQB1*0201/*0302 patients and the six first-degree relatives for secretion of PGE-2. After 6 h of LPS stimulation there was no difference in PGE-2 secretion among the subjects (Fig. 4). At higher concentrations of LPS (≥1 µg/ml) and after 24 h incubation, the patients showed increased PGE-2 secretion compared with the first-degree relatives (P < 0.025 and P < 0.05, respectively). This increase was also observed at 48 h of stimulation (P < 0.001 and P < 0.02, respectively; Fig. 4).
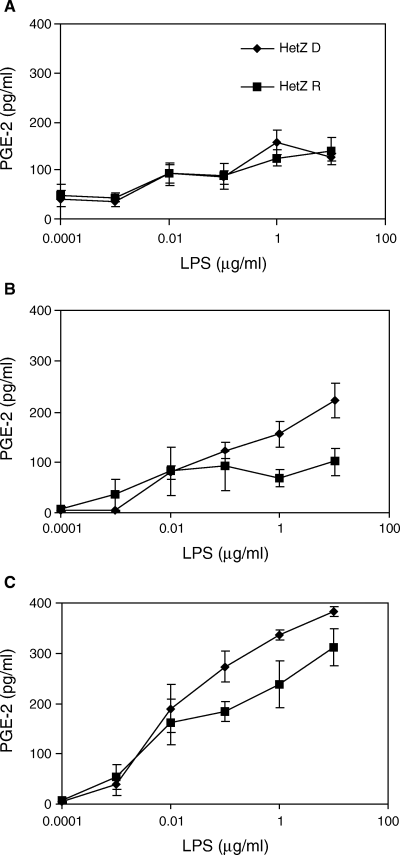
Prostaglandin E-2 (PGE-2) (pg/ml) in culture supernatants harvested from macrophage-colony stimulating factor (M-CSF) generated human macrophages after 6 (4A), 24 (4B) and 48 (4C) hours lipopolysaccharide (LPS)-stimulation. Peripheral blood mononuclear cells (PBMCs) were obtained from heterozygous human leucocyte antigen (HLA) DQB1*0201 and *0302 type 1 diabetes patients (n = 3) and first-degree relatives (n = 6; Mean ± SEM). Abbreviations used: HetZ = HLA DQB1*0201/*0302; D = diabetic patient; R = relative.
Discussion
Several lines of evidence indicate that macrophages may play important roles in the pathogenesis of type 1 diabetes [22]. In our M-CSF generated macrophages, LPS stimulated the secretion of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 but not IL-12. We demonstrate that HLA DQB1*0201/*0302 diabetes patients are hypersensitive to LPS stimulation compared with HLA-matched first-degree relatives and healthy controls. The markedly higher levels of TNF-α, IL-1β and IL-6 relative to all other groups in the DQB1*0201/*0302 patient group suggest that the heterozygous diabetes patients were hypersensitive to LPS. This hypersensitivity is owing to increased production of cytokines and not an increase in basal activity. In fact, one of the important features of our macrophage assay is that 48 h preincubation with M-CSF fully reduces the baseline cytokine secretion to show no difference between subjects. Similarly, the increase in PGE-2 secretion demonstrates that the DQB1*0201/*0302 patient group also has a significantly elevated secretion of this anti-inflammatory lipid derivative. It is noted that this stimulation was delayed to 24 and 48 h incubation. In fact, at 24 and 48 h the secretion of pro-inflammatory TNF-α and IL-1β had decreased markedly. It is possible that this may be owing to downregulation by PGE-2 and in part owing to instability of the cytokines. Our data indicate that patients, but not relatives, expressing the high-risk allele DQB1*0201/*0302 may be hypersensitized to secrete both pro- and anti-inflammatory mediators upon LPS stimulation.
Our results support the possible role of APC dysfunctions in type 1 diabetes. Indeed, numerous reports have indicated APC defects ranging from bone marrow abnormalities in monocyte differentiation [37, 38] to impaired secretion of cytokines presumably deriving from myelopoietic defects [25, 39], increased levels of PGE-2 expression [8] and poor peptide-binding owing to unusual instability of the HLA class II αβ heterodimers. In addition, insufficient support from costimulatory molecules in the spontaneous animal model of the disease, the NOD mouse as well as in humans [12, 40–42]. It has been hypothesized that these defects result in T-cell unresponsiveness, possibly because of important regulatory suppressor T-cell populations that usually hinder the development of diabetes. Alternatively, there may be an induction of otherwise silent, autoreactive T cells [43, 44]. These T cells appear to circumvent deletion early in development, possibly owing to inept interaction with APC in the thymus or periphery. Our data demonstrating high TNF-α, IL-1β and IL-6 secretion upon LPS-stimulation in the heterozygous diabetes subjects is consistent with the possibility that cytokine secretion may affect T-cell proliferation following APC activation. In addition, the increased levels of PGE-2 in the heterozygous patients may have the same effect on T-cell proliferation [8].
The role of macrophages in the β-cell destructive process is altogether multifaceted. Macrophages may not only participate in removing β-cell debris caused by other immune assaults, but may actually contribute to β-cell destruction by an increased secretion of pro-inflammatory cytokines. A defect or decreased rate in the clearance of cell debris and apoptotic cells owing to inept macrophage populations has also been proposed [45]. To this end it is interesting to note that LPS has been reported to have an inhibitory effect on apoptosis suggested to result from an enhanced nitric oxide-mediated bactericidal activity [46]. Our data on increased cytokine secretion from M-CSF-generated macrophages are consistent with the possibility that local cytokine secretion contributes to inhibition of β-cell function, and perhaps β-cell necrosis, apoptosis, or both [22, 24].
Upon LPS stimulation macrophages also synthesize PGE-2 from arachidonic acid under the control of inducible cyclooxygenase. This is illustrated by the data in Fig. 4 showing a linear increase in PGE-2 secretion over time. Increased levels of PGE-2 have previously been reported in diabetes patients and high-risk relatives [8]. We partly confirm their data in this study because we demonstrate that M-CSF generated macrophages from DQB1*0201/*0302 diabetes patients secrete increased amounts of PGE-2 in response to LPS compared with HLA-matched relatives.
PGE-2 seems to exert dual effects on β cells as it has been shown that PGE-2 inhibits insulin secretion, which leads both to an exacerbated hyperglycemia as well as β-cell rest [47]. Furthermore, a higher production of PGE-2 provides a plausible explanation for the increased severity of periodontal disease found in diabetes patients as a result of an increased sensitivity to gram-negative bacterial infections [26–28]. In contrast to the reports on increased levels of LPS-induced PGE-2 in human diabetes, a 10 times lower PGE-2 response to LPS stimulation has been reported in diabetes-prone BioBreeding rats [48]. Whether the increase in LPS-induced PGE-2 secretion over time in type 1 diabetes patients may be owing to an association with PGE-2 cyclooxygenase gene polymorphism remains to be determined.
Our data demonstrating that HLA DQB1*0201/*0302 diabetes patients are hypersensitive to LPS stimulus compared with HLA-matched first-degree relatives is important for the understanding of the multifactorial mechanisms involved in the pathogenesis of type 1 diabetes. Future studies on macrophage dysfunctions among diabetes patients are important to test novel therapeutic approaches for preventing and treating this inflammatory disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK53004 and DK17047), the American Diabetes Association (mentor-based fellowship to AP) and a supplementary grant from Leo and Karen Margrethe Nielsen's Fund for Medical Research.




