afsS is a target of AfsR, a transcriptional factor with ATPase activity that globally controls secondary metabolism in Streptomyces coelicolor A3(2)
Summary
AfsR is a pleiotropic, global regulator that controls the production of actinorhodin, undecylprodigiosin and calcium-dependent antibiotic in Streptomyces coelicolor A3(2). AfsR, with 993 amino acids, is phosphorylated on serine and threonine residues by a protein serine/threonine kinase AfsK and contains an OmpR-like DNA-binding fold at its N-terminal portion and A- and B-type nucleotide-binding motifs in the middle of the protein. The DNA-binding domain, in-dependently of the nucleotide-binding domain, contributed the binding of AfsR to the upstream region of afsS that locates immediately 3′ to afsR and encodes a 63-amino-acid protein. No transcription of afsS in the ΔafsR background and restoration of afsS transcription by afsR on a plasmid in the same genetic background indicated that afsR served as a transcriptional activator for afsS. Interestingly, the AfsR binding site overlapped the promoter of afsS, as determined by DNase I protection assay and high-resolution S1 nuclease mapping. The nucleotide-binding domain contributed distinct ATPase and GTPase activity. The phosphorylation of AfsR by AfsK greatly enhanced the DNA-binding activity and modulated the ATPase activity. The DNA-binding ability of AfsR was independent of the ATPase activity. However, the ATPase activity was essential for transcriptional activation of afsS, probably because the energy available from ATP hydrolysis is required for the isomerization of the closed complex between AfsR and RNA polymerase to a transcriptionally competent open complex. Thus, AfsR turns out to be a unique transcriptional factor, in that it is modular, in which DNA-binding and ATPase activities are physically separable, and the two functions are modulated by phosphorylation on serine and threonine residues.
Introduction
afsR, encoding a 993-amino-acid protein, was cloned from Streptomyces coelicolor A3(2) as a gene that caused Streptomyces lividans to overproduce the pigmented antibiotics actinorhodin and undecylprodigiosin and A-factor (Horinouchi et al., 1983; Horinouchi and Beppu, 1984). The cloned gene ‘awakened’ transcription of the actinorhodin biosynthetic pathway in S. lividans, thus causing the host to produce actinorhodin (Horinouchi et al., 1989). The cloned gene had originally been thought to represent afsB, as it phenotypically complemented an afsB mutation (Horinouchi et al., 1983). Later, Stein and Cohen (1989) found by genetic analysis that the cloned DNA did not correspond to the afsB locus and renamed it afsR. It also induced overproduction of the calcium-dependent antibiotic (CDA) (Horinouchi et al., 1989; 1991). Disruption of the S. coelicolor A3(2) chromosomal afsR gene resulted in significant, but not complete, loss of pigment production. Floriano and Bibb (1996) also observed reduced production of actinorhodin, undecylprodigiosin and CDA in an afsR disruptant. We have thus called afsR a global regulatory gene for secondary metabolite formation.
The N-terminal portion of AfsR shows similarity to ActII-ORF4 (Fernández-Moreno et al., 1991), RedD (Narva and Feitelson, 1990), DnrI (Stutzman-Engwall et al., 1992) and CcaR (Pérez-Llarena et al., 1997), all of which are pathway-specific transcriptional regulators that activate transcription of the genes in the respective gene clusters through DNA binding to specific nucleotide sequences (Wietzorrek and Bibb, 1997). In fact, ActII-ORF4 has been shown to recognize and bind specific regions in the actinorhodin biosynthetic gene cluster, thus activating the transcription of act genes (Arias et al., 1999). DnrI also binds promoter regions in the daunorubicin biosynthetic gene cluster and activates their transcription (Tang et al., 1996). However, afsR cannot substitute for the pathway-specific regulators actII-ORF4 and redD, but may operate independently of these regulatory proteins to influence antibiotic production (Floriano and Bibb, 1996). AfsR was therefore thought to bind some specific DNA sequences and activate their transcription, as a result of which transcription of actII-ORF4 and redD is stimulated, thereby overproducing actinorhodin and undecylprodigiosin.
A particular difference between AfsR and the above-described pathway-specific transcriptional activators is that AfsR contains A- and B-type ATP-binding consensus sequences in the middle of the protein. Furthermore, mutational analyses of the two ATP-binding sequences by site-directed mutagenesis revealed their functional importance, because the mutated afsR genes failed to enhance the production of the secondary metabolites (Horinouchi et al., 1990). Another difference is that AfsR is phos-phorylated on its serine and threonine residues by a protein serine/threonine kinase AfsK (Hong et al., 1991; Matsumoto et al., 1994). We have assumed that phosphorylation of AfsR by AfsK is important for the regulation of secondary metabolism because disruption of either afsR or afsK reduces actinorhodin production (Horinouchi et al., 1990; Matsumoto et al., 1994). However, it was unclear how the ATP-binding motifs and the phosphorylation at serine and threonine residues contribute to the regulatory function of AfsR. We found a clue to understanding their roles by analysing afsS that locates im-mediately 3′ to afsR and encodes a 63-amino-acid protein. Introduction of afsS on a high-copy-number plasmid conferred overproduction of actinorhodin and A-factor on S. lividans, which suggested a functional relationship between AfsR and AfsS (Matsumoto et al., 1995). Vögtli et al. (1994) also reported similar observations using the afsS counterpart, afsR2, in S. lividans; over-expression of afsR2 stimulated actinorhodin production by activating transcription of biosynthetic and regulatory genes in the act gene cluster in S. lividans and also stimulated undecylprodigiosin production. Floriano and Bibb (1996) found that, in S. coelicolor A3(2), afsS also stimulated transcription of actII-ORF4 and biosynthetic genes in the act gene cluster. These observations, together with the adjacent location of afsR and afsS, prompted us to examine a possible relationship between AfsR and AfsS.
We report here that AfsR, as a transcriptional activator, recognizes and binds the promoter region of afsS. Phosphorylation of AfsR enhances its DNA-binding activity. The ATP-binding motifs of AfsR turn out to be essential for its ATPase activity, but not for its DNA-binding activity. AfsR with a modular structure, in which the DNA-binding and ATPase domains are physically separable, is comparable with an enhancer-binding protein NtrC (nitrogen regulatory protein) called a ‘molecular machine’ (Wedel and Kustu, 1995). These in vitro observations are consistent with phenotypes of strains having a mutation in afsS or afsR and those harbouring extra copies of these genes or their mutated genes. Thus, we have identified a target of a transcriptional factor AfsR and revealed the significance of the phosphorylation of AfsR and its ATP-binding motifs. We have also found that the AfsR binding site overlaps the promoter region including the −35 sequence, to which the majority of repressors bind. Although the target of AfsS is still unknown, we discuss the regulation of secondary metabolism by the AfsK–KbpA– AfsR–AfsS system. KbpA is an AfsK-binding protein that inhibits the autophosphorylation of AfsK (Umeyama and Horinouchi, 2001).
Results
Involvement of afsS in actinorhodin production
We observed previously that overexpression of afsS led to overproduction of actinorhodin and undecylprodigiosin in S. lividans (Matsumoto et al., 1995). Floriano and Bibb (1996) also observed that multiple copies of afsS caused overproduction of these pigments in S. coelicolor A3(2) as well as S. lividans. They also found that afsR is a pleiotropic but conditionally required gene for antibiotic production in S. coelicolor A3(2) M145, as the regulatory function of afsR was affected by nutritional conditions, especially by the phosphate concentration. To determine the phenotype of an afsS null mutant, we disrupted the chromosomal afsS gene of S. coelicolor A3(2) M130 by deleting the afsS coding sequence (Fig. 1A). The chromosomal afsR gene was also disrupted by replacing the afsR coding sequence with the kanamycin resistance gene so that the coding region from Ala-43 to Ala-769 was deleted. This construction does not exert a polar effect on afsS, because afsS is transcribed by its own promoter, as described below. Although we previously generated afsR disruptants using phage φC31 KC515 (Horinouchi et al., 1990), these disruptants still expressed an N-terminal portion including the OmpR-like DNA-binding domain. The ΔafsS mutant produced less actinorhodin than the parental strain M130 on TSB agar, but still a detectable amount (Fig. 1B). We used TSB agar because the dif-ference in actinorhodin production was clearer on this medium than on other media such as YMPD and R2YE. Introduction of afsS on a low-copy-number plasmid (plasmid pKU209-afsS) into this mutant restored actinorhodin production to the level of strain M130 (Fig. 1C), indicating that the reduced actinorhodin production re-sulted solely from the afsS mutation. Reduction of undecylprodigiosin production by the ΔafsS mutant was also detected, but to a very small extent. We therefore used actinorhodin production as the phenotype of various mutations in the following experiments. On the other hand, the ΔafsR mutant, as a control, produced no detectable amount of actinorhodin, as was observed for the afsR disruptant generated with φC31 KC515 (Horinouchi et al., 1990; Floriano and Bibb, 1996). afsR on the low-copy-number plasmid (plasmid pKU209-afsR) restored actinorhodin production in the ΔafsR mutant.

Reduced actinorhodin production by an afsS null mutant of S. coelicolor A3(2).
A. Gene organization of the afs loci of mutants ΔafsS and ΔafsR and their parental strain M130. In mutant ΔafsS, the whole afsS coding sequence is deleted. In ΔafsR, the coding sequence from Ala-43 to Ala-769, which contains an OmpR-like DNA-binding fold and two ATP/GTP-binding motifs, is replaced by the kanamycin resistance gene. Abbreviations for restriction enzymes are: B, BalI; Bm, BamHI; F, FbaI; and H, HpaI.
B. Actinorhodin production by strains M130, ΔafsS and ΔafsR. The three strains were grown on TSB agar at 30°C for 6 days.
C. Complementation of the afsS and afsR mutations by the respective genes on a low-copy-number plasmid pKU209. The vector plasmid causes no detectable changes in actinorhodin production. The strains were grown on TSB agar at 30°C for 6 days.
D. Stimulation of actinorhodin production by multicopies of afsS and afsR in both ΔafsS and ΔafsR genetic backgrounds. The amount of actinorhodin produced by mutant ΔafsS harbouring pIJ486-afsR is apparently larger than any other strains tested. The strains were grown on TSB agar at 30°C for 6 days.
Both afsR and afsS caused S. coelicolor A3(2) M130 to overproduce actinorhodin when introduced on a high-copy-number plasmid (data not shown), as observed by Floriano and Bibb (1996). We introduced these genes into the ΔafsS and ΔafsR mutants to determine the possible hierarchy in the regulatory pathway. Multicopies of afsS (plasmid pIJ702-afsS) caused the ΔafsR mutant to produce almost the same amount of actinorhodin as strain M130 (Fig. 1D), indicating that afsS stimulated actinorhodin production in the absence of the intact AfsR protein. Similarly, multicopies of afsR (plasmid pIJ486-afsR) stimulated actinorhodin production in the ΔafsS background because mutant ΔafsS harbouring pIJ486-afsR produced a greater amount of actinorhodin than strain M130. The greater stimulatory effect of afsR compared with afsS was also observed when these were introduced in S. coelicolor A3(2) (afsS+) (Floriano and Bibb, 1996). These in vivo results did not reveal the hierarchy of afsR and afsS in the regulation of actinorhodin biosynthesis, although the following in vitro experiments showed that afsS is just downstream from afsR in the regulatory hierarchy.
The apparently independent regulation by afsS and afsR of actinorhodin production contrasts with the observations made by Floriano and Bibb (1996). They observed no stimulation by afsS of actinorhodin production in an ΔafsR background and concluded that afsS depended on afsR for its stimulatory properties in S. coelicolor A3(2). The discrepancy may result from the difference in genetic background between the two strains, as discussed below. However, in S. lividans, multicopies of afsR2 led to overproduction of actinorhodin even when most of the chromosomal copy of the afsR gene was deleted, which suggested that the stimulation of actinorhodin production by afsR2 does not require the intact AfsR function (Vögtli et al., 1994).
Control of afsS transcription by afsR
In prokaryotes, functionally related genes are generally found organized together as an operon. We examined possible control of afsS transcription by AfsR. To obtain an overall picture of transcription of the genes probably related to afsS, we first determined transcription of afsS, afsR and afsK in different genetic backgrounds such as the parental strain M130, ΔafsR and ΔafsS (Fig. 2). mRNA was prepared from cells grown on cellophane on the surface of agar medium. Under these conditions, the three strains grew as substrate mycelium at day 1, as a mixture of substrate and aerial mycelium at day 2 and as a mixture of aerial mycelium and spores at day 3. At day 2, a blue pigment actinorhodin became detectable. hrdB encoding a σ factor of RNA polymerase that is transcribed throughout growth was used to monitor the quantity and quality of the mRNA used. Transcription of afsK was not affected by the ΔafsR or ΔafsS mutations. Similarly, transcription of afsR was not affected by the ΔafsK or ΔafsS mutations. afsK and afsR had two and a single transcriptional start points respectively. The transcriptional start point of afsR was 28 nucleotides (nt) upstream of the start codon, as determined previously (Horinouchi et al., 1990). afsK had two promoters: the transcriptional start points were 92 and 51 or 52 nt upstream of the start codon, as determined by high-resolution S1 mapping (data not shown). On the other hand, afsS transcription was almost completely abolished in mutant ΔafsR and was delayed in mutant ΔafsK. In the ΔafsR mutant harbouring pKU209-afsR, afsS was transcribed to the same extent as in strain M130 (data not shown), as was observed in ΔafsR harbouring pIJ486-afsR (see below, Fig. 10B). The loss of afsS transcription in ΔafsR and the delay in ΔafsK were always observed in repeated experiments. In the parental strain M130, transcription of afsS was observed until day 3 with a peak at day 2, although the afsS transcript was not detected clearly at day 3 in this particular experiment (the average pattern of afsS transcription is seen in Fig. 10B). We therefore concluded that afsS transcription was under the control of afsR.

Low-resolution S1 nuclease mapping of afsS in various genetic backgrounds. The autoradiograms shown represent the average transcription patterns that were observed in several independent experiments. First and second blots: afsK and afsR are constantly transcribed in mutants ΔafsS and ΔafsR, as in the parental strain M130. Third blot: no transcription of afsS was detected in ΔafsR, suggesting transcriptional control of afsS by AfsR. Transcription of afsS at day 1 in mutant ΔafsK is low. Fourth blot: hrdB that encodes σHrdB and is transcribed throughout growth was used to monitor the amount and quality of the mRNA used.

No ability of the AfsR mutant without ATPase activity to activate transcription of afsS.
A. Inability of afsRΔATPase to restore actinorhodin production in mutant S. coelicolor A3(2) ΔafsR. Mutant ΔafsR harbouring pIJ486, pIJ486-afsR or pIJ486-afsRΔATPase was grown on TSB agar at 30°C for the indicated periods, together with strain M130 harbouring pIJ486 as a control. afsR on pIJ486 restored actinorhodin production in mutant ΔAfsR, whereas afsRΔATPase did not.
B. Inability of afsRΔATPase to activate afsS transcription in mutant S. coelicolor A3(2) ΔafsR, as determined by low-resolution S1 mapping. RNA was prepared from cells grown at 30°C for the indicated periods on TSB agar. As a control, the afsS transcription pattern in strain M130 is shown. afsR on pIJ486 restored afsS transcription in mutant ΔafsR, whereas afsRΔATPase did not.
We determined the transcriptional start point of afsS by high-resolution S1 mapping (Fig. 3A). The start point was determined to be the C residue 143 nt upstream of the start codon, as the fragments generated by the chemical sequencing reactions migrate 1.5 nt further than the corresponding fragments generated by S1 nuclease digestion of the DNA–RNA hybrids (half a residue from the presence of the 3′-terminal phosphate group and one residue from the elimination of the 3′-terminal nucleotide) (Sollner-Webb and Reeder, 1979). Vögtli et al. (1994) assigned the transcriptional start point of afsR2 in S. lividans to be the G 142 nt upstream of the start codon by primer extension. The TTCAGC sequence indicated in Fig. 3B is somewhat similar to the −35 consensus sequence, TTGACA, found in many bacteria including Streptomyces (Strohl, 1992). The CACTGT sequence is also similar to the −10 consensus sequences, TATAAT in many bacteria or TAGRRT (R: A or G) in Streptomyces. The two promoter elements are somewhat deviated from the promoter elements of housekeeping genes, and it is not clear which σ factor is responsible for transcription of afsS. Furthermore, the spacing between the −35 and −10 sequences is 20 bp, which is longer than the standard spacing, 17–18 bp (Strohl, 1992).

High-resolution S1 mapping of afsS and RT–PCR analysis of the afsR–afsS region.
A. RNA prepared from S. coelicolor A3(2) M130 grown at 30°C for 3 days on TSB agar was used. The arrowhead indicates the position of the S1-protected fragment, and the transcriptional start point is assigned to the C residue indicated by the arrow.
B. Nucleotide sequence of the promoter region of afsS. This fragment of 360 bp was used for all the gel mobility shift assays. Probable –35 and –10 sequences are indicated. The DNA sequence protected by DNase I digestion (see Fig. 5) is shown by thick lines.
C. RT–PCR for detection of specific mRNA. Four pairs of primers (RS-1, R, S and RS-2) to detect the specific mRNAs were subjected to RT–PCR, and the products were run on agarose gel electrophoresis. With primers R and S, amplified DNA fragments of 420 bp and 200 bp, respectively, are seen. With primers RS-1 or RS-2, no amplification occurs. No amplification occurred when reverse transcriptase (RT) was omitted from the reaction mixture, indicating no contamination of DNA in the mRNA samples. Size markers are HindIII-digested λ DNA (λ) and HincII-digested φC174 DNA (φ).
We examined possible transcriptional readthrough from the promoter of afsR into the afsS gene by reverse transcription–polymerase chain reaction (RT–PCR) (Fig. 3C). A pair of primers to detect mRNA for afsR (lane R) and afsS (lane S) yielded amplified DNA fragments with expected sizes. However, a pair of primers to detect the mRNA covering both afsR and afsS yielded no amplified fragment of 1000 bp (lane RS-1). Another pair of primers to detect a shorter mRNA covering both genes did not yield any amplified fragment (lane RS-2). These results indicated that no readthrough from afsR into afsS occurred.
Binding of AfsR to the upstream region of afsS
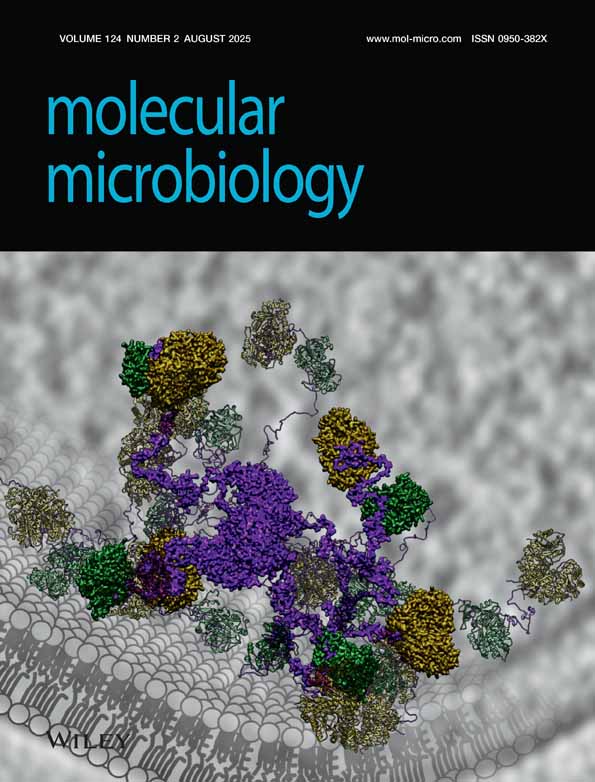
Because AfsR was suggested to be a DNA-binding protein containing an OmpR-like DNA-binding fold (Wietzorrek and Bibb, 1997) and because ActII-ORF4 showing similarity in amino acid sequence to the N-terminal portion of AfsR bound specific sequences in the act gene cluster (Arias et al., 1999), we examined the possibility of AfsR binding to the promoter region of afsS by gel mobility shift assay. afsR was placed in pET16, and AfsR was produced in the soluble fraction of Escherichia coli cells as a fusion protein with a structure of Met–Gly–His10–Ser2–Gly–His–Ile–Glu–Gly–Arg–His– AfsR (1014 amino acids, 108 kDa). The fusion protein (H-AfsR) was purified to near homogeneity with a Ni-NTA column (Fig. 4A). H-AfsR thus prepared from E. coli cells was an unphosphorylated form. As a promoter region of afsS, a 360 bp fragment from positions −178 to +182, with respect to the transcriptional start point of afsS (Fig. 3B), was used. A mobility shift, indicative of H-AfsR binding to the DNA, was detected when more than 3 μg of H-AfsR was used (Fig. 4B). In addition, the binding of H-AfsR to the DNA was not clearly competed even by a ×800 amount of cold probe. The gel mobility shift assay we used was a standard procedure for the detection of DNA–protein interaction and, under these conditions, we detected, for example, AdpA binding to a target DNA with only 0.04 μg of protein (Yamazaki et al., 2000) and ArpA binding to a target DNA with 0.5 μg of ArpA (Onaka et al., 1997). The low binding ability of H-AfsR resulted from neither the attachment of the histidine tag to its N-terminus nor some conformational change in H-AfsR, if any, produced in E. coli cells, but from the unphosphorylated form of H-AfsR. As described below, phosphorylation of H-AfsR greatly enhanced its DNA-binding activity.
Binding of H-AfsR to the promoter region of afsS, as determined by gel mobility shift assay.
A. Histidine-tagged AfsR was produced in the soluble fraction of E. coli cells harbouring pET16-afsR and purified with an Ni-bind column. Purification was monitored by SDS–PAGE. Lane 1, molecular size markers; lane 2, soluble fraction; lane 3, the first eluate in a total of 200 μl from the Ni column that had been washed thoroughly; and lane 4, the second eluate in a total of 200 μl. The second eluate was used for gel mobility shift assays.
B. Gel mobility shift assay with the purified H-AfsR and 32P-labelled 360 bp DNA fragment at nucleotide positions from +182 to –178 with respect to the transcriptional start point of afsS. The positions of H-AfsR-bound (solid triangle) and free (open triangle) probes are shown. The amounts of H-AfsR were 0, 1, 3, 5, 7, 10 and 14 μg for lanes 1–7 respectively. Binding of 14 μg of H-AfsR was examined in the presence of an excess amount (×800) of unlabelled probe (lane 8).
Determination of the AfsR binding site in afsS
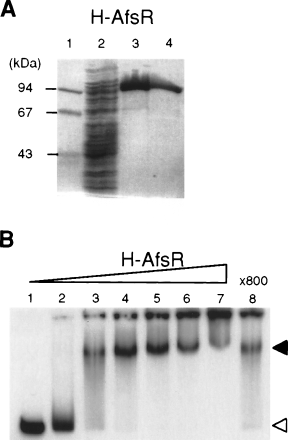
The AfsR binding site was predicted to be in positions −178 to +182, with respect to the transcriptional start point of afsS. We prepared 32P-labelled DNA probe of positions −288 to +52 to determine the AfsR binding site by DNase I footprinting (Fig. 5). H-AfsR protected a sequence, from positions −20 to −42, of the antisense strand from DNase I digestion. A similar DNase I footprinting assay showed that a sequence, from positions −40 to −15, of the sense strand was protected. The −35 and −10 sequences of promoters are the sites bound by the majority of repressors (discussed below).
Analysis of AfsR binding to afsS by DNase I protection assay. The DNase I digests were run with the same probes that were chemically cleaved (G + A lane). The amounts of H-AfsR were 0.1 μg (lane 2), 1 μg (lane 3), 3 μg (lane 4) and 6 μg (lane 5). Lanes 1 and 6 are control lanes without DNase I. The antisense strand from positions –20 to –42 with respect to the transcriptional start point of afsS and the sense strand from positions –40 to –15 are protected from DNase I digestion.
Phosphorylation of AfsR enhances its DNA-binding activity
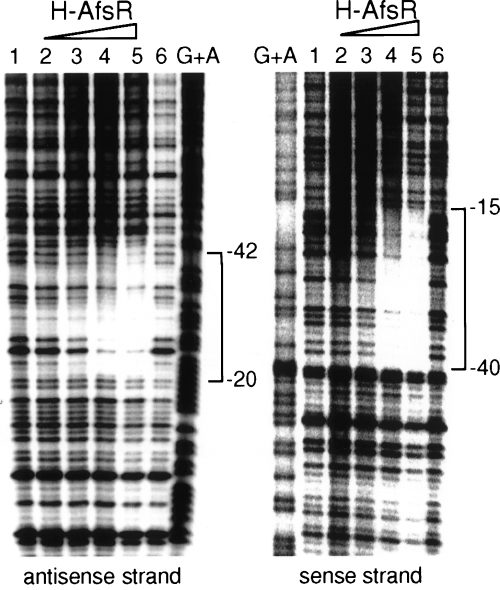
AfsR is phosphorylated on its serine and threonine residues by AfsK (Matsumoto et al., 1994). To assess the effect of phosphorylation of AfsR on its DNA-binding activity, we prepared the phosphorylated form of H-AfsR by in vitro phosphorylation of H-AfsR with TRX-KΔCwt. TRX-KΔCwt (478 amino acids, 51 kDa) contained the kinase catalytic domain (Met-1 to Arg-311) of AfsK fused to thioredoxin and was capable of phosphorylation of serine and threonine residues of AfsR (Umeyama and Horinouchi, 2001). We monitored the in vitro phosphorylation of H-AfsR by TRX-KΔCwt by adding [γ-32P]-ATP to 1/20th the amount of the reaction mixture (Fig. 6A). Although a small population of TRX-KΔCwt produced in E. coli cells was an autophosphorylated form (Umeyama and Horinouchi, 2001), the in vitro reaction without H-AfsR yielded a newly autophosphorylated form (Fig. 6A, lane 1). The addition of H-AfsR to the reaction mixture containing the autophosphorylated form of TRX-KΔCwt yielded a phosphorylated form of H-AfsR (Fig. 6A, lane 2). Phosphoamino acid analysis (Kamps and Sefton, 1989) of the HCl-hydrolysed product of 32P-labelled H-AfsR-P showed that it contained phosphoserine and phosphothreonine (data not shown). After the phosphorylation reaction on a large scale, the mixture was passed through a Nanosep 30K filtration device with a membrane to remove small molecules. Because of the small size of TRX-KΔCwt in comparison with H-AfsR, a considerable portion of TRX-KΔCwt was removed. We used the material that was retained on the membrane as the source of phosphorylated H-AfsR (H-AfsR-P). The sample was expected to contain only a small population of the phosphorylated form of H-AfsR, as the in vitro phosphorylation of AfsR by AfsK yielded a very small population of the phosphorylated form of AfsR (Umeyama et al., 1999; Umeyama and Horinouchi, 2001).
Enhancement of DNA-binding activity of AfsR by phosphorylation.
A. Phosphorylation of H-AfsR by TRX-KΔCwt. Proteins were separated by SDS–PAGE, and the gel was dried and analysed on an image analyser. Autophosphorylation of TRX-KΔCwt (lane 1) and phosphorylation of H-AfsR by TRX-KΔCwt (lane 2) are apparent. TRX-KΔCK44A with an amino acid replacement at Lys-44 by Ala failed to autophosphorylate (lane 3) and to phosphorylate H-AfsR (lane 4).
B. Gel mobility shift assay with H-AfsR-P and the promoter region of afsS as 32P-labelled probe. After in vitro phosphorylation of H-AfsR by TRX-KΔCwt, the reaction mixture was passed through a membrane filtration system to remove small molecules. The H-AfsR-P sample thus prepared was used for gel mobility shift assay. The positions of H-AfsR-P-bound (solid triangle) and free (open triangle) probes are shown. The amounts of proteins in the sample were 0, 0.1, 0.3, 0.5 and 1.5 μg for lanes 1–5 respectively. Lanes 9 and 10 show the mobility shift by 2 and 5 μg of protein respectively. Lane 11 shows competition against binding of 5 μg of protein and the probe by an excess amount (×500) of unlabelled probe. As a control, the H-AfsR sample was similarly prepared by incubation with TRX-KΔCK44A and passage through the membrane. The amounts of proteins in the H-AfsR sample were 0.3, 0.5 and 1.5 μg for lanes 6–8 respectively.
A similar gel mobility shift assay with the H-AfsR-P sample and the promoter region of afsS revealed ap-parent DNA–protein binding with 0.3–0.5 μg of protein (Fig. 6B, lanes 3 and 4). The strong retarded signal observed with 5 μg of protein (Fig. 6B, lane 10) was lost when competed with a × 500 amount of cold probe (Fig. 6B, lane 11). We can safely say that H-AfsR-P in a very small amount causes a mobility shift, because the protein sample contained only a small population of the phosphorylated form of H-AfsR-P as well as TRX-KΔCwt. To exclude the possibility that TRX-KΔCwt in the protein sample affects the binding between H-AfsR-P and the DNA, we prepared the protein sample similarly using TRX-KΔCK44A and used this for the gel mobility shift assay (Fig. 6B). TRX-KΔCK44A had an amino acid replacement at the catalytic Lys-44 residue by Ala and lost the ability to autophosphorylate (Fig. 6A, lane 3) or to phosphorylate H-AfsR (Fig. 6A, lane 4). The gel mobility shift assay with this sample (0.3–1.5 μg of protein; Fig. 6B, lanes 6–8) showed almost no retarded signal, in agreement with the idea that H-AfsR itself in the phosphorylated form shows strong DNA-binding activity. In addition, a single retarded signal and the same position of the signal for H-AfsR and H-AfsR-P suggest that phosphorylation of AfsR causes no induction of multimerization upon binding or no binding to additional DNA sequences.
ATPase and GTPase activity of AfsR
One of the characteristic features of AfsR is the presence of the A- and B-type ATP/GTP-binding motifs in the middle part. We noticed the importance of these motifs previously from the observation that mutant AfsR proteins with site-directed mutations at these sites had no ability to stimulate actinorhodin production in S. lividans (Horinouchi et al., 1990). The nucleotide-binding domain of AfsR showed similarity in amino acid sequence to ATPase domains of transcriptional factors (Fig. 7). We therefore examined possible ATPase and GTPase activities of AfsR. As a negative control, we constructed pET16-afsRΔATPase, which directed the synthesis of H-AfsRΔATPase with amino acid replacement at four positions, Gly-335 to Phe and Lys-336 to Glu in the A-type ATP/GTP-binding motif and Leu-412 to Gln and Asp-413 to Asn in the B-type ATP/GTP-binding motif (Fig. 8A). We also constructed pET16-afsRΔC, which directed the synthesis of H-AfsRΔC (291 amino acids, 32 kDa; containing the N-terminal portion of AfsR from Met-1 to Ala-270) with a similar structure to H-AfsR but with a large C-terminal truncation. These proteins were purified to homogeneity with Ni-bind resin from the soluble fraction of E. coli cells. Figure 8B shows SDS–PAGE of the H-AfsRΔATPase and H-AfsRΔC proteins. As expected, H-AfsR showed distinct ATPase and GTPase activities (Figs 8C and D). H-AfsR preferred ATP as a substrate to GTP. However, H-AfsR showed no hydrolysing activity towards CTP or TTP. H-AfsR is not a phosphatase because it released no phosphate when ADP was used as a substrate. On the other hand, H-AfsRΔATPase or H-AfsRΔC showed no ATPase/GTPase activities even when 10 μg of protein was used (data not shown). These results indicated that the ATP/GTP-binding motifs in AfsR contributed to its ATPase and GTPase activities. In addition, the DNA-binding domain at the N-terminal portion of AfsR exerted its intrinsic DNA-binding activity independently of the ATPase/GTPase domain, because AfsRΔC retained DNA-binding activity as described below.
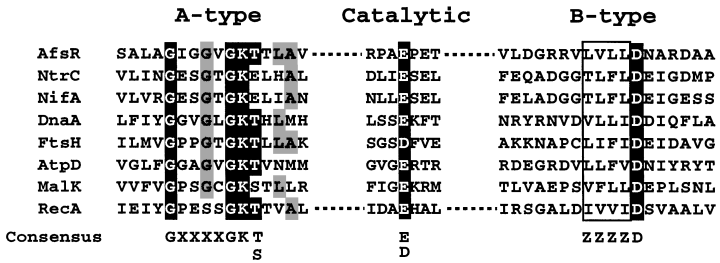
Alignment of the nucleotide-binding domains of DNA-binding proteins with ATPase activity. These include AfsR in S. coelicolor A3(2) (the DNA data base accession no. P2594), NtrC in Salmonella typhimurium (P41789), NifA in Klebsiella pneumoniae (RGKBAP), DnaA in Bacillus subtilis (IQBSOC), FtsH in Pseudomonas multocida (AAK02522), AtpD in E. coli (J01594), MalK in E. coli (P02914) and RecA in Mycobacterium tuberculosis (X58485). The consensus sequence in the A-type nucleotide-binding fold is GX4GK(T/S) (X, any amino acid; Yoshida and Amano, 1995) and that in the B-type is Z4D (Z, hydrophobic amino acid). The amino acid sequence covering the catalytic residue, E or D, is not conserved.
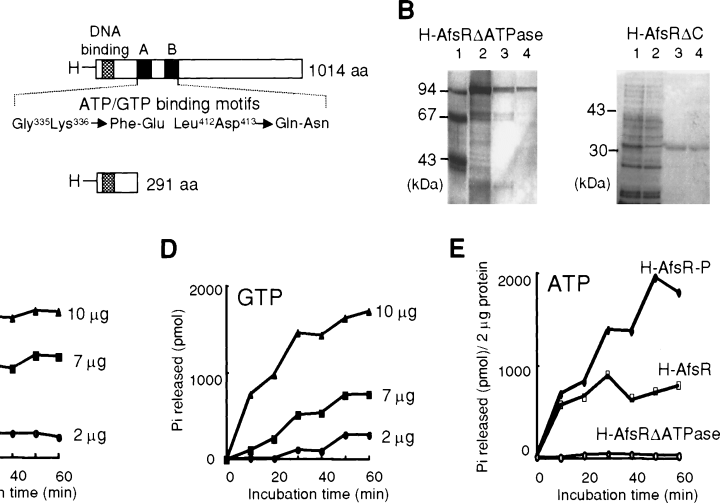
Time courses of ATP and GTP hydrolysis by H-AfsR and H-AfsR-P. Different amounts of H-AfsR (2, 7 and 10 μg) were used in this assay. The release of phosphate (Pi) was measured by the malachite green method every 10 min for 60 min. Each point is the average of the values obtained from three independent experiments.
A. Schematic representation of the AfsR mutants lacking ATPase activity. H-AfsR contains 21 extra amino acids including a histidine tag at the N-terminus of AfsR, an OmpR-like DNA-binding fold at the N-terminal portion of AfsR and A-type and B-type ATP/GTP-binding motifs in the middle of AfsR. H-AfsRΔATPase has amino acid replacement at four positions in the ATP/GTP-binding motifs. H-AfsRΔC contains only the DNA-binding fold.
B. SDS–PAGE of AfsRΔATPase and AfsRΔC. For the AfsRΔATPase blot, molecular size markers (lane 1) and the first (lane 2), second (lane 3) and third (lane 4) eluates from the Ni column are shown. For the AfsRΔC blot, the soluble fraction of E. coli harbouring pET-afsRΔC (lane 1), the pass-through from the Ni column (lane 2) and the first (lane 3) and second (lane 4) eluates are shown.
C. ATP was used as a substrate.
D. GTP was used as a substrate.
E. ATPase activities of H-AfsR and H-AfsR-P (2 μg of each protein) were compared. The H-AfsR-P sample was prepared by in vitro phosphorylation by TRX-KΔCwt, followed by membrane filtration. H-AfsRΔATPase with mutations in the ATP/GTP-binding motifs shows no ATPase activity. The ATPase activity of the H-AfsR sample used here is slightly higher than that used in (C), which was a different batch.
Phosphorylation of AfsR modulates its ATPase activity
We also examined the effect of phosphorylation of AfsR on the ATPase activity (Fig. 8E). When 2 μg each of H-AfsR and H-AfsR-P was used, the initial rates of ATP hydrolysis by both proteins were similar. After 10 min incubation, however, the ATP hydrolysis by H-AfsR was almost saturated, whereas that by H-AfsR-P continued almost linearly until 50 min. After 40 min incubation, H-AfsR-P showed two- to threefold higher ATPase activity than H-AfsR, indicating that phosphorylation of AfsR modulated its ATPase activity. It should be noted that the degree of enhancement is much underestimated because the H-AfsR-P sample contained only a very small population of the phosphorylated form. We suppose that the saturation of the ATPase activity of H-AfsR is ascribed to an inhibitory effect of the ADP produced by the hydrolysis of ATP, as is observed for an enhancer-binding protein, NtrC, whose ATPase activity is greatly inhibited by ADP (Austin and Dixon, 1992). The phosphorylated form of AfsR probably has a lower affinity to ADP than the unphosphorylated form.
The ATPase activity of NtrC is strongly stimulated in the presence of DNA containing cognate binding sites (Weiss et al., 1991; Austin and Dixon, 1992). We added 0.1– 10 μg of the 360 bp afsS upstream region to the reaction mixture containing H-AfsR or H-AfsR-P in a total volume of 450 μl and similarly measured ATPase activity. However, no significant enhancement was detected. The ATPase activity of AfsR therefore appears to be DNA independent but modulated by phosphorylation, whereas that of NtrC is DNA and phosphorylation dependent.
ATPase activity of AfsR is non-essential for its DNA-binding activity
We examined the effect of ATPase/GTPase activities of AfsR on its DNA-binding ability by gel mobility shift as-says with H-AfsRΔATPase and H-AfsRΔC. As shown in Fig. 9A, H-AfsRΔATPase retarded the DNA, which indicated that the ATPase activity of AfsR was non-essential for its binding to the promoter region of afsS. Consistent with this idea, ATP or GTP exogenously added at final concentrations of 1 pM to 100 nM to the incubation mixture for gel mobility shift assay did not affect the binding between H-AfsR and the DNA (data not shown). H-AfsRΔC also retarded the DNA (Fig. 9B). The size of the H-AfsRΔC–DNA complex is reduced because of the smaller size of AfsRΔC than of H-AfsRΔATPase.
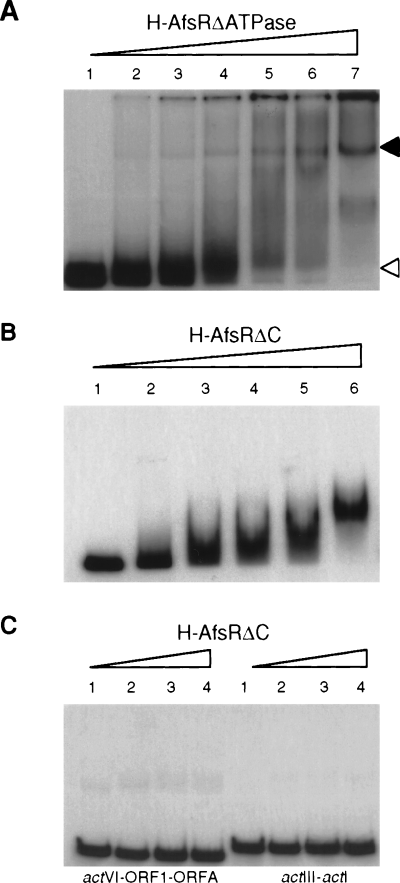
No requirement of ATPase activity for DNA binding of AfsR.
A. Gel mobility shift assay with H-AfsRΔATPase and the promoter region of afsS as 32P-labelled probe. The amounts of H-AfsRΔATPase were 0, 1, 3, 5, 7, 10 and 14 μg for lanes 1–7 respectively.
B. Gel mobility shift assay with H-AfsRΔC and the 32P-labelled probe. The amounts of H-AfsRΔC were 0, 0.6, 1.2, 2, 2.4 and 4 μg for lanes 1–6 respectively.
C. No binding of H-AfsRΔC to the DNA targets of ActII-ORF4. The actVI-ORF1-ORFA (158 bp) and actIII-actI (188 bp) probes were used. The amounts of H-AfsRΔC were 0, 1.2, 2.4 and 4 μg for lanes 1–4 respectively.
The OmpR-like DNA-binding fold in the N-terminal portion of AfsR (Wietzorrek and Bibb, 1997) shows similarity in amino acid sequence to ActII-ORF4, which has been shown to recognize and bind specific DNA sequences covering promoters in the act gene cluster (Arias et al., 1999). As probes, we prepared the two DNA fragments used by Arias et al. (1999); one was actVI-ORFA-ORF1 containing the promoters for actII-ORFA and -ORF1, and the other was actIII-actI containing the promoters of actIII and actI-ORF1. As an AfsR source, we used AfsRΔC because this protein contained the DNA-binding domain and lacked the ATPase domain. Gel mobility shift assay showed that even 4 μg of AfsRΔC caused no mobility shift (Fig. 9C). This means that the N-terminal domain of AfsR recognizes specific DNA sequences, different from those recognized by ActII-ORF4, and binds to them in the absence of the remaining C-terminal portion including the ATPase domain. No retardation of these DNA fragments by 5 μg of H-AfsR was also confirmed (data not shown). This is consistent with the observation that afsR cannot substitute for actII-ORF4 (Floriano and Bibb, 1996).
ATPase activity of AfsR is essential for activation of afsS transcription
We observed previously that pIJ41-AP105 directing the synthesis of AfsRΔATPase failed to cause S. lividans to produce actinorhodin (Horinouchi et al., 1990). As described above, AfsRΔATPase showed no ATPase activity but still retained DNA-binding activity. Introduction of pIJ486-afsRΔATPase into mutant ΔafsR failed to restore actinorhodin production, whereas, as described above, pIJ486-afsR restored actinorhodin production in ΔafsR (Fig. 10A). pIJ486-afsRΔATPase slightly reduced actinorhodin production by strain M130, probably due to a dominant-negative effect (data not shown). In agreement with these in vivo results, low-resolution S1 mapping showed that afsR on pIJ486 restored the transcription of afsS in mutant ΔafsR, whereas afsRΔATPase did not (Fig. 10B). The mRNA representing a chromosomal afsS copy in the ΔafsR mutant is not perceptible (Fig. 10B), although pIJ486-afsS appeared to restore actinorhodin production in the ΔafsR mutant (Fig. 1D). We assume that the gene dosage effect of afsS, resulting from the copy number of pIJ486 with its copy number of 40–100 per genome, supplies a sufficient amount of the afsS mRNA. These results clearly indicate that the ATPase activity of AfsR is essential for transcriptional activation of afsS.
Discussion
The present study has demonstrated that afsS, in addition to afsR, is a pleiotropic regulatory gene for antibiotic production in S. coelicolor A3(2) M130. This accords with the previous observations in this species (Horinouchi et al., 1990; Floriano and Bibb, 1996) and in S. lividans (Vögtli et al., 1994; Matsumoto et al., 1995). Our complementation experiments with the ΔafsS and ΔafsR mutants and the intact afsS and afsR genes on plasmids did not reveal the hierarchy of these genes in the regulatory pathway, although Floriano and Bibb (1996) observed that the stimulatory effect of multiple copies of afsS depended on AfsR in S. coelicolor A3(2) M145. The contrasting results may result from the difference in the strains used. Strain M145 has a mutation, sre-1 (suppression of relC effect) that leads to overproduction of actinorhodin, but strain M130 does not (Ochi and Hosoya, 1998). Because of this mutation, strain M145 sometimes gave ambiguous phenotypes, especially when actinorhodin production was followed. For example, amfC mutations do not apparently affect the yield of actinorhodin produced by strain M145 but do affect that produced by strain M130 (Yonekawa et al., 1999). The extent of overproduction of actinorhodin as a result of sre-1 in strain M145 may be dependent on nutritional conditions. In S. lividans, however, afsR2 is under the control of afsR; the stimulation of actinorhodin production by multiple copies of afsR2 does not require the intact AfsR protein (Vögtli et al., 1994), and transcription of afsR2 does not occur in ΔafsR backgrounds (Kim et al., 2001). Despite the ambiguous relation of afsS and afsR in vivo in S. coelicolor A3(2), the transcriptional analyses of afsS in the afsR+ and ΔafsR backgrounds clearly indicate the dependence of afsS on afsR. The enhancement of actinorhodin production by afsR in the absence of the intact afsS function may result from the activation of some additional gene(s) by afsR. This idea is in agreement with the observations that mutant ΔafsR produces almost no detectable amount of actinorhodin but mutant ΔafsS produces actinorhodin in a reduced amount, and that multiple copies of afsR induce actinorhodin production to a greater extent than that of afsS in both S. coelicolor A3(2) and S. lividans.
The control of afsS transcription by afsR turns out to be direct because AfsR recognizes and binds the promoter region of afsS. Binding of AfsRΔC and AfsRΔATPase to the DNA implies that the N-terminal portion containing an OmpR-like DNA-binding fold serves as a DNA-binding domain independently of the A- and B-type nucleotide-binding domain adjacent to the DNA-binding domain. The lack of ATPase activity of AfsRΔC and AfsRΔATPase suggests that the nucleotide-binding region serves as the ATP hydrolysis domain. AfsR thus appears to be modular, in that it is composed of physically separable DNA-binding and ATPase domains that can function independently of one another, as found in many eukaryotic enhancer-binding proteins and some prokaryotic transcriptional factors including NtrC and NifA (nitrogen fixation) (for a review, see Frankel and Kim, 1991; North et al., 1993). NtrC, a response regulator of a two-component system found in enteric bacteria, accepts a phosphate at aspartate-54 in the receiver domain from its sensor-autokinase NtrB. Phosphorylation of AfsR on serine and threonine residues by AfsK greatly enhances both DNA-binding and ATPase activities, although the sites of phosphorylation in AfsR have not been identified. The observed enhancement by phosphorylation is much underestimated because the H-AfsR-P preparation is actually a mixture of a large population of the unphosphorylated form of H-AfsR and a very small population of the phosphorylated form. The failure of afsRΔATPase to activate afsS transcription in the ΔafsR mutant indicates the essential role of the ATPase activity of AfsR in transcriptional activation of afsS. As expected for AfsR, AfsRΔATPase binds the upstream region of afsS in vivo, presumably contacting the RNA polymerase holoenzyme with a certain σ factor. In fact, pIJ486-afsRΔATPase slightly reduced actinorhodin production by strain M130, probably due to dominant-negative effect. AfsR is supposed to catalyse the isomerization of the closed complex between AfsR and the RNA polymerase to a transcriptionally competent open complex in which the DNA around the transcriptional start point is partially denatured. NtrC and NifA interact with the RNA polymerase with RpoN-sigma (σ54). The energy available from ATP hydrolysis is coupled to the formation of the open complex by the RNA polymerase, as is known for NtrC (Austin and Dixon, 1992; Wedel and Kustu, 1995; Lee et al., 2000). The energy supplied by the intrinsic low ATPase activity of unphosphorylated AfsR is thought to be insufficient to overcome the activation energy barrier to open complex formation or to change thermodynamically favourable conditions of the RNA polymerase configuration. The association of phosphorylation-modulated ATPase activity with site-specific DNA binding ensures that ATP hydrolysis by the transcriptional factors is primarily coupled to the formation of open complexes during transcriptional initiation (Austin and Dixon, 1992). From the present study, together with the analogy of AfsR with NtrC, we infer the roles of phosphorylation and ATPase activity of AfsR; phosphorylation enhances its DNA-binding activity and modulates ATPase activity, and the ATP hydrolysis generates the energy required for the formation of open complexes of AfsR and RNA polymerase to initiate transcription.
In transcriptional activation by NtrC, its subunit structure is important at every dissected step for achieving efficient activation. NtrC is a dimer in solution, and phosphorylation at Asp-54 induces dimerization of the receiver module (Fiedler and Weiss, 1995). In addition, phosphorylation of NtrC induces tetramerization on DNA binding, which facilitates strong co-operative binding to DNA essential for transcriptional activation (Weiss et al., 1992; Porter et al., 1993; Wyman et al., 1997). We could not determine the subunit structure of H-AfsR even in solution because H-AfsR readily formed aggregates. The aggregate of H-AfsR was recovered in the pass-through fraction when applied to gel filtration columns. To elucidate in more detail the molecular mechanism by which AfsR activates afsS transcription, we have to overcome the difficulties in handling AfsR and to establish an efficient in vitro transcription system.
AfsR binds the promoter region of afsS, including the −35 sequence. Although the binding sites of the majority of repressors overlap the promoter elements, several activators have been shown to bind the promoter regions. Examples are MerR mediating mercury resistance by controlling the mer operon on Tn501 (Ansari et al., 1992; 1995), SoxR mediating a global response against superoxide-generating agents by controlling soxS in E. coli (Hidalgo and Demple, 1994; 1997) and PcaR mediating protocatechuate degradation by controlling the pca operon in Pseudomonas putida (Guo and Houghton, 1999). MerR and SoxR bind the target −35 and −10 promoter elements with a space of 19 bp, and PcaR binds the two elements with a space of 16 bp. In E. coli and related species, RNA polymerase recognizes and binds the two elements with non-conserved spacers of 17 ± 1 bp (Russell and Bennett, 1982). All these transcriptional factors are believed to optimize the spacing between the two promoter elements by introducing a bend and untwisting of the DNA. The spacing between the probable −35 and −10 sequences of the afsS promoter is 20 bp, and we therefore speculate that AfsR binding to this promoter induces a DNA bend to optimize the spacing. We need further study to elucidate the mechanism by which AfsR-P binds the afsS promoter with a higher affinity than AfsR and induces transcription, probably by forming an open complex with RNA polymerase by the energy supplied by ATP hydrolysis.
The mechanism by which AfsS and AfsR2 function as regulatory proteins also remains to be elucidated. The presence of three repeats of Thr-Xaa2–Asp–Asn–His– Met–Pro–Xaa2–Pro–Ala (Xaa represents a non-conserved amino acid) in AfsS and AfsR2 of only 63 amino acids tempts us to speculate that they interact with some protein via the repeats, thereby exerting their regulatory function. An afsR–afsS counterpart in Streptomyces noursei, orf1–ssmA, which was cloned as a DNA frag-ment that activated actinorhodin production in S. lividans (Sekurova et al., 1999), also contains two repeats of Pro–Xaa–Asp–Asn–His–Thr–Pro–Ile–Xaa–Pro in SsmA of 55 amino acids, which suggested the importance of the repeats in these small proteins.
On the basis of the results obtained so far and our unpublished data, we would like to present a hypothesis for the regulation of actinorhodin biosynthesis by the AfsK–AfsR–AfsS system (Fig. 11). AfsK produced from early to late growth phase is attached loosely to the inner side of the membrane (Matsumoto et al., 1994). An AfsK-binding protein, KbpA, which is encoded 5′ to afsK (Fig. 1A) and actively produced when actinorhodin production has already begun, binds the kinase catalytic domain of unphosphorylated AfsK or AfsK dephosphorylated by phosphatases and inhibits its kinase activity (Umeyama and Horinouchi, 2001). KbpA thus modulates the population of phosphorylated AfsK, controlling the degree of phosphorylation of AfsR. afsK may be induced by some external signal, although transcription of afsK is apparently constant in cells grown on agar medium under laboratory conditions. AfsR is also phosphorylated by an additional kinase (Matsumoto et al., 1994), here named kinase A, which has been shown recently to autophosphorylate its serine and threonine residues and phosphorylate serine and threonine residues of AfsR (unpublished data). Some other kinases among about 30 kinases containing a kinase catalytic domain similar to that of AfsK [predicted by the genome sequence of S. coelicolor A3(2); www.sanger.ac.uk/Projects/S_coelicolor/] may also phosphorylate AfsR. These kinase genes may be activated by external signals different from that for afsK. However, the phosphorylation of AfsR by AfsK, rather than by other kinases, seems to contribute more to its regulatory function, because the ΔafsK produces a reduced amount of actinorhodin (Matsumoto et al., 1994), and the transcription of afsS in the ΔafsK mutant is delayed. It is unclear whether the autophosphorylated form of AfsK is still attached to the membrane. Phosphorylation of AfsR enhances its DNA-binding and ATPase activities. The phosphorylated form of AfsR, together with the RNA polymerase holoenzyme with a certain σ factor, binds the upstream region of afsS to form a closed complex, which is then converted to an open complex by the energy available from ATP hydrolysis. The subunit structure of AfsR at these steps is not known. Our speculation on the overlap of the promoter and the AfsR binding site is that AfsR bends the DNA to optimize the spacing of the −35 and −10 promoter elements. The small afsS product with three repeats of the 12-amino-acid sequence (Matsumoto et al., 1995) influences secondary metabolism, including actinorhodin biosynthesis, in a still unknown way. Overexpression of either afsR or afsS thus enhances actinorhodin production by stimulating transcription of actTI-ORF4 (Horinouchi et al., 1989; Floriano and Bibb, 1996). The phosphorylated form of AfsR appears to activate other genes that influence secondary metabolism.
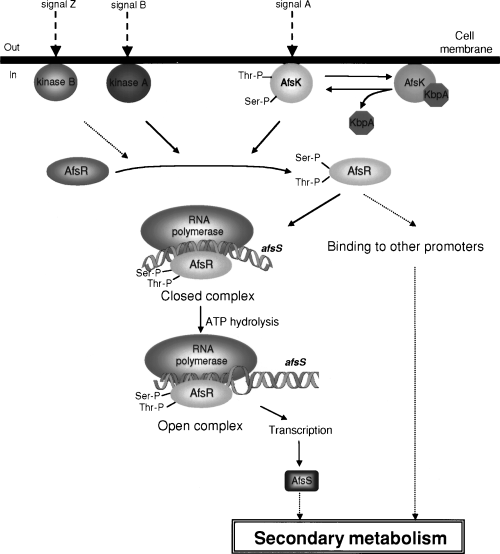
A hypothetical scheme for the regulation of secondary metabolism by the AfsK–KbpA–AfsR–AfsS system. See text for details.
Experimental procedures
Bacterial strains, plasmids and growth conditions
Streptomyces coelicolor A3(2) M130 (hisA1 uraA1 strA1, SCP1, SCP2) was obtained from D. A. Hopwood (Bibb and Hopwood, 1981), John Innes Research Centre, Norwich, UK. pGST-afsR contained the afsR coding sequence in the cloning site of pGEX-5X-1 (Umeyama and Horinouchi, 2001). pIJ41-AP105 directed the synthesis of a mutated AfsR protein, in which four amino acids, Gly-335 to Phe and Lys-336 to Glu in the A-type ATP-binding motif and Leu-412 to Gln and Asp-413 to Asn in the B-type ATP-binding motif, were changed (Horinouchi et al., 1990). pTRX-KΔCwt containing a 5′ region of afsK directed the synthesis of a truncated AfsK protein with kinase activity (Umeyama and Horinouchi, 2001). pTRX-KΔCK44A had a similar structure to pTRX-KΔCwt, but the protein encoded was unable to phosphorylate AfsR because of amino acid replacement at Lys-44 with Ala (Umeyama and Horinouchi, 2001). As Streptomyces plasmids, high-copy-number plasmids pIJ486 containing the kanamycin and thiostrepton resistance genes (Ward et al., 1986) and pIJ702 containing the melanin production and thiostrepton resist-ance genes (Katz et al., 1983), with their copy numbers of 40–100 per genome, were used. A low-copy-number plasmid pKU209 with its copy number of one or two per genome containing the ampicillin and thiostrepton resistance genes (Kakinuma et al., 1991) was also used. E. coli JM109 and pUC19 (Yanisch-Perron et al., 1985) were used for DNA manipulation. E. coli BL21(DE3)pLysS and pET16a(+), purchased from Novagen, were used for the production of histidine-tagged AfsR proteins. S. coelicolor A3(2) was grown routinely on TSB (tryptic soya broth; Nissui Pharmaceutical) containing 2% agar. YMPD (Umeyama et al., 1999) and R2YE (Hopwood et al., 1985) have been described earlier. Thiostrepton (50 μg ml−1) and kanamycin (10 μg ml−1) were added when necessary.
General recombinant DNA studies
Restriction enzymes, T4 DNA ligase and other DNA-modifying enzymes were purchased from Takara Shuzo. Superscript II reverse transcriptase was purchased from Gibco/Invitrogen. [γ-32P]-ATP (220 TBq mmol−1) for end-labelling at 5′ ends with T4 polynucleotide kinase was purchased from Amersham Pharmacia Biotech. DNA was manipulated in Streptomyces (Hopwood et al., 1985) and in E. coli (Maniatis et al., 1982; Ausubel et al., 1987), as des-cribed earlier. Nucleotide sequences were determined by the dideoxy chain termination method with the DYEnamic ET terminator cycle sequencing kit on an automated DNA sequencer.
Disruption of afsS and afsR
An 800 bp region upstream of afsS was amplified by PCR with primers 5′-GCCGAATTCCGGTACGCCGAGGGCAAGG GCC-3′ (the underlined letters indicate an EcoRI site) and 5′-GGCAAGCTTGTTAACGAACTTCGCTCCTCATGG-3′ (the underlined and italic letters indicate HindIII and HpaI sites respectively). Another 800 bp region downstream of afsS was amplified with 5′-GCCGAATTCGTTAACAACTTCCTCC ACACGGG-3′ (the underlined and italic letters indicate EcoRI and HpaI sites respectively) and 5′-GGCAAGCTTGAAGCTC GCCACCGGCGGCGAG-3′ (the underlined letters indicate a HindIII site). These fragments were then inserted between the EcoRI and HindIII sites of pUC19 by three-fragment ligation. The 3.5 kb HindIII fragment containing the neomycin resistance gene aphII (Beck et al., 1982) was then inserted in the HindIII site of the recombinant pUC19 plasmid to construct pUD-Km. The recombinant plasmid was used to transform protoplasts of S. coelicolor A3(2) M130. Transformants carrying pUD-Km in the chromosome as a result of single cross-over were first selected on TSB agar containing 10 μg ml−1 kanamycin. These kanamycin-resistant strains were grown for 1 week on TSB agar without kanamycin. Spores recovered were spread on TSB agar without kanamycin. Kanamycin-sensitive colonies, which were generated as a result of homologous recombination, were selected. Correct afsS disruptants were selected by Southern hybridization with the 1600 bp fragment on pUD-Km as 32P-labelled probe against the BamHI-digested chromosome.
For disruption of afsR, the BamHI–FbaI fragment (see Fig. 1A) was cloned in the BamHI site of pUC19. The BalI fragment corresponding to Leu-42 to Ala-769 of AfsR was then replaced by the SmaI fragment carrying aphII. The pUC19 recombinant plasmid was linearized by DraI digestion, alkali-denatured with 0.1 M NaOH (Oh and Chater, 1997) and introduced by protoplast transformation into strain M130. Among kanamycin-resistant transformants, true afsR disruptants were selected by Southern hybridization with the kanamycin resistance gene and part of the afsR coding sequence as probes against the chromosomal fragments digested with BamHI plus FbaI.
S1 nuclease mapping
Methods for RNA isolation from cells grown on cellophane on the surface of TSB agar medium and S1 nuclease mapping have been described by Kelemen et al. (1996). Hybridization probes were prepared by PCR with a pair of 32P-labelled and unlabelled primers. Primers were labelled at the 5′ ends with [γ-32P]-ATP and T4 polynucleotide kinase. For the 360 bp afsS probe, 5′-CTGCGGTGTGGCGTCCGCGTC-3′ (nucleotide positions +163 to +182, taking the transcriptional start point of afsS as +1, which was determined later), 32P-labelled at the 5′ end, and 5′-GGACCGCGCGCGA GCGTGCT-3′ (positions −178 to −159) were used. For the 360 bp afsR probe, 5′-GTCCCGCCAGGCGCGCACCGG-3′ (nucleotide positions +114 to +94, with respect to the start codon), 32P-labelled at the 5′ end, and 5′-CCCCGGGGACG GCCACCTCC-3′ (positions −246 to −237) were used. For the 480 bp afsK probe, 5′-CGCCACGCGCCGCCCGGACG-3′ (nucleotide positions +117 to +137, with respect to the start codon), 32P-labelled at the 5′ end, and 5′-GACATGGACGC CGGGCTCGC-3′ (positions −352 to −333) were used. For high-resolution S1 mapping of afsS, 5′-AACTGGTGGGCTC GGTCGTC-3′ (nucleotide positions −52 to −33, taking the transcriptional start point of afsS as +1), 32P-labelled at the 5′ end, and 5′-GACCGGCGGTAGCCGGAGCG-3′ (positions −58 to −39) were used to prepare the 100 bp probe. hrdB encoding a σ factor of RNA polymerase was used to check the purity and amount of the RNA used, as described previously (Umeyama and Horinouchi, 2001). Protected fragments were analysed on 6% DNA sequencing gels according to the method of Maxam and Gilbert (1980).
RT–PCR
Transcription of the afsRS region was analysed by RT–PCR with pairs of the following primers (see Fig. 3C). R1 (5′-G CACCAGCGCCGGTACGCCGAGGGCAAGGGCC-3′; nucleotide positions −660 to −629, taking the transcriptional start point of afsS as +1) and S2 (5′-GAAGGTCTACTTGCC GTCG-3′ (nucleotide positions +341 to +323; the bold letters indicate the stop codon of afsS); R1 and R2 (5′-GCATC CCAGGGCGAGTGCCTGCTCGGCGT-3′; nucleotide positions −240 to −268); S1 (5′-CCATGAGCGACAAGATGA AGGACGC-3′; nucleotide positions +142 to +166; the bold letters indicate the start codon of afsS) and S2; and R3 (5′-GGACCGCGCGCGAGCCTTGCT-3′; nucleotide positions −178 to −159) and S3 (5′-CTGCGGTGTGGCGTCCGCGTC-3′; nucleotide positions +182 to +163). First-strand synthesis was performed with Superscript II reverse transcriptase according to the supplier’s manual. PCR conditions were 94°C for 30 s, 60°C for 20 s and 72°C for 1 min in a total of 30 cycles. PCR products were analysed by 2% agarose gel electrophoresis.
Construction of plasmids
pIJ2476 containing afsR and its promoter was obtained from M. J. Bibb (Floriano and Bibb, 1996) and called here pIJ486-afsR for clarity. For the construction of pIJ702-afsS, a 710 bp FbaI–SalI fragment containing the whole afsS gene was inserted between the BamHI and SalI sites of pUC19. The afsS sequence was excised as a KpnI–PstI fragment and inserted between the KpnI and PstI sites of pIJ702 to construct pIJ702-afsS. For construction of pKU209-afsS, the afsS sequence on pUC19 was excised as a HincII–SmaI fragment, and both ends were converted to EcoRI sites with an 8-mer EcoRI linker. The EcoRI fragment was inserted in pUC19, and the afsS sequence was excised as an EcoRI fragment. The EcoRI fragment was then inserted in the EcoRI site of pKU209 to construct pKU209-afsS. For the construction of pKU209-afsR, the BamHI–PstI fragment from pIJ41-AP3, containing the whole afsR sequence including the promoter, was cloned between the BamHI and PstI sites of pUC19. The afsR sequence was then excised as an EcoRI–HindIII fragment, and the ends were converted into EcoRI sites with an 8-mer EcoRI linker. The EcoRI fragment was inserted in the EcoRI site of pKU209, resulting in pKU209-afsR.
For the construction of pET16-afsR and pET16-afsRΔC, a 5′ region of 810 bp encoding an N-terminal portion of AfsR was amplified by PCR with two primers; 5′-GCCGAATTCCATATGGACGGTGGACCGC-3′ (the underlined and italic letters indicate EcoRI and NdeI sites respectively; the bold letters indicate the start codon of afsR) and 5′-GCCAAGC TTGGATCCTCAGGCCAGGGCCGGGTC-3′ (the under-lined and italic letters indicate HindIII and BamHI sites re-spectively; the bold letters serve as a stop codon just after Ala-270 of AfsR). No errors in amplification were checked by nucleotide sequencing. A single MluI site was present in this region. The amplified fragment was trimmed into an NdeI–BamHI fragment and inserted in pET16 to construct pET16-afsRΔC. A MluI–XhoI fragment encoding a C-terminal portion was excised from pGST-afsR and inserted between the MluI and XhoI sites of pET16-afsRΔC, resulting in pET16-afsR.
For the construction of pET16-afsRΔATPase, a mutated afsR sequence on pIJ41-AP105 (Horinouchi et al., 1990) was used. A 1.5 kb SacI–SphI fragment representing an internal region of afsR encoding Leu-272 to Ala-762 was excised from pIJ41-AP105 and replaced with the native sequence on pET16-afsR by standard DNA manipulation. The resulting plasmid, pET16-afsRΔATPase, would direct the synthesis of a mutant AfsR protein with a histidine tag (H-AfsRΔATPase) in which four amino acids in the A-type and B-type ATP/GTP-binding motifs are changed.
DNase I footprinting
For preparation of the antisense strand by PCR for DNase I footprinting, 5′-CCCCTCCTCGGCGGCCCAGC-3′ (nucle-otide positions −288 to −269, with respect to the transcriptional start point of afsS) and 5′-AACTGGTGGGCTC GGTCGTC-3′ (nucleotide positions +52 to +33) labelled at the 5′ end were used. For preparation of the sense strand, 5′-GGAGGTCGGCGAGGTAAGGGCG-3′ (nucleotide positions −118 to −97) labelled at the 5′ end and 5′-CTGCGGT GTGGCGTCCGCGTC-3′ (nucleotide positions −182 to −163) were used. The reaction mixture contained 10 kc.p.m. of 32P-labelled DNA probe, 0.1–6 μg of H-AfsR, 25 mM HEPES– KOH (pH 7.9), 0.5 mM EDTA–NaOH (pH 8.0), 50 mM KCl and 10% glycerol in a total volume of 50 μl. After incubation of the mixture for 30 min at 25°C, DNase I was added at a final concentration of 5 μg ml−1, and the mixture was incubated further for 1 min. The reaction was stopped by adding 100 μl of stop solution (100 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1% sodium N-lauroyl sarcosinate, 10 mM EDTA, 25 μg ml−1 salmon sperm DNA) and 300 μl of phenol–CHCl3 (1:1). After ethanol precipitation, the pellet was washed with 80% ethanol, dissolved in 6 μl of the formamide–dye mixture and run on a 6% polyacrylamide gel (Maxam and Gilbert, 1980).
Production and purification of H-AfsR, H-AfsRΔC and H-AfsRΔATPase
An overnight preculture (0.5 ml) of E. coli BL21(DE3)pLysS harbouring pET16-afsR, pET16-afsRΔC or pET16-afsRΔATPase was used to inoculate 50 ml of LB broth supplemented with 100 μg ml−1 ampicillin. After incubation at 37°C for 3 h, IPTG was added to a final concentration of 0.5 mM to induce the T7 promoter, and incubation was continued for another 3 h. Cells harvested by centrifugation were washed once with 10 mM Tris-HCl (pH 7.0) buffer and disrupted by mild sonication. The lysate was cleared of debris by centrifugation at 12 000 g for 10 min. The supernatant was then centrifuged at 23 500 g for 15 min. H-AfsR, H-AfsRΔC and H-AfsRΔATPase in the soluble fraction were purified with an Ni-NTA spin column (Qiagen), according to the manufacturer’s manual. The purified samples were dialysed overnight against 10 mM Tris-HCl (pH 7.0) and 10% glycerol. Protein concentrations were measured with a dye-binding protein assay kit (Bio-Rad) using bovine serum albumin (BSA) as standard.
Preparation of phosphorylated AfsR (H-AfsR-P)
H-AfsR (100 μg; 108 kDa; 1014 amino acids) was phosphorylated in vitro by incubation at 30°C for 20 min with TRX-AfsKΔCwt (60 μg; 478 amino acids, 51 kDa) in 10 mM Tris-HCl (pH 7.0), 10 mM MnCl2, 10 mM MgCl2, 1 mM ATP and 1 mM dithiothreitol (DTT) in a total volume of 400 μl. The reaction was terminated by adding 10 μl of 100 mM EDTA. The reaction mixture was applied to a Nanosep 30K device (Pallfiltron), and the membrane was washed five times with 500 μl of 10 mM Tris-HCl (pH 7.5) and 10% glycerol to change buffer and to remove small molecules such as inorganic phosphate, ATP and small proteins. The material retained on the membrane was recovered in 50 μl of 10 mM Tris-HCl (pH 7.5) and 10% glycerol. H-AfsR was recovered at almost 100%, whereas some proportion of TRX-AfsKΔCwt passed through the membrane. For monitoring the phosphorylation, 1/20th volume (20 μl) of the reaction mixture without TRX-AfsKΔCwt was taken, and 10 μCi (370 kBq) [γ-32P]-ATP, together with 3 μg of TRX-AfsKΔCwt, was added. After incubation at 30°C for 15 min, the reaction was terminated by adding 4 μl of 375 mM Tris-HCl (pH 6.8), 60% glycerol, 12% SDS, 6% 2-mercaptoethanol and 0.003% bromophenol blue and by subsequent boiling for 5 min. The mixture was then separated by 10% SDS–PAGE. The gel was dried and analysed using an image analyser. TRX-AfsKΔCK44A with no ability to phosphorylate AfsR was used similarly as a negative control.
Gel mobility shift assay
For protein–DNA binding assay (Vujaklija et al., 1993), a 32P-labelled probe DNA of 360 bp (nucleotide positions −178 to +182, taking the transcriptional start point of afsS as +1) was incubated with various amounts of H-AfsR, H-AfsRΔATPase, H-AfsRΔC or His-AfsR-P at 25°C for 30 min in the buffer containing 10 mM Tris-HCl (pH 7.0), 1 mM DTT, 1 mM EDTA, 10% glycerol and 1 μg poly-(dI–dC)-poly(dI–dC) in a total volume of 30 μl. For assay of H-AfsRΔC binding to two regions within the act gene cluster, 32P-labelled probes were prepared by the method described by Arias et al. (1999). Probes actVI-ORFA-ORF1 and actIII-actI were 158 bp and 188 bp in size respectively. After incubation, complexes and free DNA were resolved by 6% non-denaturing polyacrylamide gels (mono/ bis, 79:1) with a running buffer containing 40 mM Tris-HCl (pH 7.8), 20 mM sodium acetate and 1 mM EDTA. Gels were dried and subjected to autoradiography.
Assay of ATPase
ATPase activity was measured by a modification of the malachite green ATPase assay described by Eichelberg et al. (1994). The standard reaction contained 2–10 μg of H-AfsR or H-AfsRΔATPase, 5 mM HEPES, 30 mM KCl, 30 mM NH4Cl, 1 mM DTT, 5 mM magnesium acetate, 2 mmol of ATP or GTP and 5 μg ml−1 BSA in a total volume of 450 μl. The mixture was placed on ice, and the reaction was started by incubation at 30°C. Samples (50 μl each) were withdrawn every 10 min, and the reaction was stopped by adding 800 μl of malachite green–ammonium molybdate reagent. The amounts of enzymatically released inorganic phosphate in triplicate samples were measured photometrically by referring to a standard curve, which was prepared with dilutions of a standard solution.
Acknowledgements
P.-C. Lee was supported by the Monbusho Fellowship. T. Umeyama was supported by the Japan Society for the Promotion of Science (JSPS). This work was supported by the Asahi Glass Foundation, by the ‘Research for the Future’ Program of JSPS and by the Bio Design Program of the Ministry of Agriculture, Forestry and Fisheries of Japan (BDP-01-VI-2-4).