OmpR-dependent and OmpR-independent responses of Escherichia coli to sublethal attack by the neutrophil bactericidal/permeability increasing protein
Summary
Bactericidal/permeability-increasing protein (BPI) of neutrophils is a lipopolysaccharide (LPS)-binding antibacterial protein with specificity for Gramnegative bacteria. BPI binding to the bacterial surface rapidly triggers potentially reversible bacterial growth inhibition and alterations of the outer membrane and, later, disruption of the inner membrane and lethal injury. Initial effects include selective OmpR-dependent changes in the synthesis of outer membrane porins (OmpF and OmpC). Because OmpR is a global transcriptional regulator, we have examined its possible role in responses of E. coli to sublethal injury caused by BPI. Early (<15 min) reversible effects of BPI on bacterial colony-forming ability and outer membrane permeability were virtually identical in isogenic wild-type (wt) and ompR−E. coli. Both strains could repair the outer membrane permeability barrier after Mg2+-induced displacement of bound BPI. However, OmpR was essential for the ability of E. coli to tolerate low doses of BPI and escape the progression of sublethal to lethal damage. Scanning electron microscopy revealed that BPI treatment produced greater membrane perturbations in the ompR− strain, apparent even before lethal injury. These findings suggest that the fate of E. coli exposed to BPI depends on both OmpR-independent mechanisms engaged in outer membrane repair and OmpR- dependent processes that modulate porin synthesis and retard progression of injury from the outer to the inner membrane.
Introduction
Polymorphonuclear leukocytes (PMN) represent the first line of cellular defence against invading bacteria (Elsbach et al., 1999) by responding chemotactically and accumulating at sites of infection. PMN contain an array of antimicrobial compounds (Levy, 1996), including the bactericidal/permeability-increasing protein (BPI) (Elsbach and Weiss, 1993). Reflecting, in part, its cationic nature, BPI is highly selective and potent against Gram-negative bacteria (GNB). Selectivity and cytotoxicity of BPI is due to its high affinity for lipopolysaccharides (LPS), which represent unique structural components of the surface of GNB. The lipid A portion of LPS is highly conserved and structurally unique, found only in GNB, and is the primary site of BPI action (Gazzano-Santoro et al., 1992).
Within 1–5 min after binding to the bacterial surface, BPI causes several changes in the outer membrane, including an increase in outer membrane permeability to small hydrophobic compounds, alteration in membrane lipid metabolism and disruption of bacterial growth (Beckerdite et al., 1974; Weiss et al., 1975; in’t Veld et al., 1988; Mannion et al., 1990a;b). These changes are sublethal and potentially reversible (Weiss et al., 1976; Mannion et al., 1990b). Later effects of BPI involve the progression of bacterial damage to the inner membrane in a dose- and time-dependent manner. These changes are apparently irreversible and coincident with bacterial death (Mannion et al., 1990b). Addition of high concentrations (≥20 mM) of Mg2+ and/or Ca2+during the sublethal phase of BPI action displaces surface bound BPI, and induces net resynthesis of membrane phospholipids and repair of the outer membrane permeability barrier in a process dependent on de novo LPS synthesis (Weiss et al., 1976; Weiss et al., 1983;1984). In contrast, addition of albumin (≥500 μg ml−1) can rescue BPI-treated bacteria allowing them to survive without displacement of bound BPI or acute repair of outer membrane damage (Mannion et al., 1990b). Thus, repair and rescue of BPI-treated bacteria appear to represent two distinct processes.
During the sublethal phase of BPI action, the metabolic integrity of the bacteria remains largely intact, suggesting that the bacteria could use their sustained biosynthetic activity to repair outer envelope damage and retard/ prevent progression of injury to the inner membrane. Little is known about the bacterial factors that may mediate and regulate such a response. Although overall bacterial protein synthesis is not appreciably altered during the sublethal phase of BPI action, selective changes are detectable (Elsbach et al., 1999; Qi et al., 1995). These include apparently reciprocal changes in the synthesis of the two major outer membrane porins, OmpF and OmpC. Here we show that the BPI-induced alterations of OmpF and OmpC synthesis are mediated by OmpR in a manner that mimics the bacterial response to hyperosmotic shock. The function of OmpR has been best studied in the osmoregulation of the OmpF and OmpC genes (Pratt and Silhavy, 1995a). However, OmpR is a global trans-criptional regulator implicated in control of a wide array of cellular processes (Gibson et al., 1987; Higashitani et al., 1993; Shin and Park, 1995; Römling et al., 1998; Bang et al., 2000). We have, therefore, also investigated the possible role of OmpR in Mg2+-induced repair and albumin-dependent rescue of BPI-treated bacteria. Our findings suggest that repair and rescue of BPI-treated bacteria are dependent upon OmpR-independent and OmpR-dependent adaptive response mechanisms respectively.
Results
Selective alterations of OmpF and OmpC synthesis during sublethal action of BPI on E. coli
To determine the effects of BPI on bacterial protein synthesis during the sublethal phase of its action, E. coli PL-2 were preincubated ± BPI for 15 min then pulse-labelled with radioactive amino acids for another 15 min. Under these conditions, BPI-treated bacteria retained the ability to form colonies in nutrient agar supplemented with 0.1% (w/v) albumin (86% of colony-forming units (CFU) of inoculum) but not in nutrient agar alone (<1% of CFU of original inoculum), a difference that reflects sublethal alterations of the bacteria (Mannion et al., 1990b). There was no difference in Coomassie blue staining of bacterial proteins in whole cell lysates resolved by SDS–PAGE from untreated and BPI-treated cells (Fig. 1, lanes 2 and 1 respectively), indicating that BPI treatment did not cause detectable release or degradation of proteins. Overall bacterial protein synthesis was not significantly affected by BPI treatment, as assessed by incorporation of radioactive amino acids into trichloroacetic acidprecipitable material and SDS–PAGE/autoradiography of bacterial extracts (Fig. 1, lanes 5 and 6). However, radio-labelling of OmpF was inhibited and OmpC stimulated in BPI-treated bacteria (lane 5) versus that of the controls (lane 6).
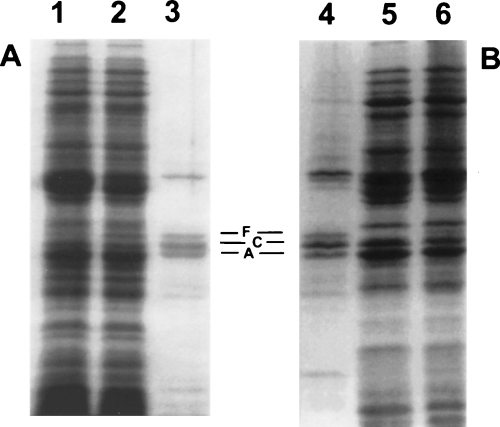
Effect of bactericidal/permeability-increasing protein (BPI) on de novo protein synthesis in E. coli PL-2. E. coli PL-2 (5 × 108 ml−1) was incubated in proteose peptone beef extract (PPBE) for 15 min with (lanes 1 and 5) or without (lanes 2 and 6) 400 nM BPI before addition of [14C]-amino acids (5 μCi ml−1) and incubation for an additional 15 min. Cell lysates were prepared as described in Experimental procedures. Aliquots were separated by 11% SDS–PAGE and autoradiograms were developed. Lanes 1–3, Coomassie blue staining; 4–6, autoradiogram. Lanes 3 and 4 represent enriched outer membrane fractions from untreated bacteria. Outer membrane proteins OmpF, OmpC and OmpA are marked as F, C and A respectively. Results shown are representative of several experiments.
To further demonstrate the effect of BPI on de novo porin synthesis, similar experiments were performed in E. coli J5 in which untreated bacteria produced a higher ratio of OmpF/OmpC than did E. coli PL-2 (compare Fig. 1, lane 6, with Fig. 2, lane 3). To verify that the effects of BPI were indeed on OmpF and OmpC, outer membrane-enriched fractions were isolated after pulse-labelling. Coomassie blue staining of SDS–PAGE resolved fractions from untreated (Fig. 2, lane 1) and BPI-treated cells (Fig. 2, lane 2) was virtually identical indicating no effect of BPI on either recovery or OmpF/C content of outer membrane-enriched fractions. However, examination of radiolabelled proteins indicated that OmpF was depressed and OmpC markedly enhanced in outer membrane fractions from BPI-treated (Fig. 2, lane 4) versus control cells (Fig. 2, lane 3). This illustrated the reciprocal effects of BPI on OmpF and OmpC synthesis.

Effect of BPI on de novo OmpF and OmpC synthesis in E. coli J5. Same conditions as described in Fig. 1 except that E. coli J5 incubated in NB buffered with 20 mM phosphate buffer (pH 7.4) were used. Samples shown represent enriched outer membrane protein-enriched fractions, prepared as described in Experimental procedures and separated by 11% SDS–PAGE. Lanes 1 and 2, Coomassie blue staining; 3 and 4, autoradiogram; 1 and 3, control samples; 2 and 4, outer membrane proteins from BPI-treated cells. Outer membrane proteins OmpF, OmpC and OmpA are marked as F, C and A respectively.
BPI-induced modulation of OmpF and OmpC synthesis is mediated by OmpR
Effects of BPI on OmpF/C resemble effects of hyperosmotic shock which are mediated by OmpR. Therefore, we tested the role of OmpR in the modulation of OmpF and OmpC production by BPI-treated bacteria by comparing the effects of BPI on E. coli MC4100 and E. coli MH760 isogenic strains containing wild-type (wt) and mutant (V203M) OmpR proteins, respectively. In comparison with wt OmpR, the mutant OmpR of MH760 binds with higher affinity to the activation sites of ompF and binds with reduced affinity to the activation sequences of ompC (Tran et al., 2000). This results in constitutive synthesis of OmpF and no OmpC. If the effects of BPI on porin synthesis are mediated via OmpR, both repression of OmpF synthesis and stimulation of OmpC production should be impaired during BPI treatment of E. coli MH760. In accordance with previous results, addition of either sucrose or BPI to MC4100 induced a decrease in OmpF synthesis and an increase in OmpC synthesis (Fig. 3, lanes 1–3). In contrast, sucrose (Fig. 3, lane 5) or BPI (Fig. 3, lane 6) had little or no effect on either OmpF or OmpC synthesis in MH760. These findings indicate that the BPI-induced decrease in OmpF levels and increase in OmpC levels are dependent on the presence of wt OmpR.
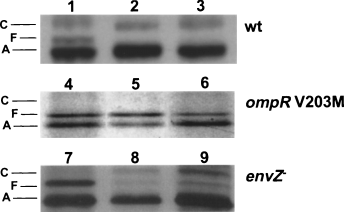
Role of OmpR and EnvZ in BPI-induced changes in porin synthesis. E. coli MC4100 (wt, lanes 1–3), MH760 (V203M, lanes 4–6) and SG477 (envZ−, lanes 7–9) (5 × 108 ml−1) were incubated for 15 min at 37°C in NB/phosphate buffer alone (lanes 1, 4 and 7) or containing 20% sucrose (lanes 2, 5 and 8) or 400 nM BPI (lanes 3, 6 and 9) and pulse-labelled with [35S]-methionine (0.5 μCi ml−1) for an additional 15 min before outer membrane protein-enriched fractions were prepared. Samples were run on 10% SDS/9 M urea PAGE and autoradiograms were subsequently developed. Note the faster migration of OmpF (compared with OmpC) in this electrophoretic system.
The phosphorylated state and functional properties of OmpR are regulated by the histidine kinase EnvZ (Pratt and Silhavy, 1995a). However, incubation of an isogenic envZ-null derivative of MC4100 (SG477) with 20% sucrose (Fig. 3, lane 8), as shown before (McCleary and Stock, 1994; Leonardo and Forst, 1996; Matsubara and Mizuno, 1999), or with BPI (Fig. 3, lane 9), stimulated production of OmpC synthesis and diminished OmpF production. Taken together, these results demonstrate that BPI, like hyperosmolarity, can modulate porin expression by an OmpR-dependent mechanism that can be independent of EnvZ.
BPI affects OmpF and OmpC mRNA levels
OmpR regulates the synthesis of OmpF/C at the transcriptional level. To test the hypothesis that BPI, like hyperosmolarity, modulates the synthesis of OmpF/C on the transcriptional level, we used RT-PCR to measure omp F/C mRNA levels with or without BPI treatment. Incubation of wt bacteria with as low as 10 nM BPI was sufficient to produce a decrease in ompF mRNA and an increase in ompC mRNA compared with that of the un-treated control (Fig. 4A). The ratio of ompC/ompF mRNA was markedly increased with increasing doses of BPI. These data suggest that the effect on porin synthesis induced by BPI occurs by changes of mRNA levels.
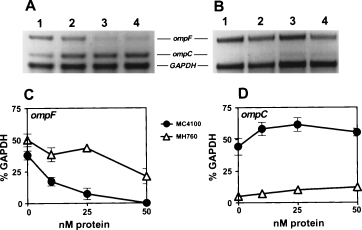
RT-PCR analysis of effects of BPI on OmpF and OmpC mRNA levels.◊E. coli MC4100 and MH760 (5 × 107 bacteria ml−1; ranging from 4 × 106 to 2 × 107 ml−1 in different experiments) were treated with increasing concentrations of BPI for 15 min. RT-PCR was performed as described in Experimental procedures. Amplified DNA segments were separated on 2% agarose (A and B). For each sample, the density of ompF and ompC bands was determined using Alpha Imager and calculated relative to the intensity of GAPDH as presented in C and D. Lanes: 1, untreated cells; 2, 10 nM BPI; 3, 25 nM BPI; 4, 50 nM BPI. Data in panels C and D are mean ± SEM of two independent experiments. Error bars are shown when larger than the symbols used.
To further demonstrate that BPI-induced changes in ompF and ompC mRNA levels are dependent on the presence of wt OmpR, we performed RT-PCR analysis with E. coli MH760 expressing mutant (V203M) OmpR. The results in Fig. 4 demonstrate that BPI-induced changes in ompF and ompC mRNA levels were diminished in MH760 as compared with MC4100, hence dependent on the presence of wt OmpR. Note, however, that at higher doses of BPI, a decrease in ompF mRNA and an increase in ompC mRNA was apparent in MH760 (Fig. 4B). Similar modest effects on ompF/C mRNA levels were also seen when this strain was treated with 15% sucrose (data not shown). Taken together, effects of BPI on OmpF/C synthesis were accompanied by parallel changes in mRNA levels and were dependent on the presence of wt OmpR.
BPI treatment enhances OmpR-dependent binding to ompF regulatory (repressor) sites
OmpR-dependent changes in OmpF and OmpC synthesis depend on induced changes in the DNA binding properties of OmpR (Russo and Silhavy, 1991). To determine whether BPI treatment affects OmpR-dependent binding to the ompF regulatory region, we measured in a gel-shift assay the binding of extracts of untreated or BPI-treated bacteria to a DNA fragment containing the –102 to –58 region of ompF. OmpR extracted from cells exposed to hyperosmotic conditions binds with higher affinity to this region of ompF, increasing formation of ‘complex b’ and generating a second, slower migrating, DNA–OmpR complex (‘complex a’) (Rampersaud et al., 1994). Similarily, in comparison with the extracts from untreated cells (Fig. 5, lane 2), extracts from BPI-treated E. coli formed ~fivefold greater amounts of ‘complex b’ and also produced the slower migrating complex (‘complex a’; Fig. 5, lane 3) not seen with extracts from untreated cells (Fig. 5, lane 2). Formation of these protein–DNA complexes was entirely dependent on the presence of OmpR; neither complex was formed when an extract from an ompR-null strain was used (Fig. 5, lanes 4 and 5). Total OmpR levels were identical in extracts from control and BPI-treated cells (data not shown). These findings demonstrate that early responses of E. coli to sublethal attack by BPI include increased OmpR-dependent binding to the up-stream region of ompF mediating repression of ompF expression.

Gel-shift assay of ompF fragment with cell extracts from untreated and BPI-treated cells. A 32P-labelled DNA fragment corresponding to the upstream sequence (−102) to (−58) from the ompF gene was incubated either alone or with extracts of E. coli MC4100 and E. coli TK821 prepared after incubation of bacteria with and without BPI. Lanes: 1, no extract added; 2, added extract from untreated cells; 3, added extract from BPI-treated cells; 4, added extract from untreated ompR-null TK821 cells; 5, added extract from BPI-treated ompR-null TK821 cells. Complexes ‘a’ and ‘b’ are marked as ‘a’ and ‘b’ respectively.
OmpR-deficient bacteria are more readily killed by BPI
As OmpR is a global regulator involved in the expression of many genes, we have investigated the possible role of OmpR in the ability of E. coli to survive attack by BPI. For this purpose, we compared sublethal and lethal effects of BPI on MC4100 and an isogenic ompR-null derivative (TK821). Sublethal and lethal effects of BPI were assayed by measuring bacterial colony-forming ability in NB agar ± albumin. Killed bacteria do not form colonies in either medium whereas sublethally damaged bacteria can form colonies when NB agar is supplemented with albumin (Mannion et al., 1990b). As shown in Fig. 6, initial (≤30 min) effects of BPI were nearly the same on both strains. Over a broad range of BPI doses (approximately 10–100 nM), both wt and ompR−E. coli were similarly sublethally injured within the first 30 min. In both strains, bacterial growth was arrested and CFU in NB agar declined by ≥90%. In contrast, nearly all bacteria retained the ability to form colonies in NB agar supplemented with albumin (Fig. 6). However, a dramatic difference in the fate of wt and ompR-null E. coli became apparent at later times (i.e. ≥60 min) of incubation with BPI: the mutant bacteria were killed whereas the wt bacteria were not killed but, instead, gradually regained the ability to form CFU in NB agar and multiply (Fig. 6). At higher concentrations (e.g. >100 nM), BPI killed both wt as well as ompR-null E. coli (data not shown). Therefore, the absence of OmpR renders E. coli more susceptible to the lethal effects of BPI. A similar effect was also observed in E. coli PL-2 (wt) and its isogenic ompR-null strain, PP-1 (data not shown). In contrast, ompR-null E. coli were not more sensitive to killing by a nonapeptide derivative of the polymyxin B which, like BPI, disrupts the bacterial outer membrane and increases bacterial sensitivity to actinomycin D (ActD) (Fig. 7).
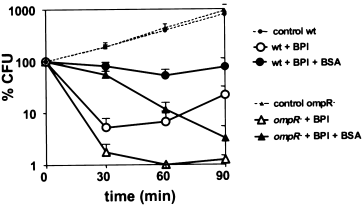
Greater susceptibility of OmpR-deficient E. coli to killing by low doses of BPI. Isogenic E. coli wt MC4100 and ompR-null TK821 (5 × 107 ml−1) were incubated in NB/0.9% NaCl buffered with 20 mM phosphate buffer pH 7.4 either alone or with BPI (see Experimental procedures for details). At the indicated times, bacterial viability was measured as described in Experimental procedures. Results are presented as a percentage of the number of initially added viable bacteria. Similar effects were seen with BPI concentrations ranging from 20 to 100 nM and also when 5 × 108 bacteria ml−1 were incubated with 200–1000 nM BPI. Results shown represent the mean of 3–5 experiments ± SEM.

Effects of polymyxin B nonapeptide on wt and ompR-null bacterial. Wild-type (MC4100) and ompR-null (TK821) bacteria were incubated with or without polymyxin B nonapeptide as indicated in the standard incubation mixture for 45 min at 37°C. After an additional incubation for 10 min with or without 50 μg ml−1 of acinomycin D (ActD), aliquots were taken to measure bacterial viability. Effects of the peptide with ActD (but not without ActD) reflect permeability increasing effects of the peptide rendering the bacteria sensitive to the toxic effects of ActD. In both strains, polymyxin B nonapeptide produced permeability-increasing effects but no bacterial killing.
In the absence of OmpR, OmpF and OmpC are not produced. To test if it was the absence of these porins in the ompR-null strain that accounted for increased sensitivity to killing by BPI, we constructed a strain PP4 that was ompF−ompC− in an ompR+ background. As shown in Fig. 8, strain PP-4 exhibited sensitivity to BPI that was closely similar to that of the wt strain and not of the ompR-null E. coli. These data indicate that the absence of porins per se is not what renders ompR-null bacteria of the TK821 strain more susceptible to killing by BPI.

Absence of porins OmpF and OmpC does not render ompR-null bacteria more sensitive to killing by BPI. Isogenic E. coli MC4100 (wt), TK821 (ompR-null) and PP4 (ompF−ompC−) were treated with 100 nM BPI as described in Experimental procedures and in a legend to Fig. 6. Diluted samples were plated in NB agar supplemented with 0.1% BSA. Results shown represent the mean of two independent experiments ± SEM.
OmpR-deficient bacteria are not more susceptible to permeability increasing effects of BPI and can also repair outer membrane permeability barrier disrupted by BPI. The preceding results (Fig. 6) suggest that whereas ompR-null E. coli are more readily killed by BPI, ompR+E. coli are as equally sensitive to the initial sublethal effects of BPI as ompR-null E. coli. To test this hypothesis more directly, we compared the permeability increasing effects of BPI in the two strains by measuring bacterial protein synthesis ± ActD (see Experimental procedures). In both strains, BPI initially had little effect on overall bacterial protein synthesis, but increased bacterial sensitivity to ActD, a reflection of increased outer membrane permeability (Table 1). BPI dose requirements for this permeability increasing effect were the same in the two strains (data not shown). In addition, BPI-treated ompR-null, as well as ompR+, regained resistance to ActD (i.e. repaired the outer membrane permeability barrier) when BPI-treated bacteria were further incubated with 40 mM MgCl2 (Table 1) before addition of ActD. These observations demonstrate that OmpR affects neither the sensi-tivity of E. coli to the permeability-increasing effects of BPI nor the ability of E. coli to repair these outer membrane alterations.
| % c.p.m. ([14C]-amino acids incorporation) | ||||
|---|---|---|---|---|
| Before Mg2+ | After Mg2+ | |||
| No ActD | + ActD | No ActD | + ActD | |
| wt | 103 ± 6 | 36 ± 6 | 98 ± 3 | 79 ± 7 |
| ompR– | 94 ± 3 | 43 ± 6 | 86 ± 2 | 86 ± 2 |
- Outer membrane permeability was measured as sensitivity to in-hibitory effects of ActD on bacterial protein synthesis as described in Experimental procedures. Isogenic wt PL-2 and ompR-null PP-1 (5 × 108 ml−1) were pre-treated with 500 nM rBPI21 for 15 min. [14C]-amino acids were added either 5 min later (after addition of medium ± ActD; before Mg2+) or after 30 min of incubation of bacteria with
- 40 mM MgCl2 followed by 5 min ± ActD (after Mg2+). After 15 min
- incubation with [14C]-amino acids, radiolabelled TCA precipitates were collected and counted as described in Experimental procedures. All results are expressed as a percentage of the incorporation of [14C]-amino acids into TCA-precipitable material by bacteria treated in the same way but without BPI. Values shown represent the mean ± SEM of at least five experiments.
Morphological evidence of greater perturbation of ompR− E. coli during sublethal phase of BPI action
The findings described above have demonstrated greater sensitivity of ompR−E. coli to killing by BPI, but have not revealed differences in either the extent of sublethal damage or the ability to repair this damage. To further compare the extent and nature of sublethal envelope alterations induced by BPI in wt and ompR-null E. coli, we examined these bacteria by scanning electron microscopy. After 15 min of incubation with BPI, wt bacteria showed only a modest increase in ‘pimpling’ of the bacterial surface (Fig. 9, A versus C). In contrast, shortly after BPI treatment, the surface of ompR− bacteria appeared more perturbed, with numerous membrane protrusions (compare B with D) up to 100 nm in length extending away from the bacterial surface. These effects were apparent in virtually all BPI-treated bacteria (Fig. 9D). The electron micrographs clearly show that the extent of BPI-induced bacterial surface alterations is greater in the OmpR deficient strain and that these differences are already apparent before bacterial death.

Scanning electron micrographs of BPI-treated bacteria. A total of 5 × 108E. coli PL-2 and PP-1 (ompR-null) bacteria ml−1 were incubated either alone or with 100 nM rBPI21 for 15 min, fixed in 2.5% glutaraldehyde, processed as described in Experimental procedures and viewed under scanning electron microscope at magnification of 30 000. (A) and (C) represent untreated or BPI-treated cells of E. coli PL-2, respectively, and (B) and (D) represent bacteria of the OmpR-deficient strain untreated (B) or treated with BPI (D).
Discussion
One of the hallmarks of the antibacterial action of BPI is the very discrete nature of the initial lesions inflicted on the bacteria. Prompt growth arrest is accompanied by discrete outer envelope alterations without initial loss of energy-dependent functions (Beckerdite et al., 1974; Weiss et al., 1978; Elsbach et al., 1979; Weiss et al., 1984). Under certain conditions, there is a pronounced temporal separation between early, potentially reversible, sublethal alterations of the bacterial outer envelope and later lethal lesions associated with damage to the bacterial inner membrane (Mannion et al., 1990b). Sev-eral host factors have been identified that can modulate, either positively or negatively, the rate of progression of BPI-dependent cytotoxicity (Mannion et al., 1990b). However, little is known about bacterial properties that could influence the progression of BPI-induced bacterial injury from reparable to irreparable alterations. The remarkable preservation of integrated biochemical functions suggests that bacteria upon exposure to BPI could activate complex adaptive responses to counter BPI- triggered bacterial damage and prevent the progression of bacterial injury.
We initially focused on alterations induced by BPI on expression of the two major non-specific outer membrane porins of E. coli, OmpF and OmpC. Our studies showed that whereas overall protein synthesis is not affected by sublethal exposure to BPI, there is a marked change in synthesis of outer membrane porins OmpF and OmpC upon BPI treatment. Exposure to BPI, for as little as 5 min, reduced OmpF synthesis and enhanced OmpC synthesis. These changes in porin synthesis were paralleled by changes in the level of mRNA for the respective porin proteins (1-4). These results suggest an alteration of de novo synthesis rather than a change in porin trimer assembly and/or translocation to the outer membrane. The fact that BPI-induced alterations in production of OmpF and OmpC were manifest only in strains capable of osmoregulation of porin expression (Fig. 3, data not shown) and that this response closely simulated the effects of exposure of the bacteria to hyperosmotic conditions (Fig. 3) is consistent with that view. Moreover, a strain of E. coli MH760 that expresses a mutant (V203M) form of OmpR that produces OmpF constitutively, with little or no osmoregulation, also showed little change in response to BPI. These findings indicate an important role of the transcriptional regulator OmpR in responses of E. coli to sublethal damage by BPI, as well as to hyperosmotic conditions.
OmpR is a DNA binding protein that binds to sites upstream of ompF and ompC genes (Tran et al., 2000). These sites are complex and OmpR binds to them with different affinities. Changes in OmpR concentration and/ or functional state alter OmpR binding to these sites and mediate differential expression of ompF and ompC (Rampersaud et al., 1991; Forst and Roberts, 1994; Pratt and Silhavy, 1995b). The functional state of OmpR is regulated by the reversible phosphorylation of Asp 55. Phosphorylation increases DNA binding affinity and increases the probability of OmpR binding to low affinity sites leading to repression of ompF expression and activation of ompC.
Increased medium osmolarity causes an elevation in the levels of phospho-OmpR that results in decreased production of OmpF and increased production of OmpC (Forst et al., 1990). We have not yet directly demonstrated similar effects of BPI. However, the similarity of the effects of BPI and 20% sucrose on porin expression and the increased OmpR-dependent binding of extracts of BPI-treated cells to upstream sequences in the ompF gene are consistent with an alteration of OmpR function in response to sublethal BPI treatment. In these experiments, the amount of OmpR present in extracts from untreated and BPI-treated E. coli is the same (data not shown). Therefore, a change of functional state of OmpR (i.e. increased phosphorylation of OmpR) induced by BPI seems most likely.
The levels of phospho-OmpR are regulated by the inner membrane sensor EnvZ, which has autokinase and phosphatase functions. EnvZ somehow senses changes in osmolarity and transmits this signal by increasing phosphorylation of OmpR at high osmolarity and increasing dephosphorylation of OmpR at low osmolarity (Forst et al., 1990; Russo and Silhavy, 1991; Waukau and Forst, 1999). However, EnvZ is not the only regulator of OmpR phosphorylation (Forst et al., 1990). In the absence of EnvZ, bacteria can still modulate OmpF and OmpC expression in response to changes in osmolarity (Forst et al., 1988; Leonardo and Forst, 1996; Fig. 3) and to treatment by BPI (Fig. 3), suggesting an alternative mechanism to phosphorylate OmpR. Acetyl-phosphate has been shown to act as a phospho-donor and to phosphorylate OmpR in vitro (Kenney et al., 1995), and to regulate OmpR-dependent porin expression in vivo (McCleary and Stock, 1994; Leonardo and Forst, 1996; Matsubara and Mizuno, 1999). The fact that BPI-induced changes are less in a strain producing mutant OmpR (V203M), which can be phosphorylated in vitro by EnvZ but not by acetyl-phosphate (Tran et al., 2000), could reflect an important role of acetyl-phosphate as well as differences in the DNA-binding properties of this mutant protein (Tran et al., 2000).
The membrane damage that accompanies the sublethal effects of BPI triggers increased lipid synthesis including de novo synthesis of LPS and phospholipids to repair the outer membrane permeability barrier and replace phospholipids catabolized by activated phospholipases (Mooney and Elsbach, 1975; Weiss and Elsbach, 1977; Weiss, 1984). Increased lipid synthesis may depend on increased intracellular pools of acetyl-CoA, a key precursor of fatty acid synthesis. We speculate (Fig. 10) that an early response of E. coli to sublethal membrane damage by BPI is an increased flux of carbon pools through acetyl-CoA which, together with enhanced expression of key biosynthetic enzyme(s) (Qi et al., 1995), may fuel the lipid synthesis needed for membrane repair (Fig. 10). An increase in acetyl-CoA would lead, via the action of phosphotransacetylase, to elevated intracellular levels of acetyl-phosphate and result in increased phospho-OmpR and alterations of porin expression (Fig. 10).
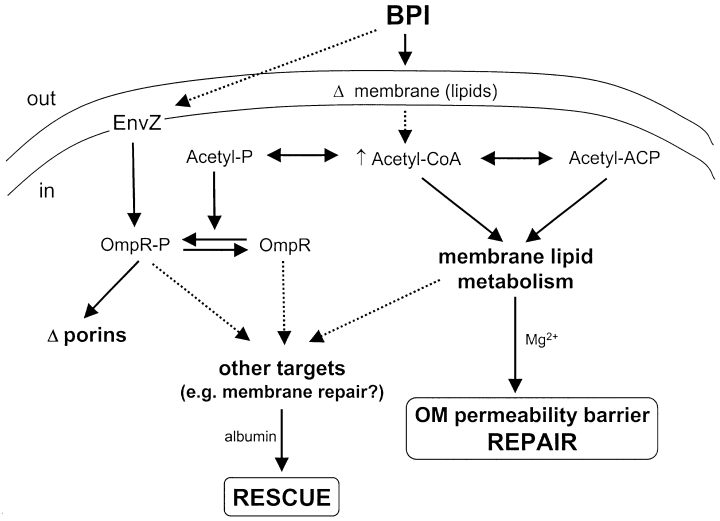
OmpR-dependent and OmpR-independent responses of E.coli to sublethal attack by BPI: Possible role of EnvZ and bacterial acetate metabolism. See Discussion for details. Events connected by solid arrows have been experimentally established whereas those denoted by broken arrows are hypothetical. Note that OmpR-independent repair occurs only when high concentrations of divalent cations are present. Conversely, OmpR-dependent rescue of sublethally damaged bacteria requires the presence of albumin. Acetyl-phosphate may affect OmpR phosphorylation directly or indirectly through either EnvZ or some other histidine kinase.
As repair of membrane damage by restoration of outer membrane permeability barrier is not dependent on OmpR, the modulation of the functional state of OmpR may be a secondary effect of bacterial responses to membrane damage inflicted by BPI. However, OmpR-deficient bacteria are more susceptible to killing by BPI and show increased envelope perturbations seen by scanning electron microscopy (SEM). These protrusions on the bacterial surface may reflect weakening of associations between the outer membrane and cell wall and represent envelope sites vulnerable to the progression of injury from the outer to the inner membrane.
Both OmpR and phospho-OmpR have transcriptional regulatory properties (Matsubara and Mizuno, 1999). We do not know whether the greater ability of OmpR+E. coli to tolerate low doses of BPI reflects protective effects of OmpR and/or of phospho-OmpR. We also do not know if such tolerance depends on acute OmpR-dependent responses to BPI or OmpR-dependent bacterial machinery made during growth, before exposure of the bacteria to BPI. The absence of OmpF and OmpC in ompR-null E. coli is not critical (Fig. 7), as the ability of these bacteria to resist killing by low doses of BPI is the same as in wt bacteria. Therefore, it is likely that changes in the levels of other targets, normally regulated by OmpR, account for the increased sensitivity of ompR-null bacteria to killing by BPI. Effects of OmpR can indeed encompass a broad array of cell functions (Bishop et al., 1998; Pruss, 1998; Vidal et al., 1998; Bang et al., 2000; Lee et al., 2000; Yamamoto et al., 2000) possibly including a salvage pathway for biosynthetic recycling of phospholipid breakdown products (R. Valdivia, personal communication). Identification in the future of OmpR-dependent bacterial components mediating tolerance to BPI should help to further define how BPI kills bacteria and the means by which, at higher doses, BPI can kill OmpR+E. coli (Mannion et al., 1990b).
Several lines of evidence suggest that the antibacterial action of BPI is not identical to that of other cationic antimicrobial compounds. The very discrete initial injury inflicted by BPI and accompanying window of sublethal damage is a unique feature of BPI action. This feature of BPI action permits one to study bacterial stress responses to this host defence agent. Although initial interactions of polymyxin B and BPI with the Gram-negative bacterial surface are generally similar, effects of polymyxin B on the outer membrane are rapidly followed by damage to the inner membrane and bacterial killing. In comparison with BPI, a nonapeptide derivative of polymyxin B produces similar sublethal alterations of the outer membrane but not the OmpR-dependent effects seen with BPI on bacterial porins (data not shown; Oh et al., 2000) and viability (Fig. 7). ompR-null E. coli are not killed by the polymyxin B nonapeptide (Fig. 7), suggesting that the increased sensitivity of these bacteria to BPI reflects an enhanced susceptibility that is specific to treatment with BPI. Furthermore, the ability of the OmpR-null bacteria to fully repair damage to the outer membrane permeability barrier to ActD indicates that increased sensitivity to killing by BPI is not a non-specific manifestation of bacteria less capable of tolerating sublethal injury.
Our data provide the first identification of bacterial machinery capable of regulating the progression of BPI-mediated cytotoxicity. Accumulating evidence implicates involvement of OmpR in modulation of virulence determinants (Dorrell et al., 1998; Mills et al., 1998; Beuzon et al., 2000; Lee et al., 2000). OmpR may play an important role in host–pathogen interaction and contribute to the virulence by enabling the bacteria to respond and adapt to bactericidal components of host environment.
Experimental procedures
Materials
Native human bactericidal/permeability-increasing protein (BPI) was purified from human polymorphonuclear leukocytes (PMN) as described previously (Mannion et al., 1989). Recombinant holo-human BPI and a bioactive N-terminal fragment of BPI comprising residues 1–193 (rBPI21) were obtained from XOMA. All effects of BPI described in this study were seen with each BPI species but most experiments used rBPI21. Polymyxin B nonapeptide was purchased from Calbiochem and [35S]-methionine (>1000 Ci mmol−1) from New England Nuclear. Actinomycin D (ActD) was obtained from Merck and Co., bovine serum albumin (BSA) from Boehringer Mannheim and GF/C glass microfibre filters from Whatman International.
Bacterial strains
Bacterial strains used in this study are listed in Table 2. All strains are E. coli K-12 derivatives except J5, which is derived from strain O111:B4. Transductions using P1vir were performed as described by Miller (1972). Transductants were selected for the presence of Tn10 on Luria–Bertani (LB) agar plates supplemented with 10 μg ml−1 of tetracycline. The absence of wild-type (wt) OmpR was confirmed by the absence of outer membrane porins OmpF and OmpC in outer membrane protein-enriched fractions. Strain PP4 lacks porins OmpF and OmpC and was constructed using one-step polymerase chain reaction (PCR) mutagenesis (Datsenko and Wanner, 2000). An ompF::cat deletion–insertion was introduced into strain BW25113 using the primer pair 5′-ATG ATGAAGCGCAATATTCTGGCAGTGATCGTCCCTGCTCTG TTAGTGTAGGCTGGAGCTGCTTC-3′ and 5′-AACTGGTAA ACGATACCCACAGCAACGGTGTCGTCTGAACCTACGCA TATGAATATCCTCCTTAGT-3′, with plasmid pKD3 as a template. The underlined nucleotides (nt) represent a 45-nt-long sequence homologous to the ompF sequence. Mutations were crossed into MH225 by P1vir transduction. The absence of ompF and ompC was verified by PCR and by the absence of the two porins in outer membrane protein-enriched fractions.
| Strain | Relevant genotype | Source/reference |
|---|---|---|
| J5 | O111:B4 galE | Gift from L.Leive |
| PL-2 | galE28 LAM-relA1 spoT1 thi-1 | Buttin (1963) |
| MC4100 | F–araD139Δ(argF-lac)U169 rpsL150 relA1 flb-5301 ptsF25 deoC1 | Casabadan (1976) |
| TK821 | MC4100 ompR::Tn10 | Silhavy et al. (1984) |
| PP-1 | PL2 ompR::Tn10 | This study |
| MH760 | MC4100 ompR472 | Hall and Silhavy (1981) |
| SG477 | MC4100 envZ22 | Garrett et al. (1983) |
| MH225 | MC4100 Φ(ompC′-lacZ+)10–25 | Hall and Silhavy (1981) |
| PP-4 | MH225 Δ(ompF::cat) | This study |
| BW25113 | lacI q rrnBT14ΔlacZWJ16hsdR514ΔaraBA-DAH33ΔrhaBADLD78 | Datsenko and Wanner (2000) |
Growth of bacteria
Bacteria were grown shaking at 37°C in either NB/0.9% NaCl or proteose peptone beef extract (PPBE). Stationary-phase overnight cultures were transferred to fresh medium (starting OD550 = 0.05) and the subcultures were grown to mid-log phase. Bacteria were harvested, washed and resuspended at the desired concentration in sterile saline.
Assay of cellular and outer membrane protein synthesis
Specific incubation conditions are indicated in the legends of appropriate figures. In brief, after incubation, bacteria were sedimented by centrifugation (6000 g, 10 min) and either resuspended in SDS–PAGE sample buffer or resuspended in 50 mM tris-HCl/2 mM EDTA to a concentration of 1.5 × 109 bacteria ml−1 and sonicated (50 W/15 s/4×). Sonicated samples were diluted 5× with 10 mM tris-HCl, pH 8.0 containing 10 μg ml−1 of DNase and 25 mM MgCl2. After 30 min incubation on ice, samples were spun at 1600 g for 20 min at 4°C and the recovered pellets were re-sonicated as above. Combined supernatants were spun at 225 000 g for 60 min at 4°C to sediment bacterial membranes. The membrane pellets were washed once with 10 mM tris-HCl, pH 7.5, resuspended in 0.5% sodium sarcosinate, vortexed for 20 min and spun at 225 000 g for 60 min to separate solu-bilized inner membrane from insoluble outer membrane. The pelleted outer membrane proteins were solubilized in SDS–PAGE sample buffer. All samples were boiled for 5 min before applying to SDS–PAGE gel (either 11% acrylamide or 9 M urea/10% acrylamide). Whole cell lysates were prepared by boiling bacteria resuspended in SDS–PAGE sample buffer for 10 min before separation by SDS–PAGE. Analogous samples from E. coli strains lacking OmpF or OmpC were used to confirm identity of OmpF and OmpC in experimental samples. Note the different migration of OmpF and OmpC proteins in the two electrophoretic systems.
Gel-shift assay
The DNA-binding assay was performed at 24°C as described by Waukau and Forst (1992). The ompF DNA fragment contained the upstream sequence (–102) to (–58) (5′-ATTT TACTTTTGGTTACATATTTTTTCTTTTTGAAACCAAATCTT-3′). Cell extracts were prepared from untreated or BPI-treated cells. Cells were washed with 20 mM sodium phosphate buffer, pH 7.1. After sonication in 400 μl of sodium phosphate buffer (4°C), the cell extracts were centrifuged (4°C) for 14 min at 390 000 g and the supernatants were collected. Soluble cell extracts were then incubated with the DNA for 20 min, loaded onto a low-ionic-strength 5% polyacrylamide gel and electrophoresed for 3–4 h. Gels were dried and exposed for autoradiography. The synthetic oligonucleotides used for creating the DNA-binding fragments were annealed and subsequently radiolabelled with [α-32P]-TTP by the Klenow fragment. The DNA fragments were gel-purified and finally suspended in 100–400 μl of sterile distilled water.
Reverse transcription-PCR (RT-PCR)
Bacteria were incubated ± BPI for 15 min at 37°C in the standard incubation mixture. Total RNA was isolated from 107 bacteria using RNeasy Kit (Qiagen) and reverse transcribed with random hexamers and AMV reverse transcriptase. Isolated RNA was treated with RNase-free DNase. PCR was then performed with cDNA derived from 2.5 × 103 bacteria. ompF, ompC and GAPDH were amplified using the following primers: 5′-TGCACTGGGTTACACCGATA-3′ and 5′-TTGTC GCGTCGTACTTCAG-3′ for ompF; 5′-CTACATGCGTCTTG GCTTCA-3′ and 5′-ATTCTGGCAGTACGTCGGTC-3′ for ompC; and 5′-AAAGACGGTCATCTGATCGTTAA-3′ and 5′-TCTTCGCACCAGCGGTGATGT-3′ for GAPDH. Primer pairs resulted in 351 (ompF), 224 (ompC) and 125 (GAPDH) nt PCR products that were resolved on 2% agarose gel. For each sample, the densities of ompF and ompC products were expressed relative to that of the band corresponding to GAPDH.
Measurement of bacterial viability
Mid-log phase bacteria were harvested and incubated with rBPI21. After indicated incubation times at 37°C, aliquots of the samples were serially diluted in sterile isotonic saline and plated in 6 ml of 1.3% (w/v) molten (50°C) Bacto-agar containing 0.8% (w/v) nutrient broth and 0.5% (w/v) NaCl and poured into a Petri dish. The agar was allowed to solidify at room temperature, and bacterial viability was measured as the number of colonies formed after incubation at 37°C for 18–24 h. To distinguish sublethal and lethal damage by BPI, bacteria were incubated in NB agar ± 0.1% albumin. Bacteria sublethally damaged by BPI can form colonies in NB agar supplemented with albumin but not in NB agar alone. Killed bacteria do not form colonies in either medium and undamaged bacteria grow equally well in both media.
Assay of outer membrane permeability
Outer membrane permeability-increasing effects of BPI and a nonapeptide derivative of polymyxin B were measured by determining the susceptibility of bacteria to ActD. The outer membrane of E. coli is normally impermeable to ActD (Beckerdite et al., 1974). Hence, this antibiotic normally does not affect RNA and protein synthesis by these bacteria nor bacterial viability. Sublethal effects of BPI and polymyxin B nonapeptide produce little or no inhibition of bacterial macromolecular synthesis or bacterial colony formation in nutrient agar supplemented with 0.1% albumin (Mannion et al., 1990b). Therefore, inhibition of either bacterial colony formation or protein synthesis by addition of ActD (50 μg ml−1) to BPI or polymyxin B nonapeptide is as a result of the inhibitory effects of ActD and reflects an increase in the outer membrane permeability to this drug. Bacterial protein synthesis was measured by incorporation of 14C-labelled mixed amino acids (0.5 μCi ml−1) into cold 10% trichloroacetic acid (TCA)-precipitable material during 15 min incubation at 37°C. Radiolabelled TCA precipitates were collected on glass microfibre GF/C filters and counted as described previously (Beckerdite et al., 1974).
Scanning electron microscopy
Bacteria incubated with or without BPI were fixed in 2.5% glutaraldehyde. Fixed samples (10 μl) were applied to silica bars, dried, rinsed with phosphate buffer, treated with 1% OsO4 in 0.1 M sodium cacodylate buffer pH 7.2 with 1.5% potassium ferrocyanide, rinsed with buffer, then water dehydrated by treatment in a graded series of ethanol solutions (25%, 50% and 75% for 15 min each, 95% for 1 h and 100% twice for 30 min) and finally treated with hexamethyldisilizane twice for 15 min.
Acknowledgements
We gratefully acknowledge Dr Peter Elsbach for many stimulating discussions throughout the course of this work and Michael Victor, Shu Chen and William Wertheim for their early experimental contributions. We thank Drs Theresa Gioannini and William Nauseef for their careful and critical review of the manuscript. We also thank Dr Stephen Carroll (XOMA Corp.) for providing recombinant BPI and Jian Shao from Central Microscopy Research Facility at the University of Iowa for help with electron microscopy. This work was support by PHS grant DK05472.