Restriction–modification system differences in Helicobacter pylori are a barrier to interstrain plasmid transfer
Abstract
Helicobacter pylori cells are naturally competent for the uptake of both plasmid and chromosomal DNA. However, we demonstrate that there are strong barriers to transformation of H. pylori strains by plasmids derived from unrelated strains. We sought to determine the molecular mechanisms underlying these barriers. Transformation efficiency was assessed using pHP1, an Escherichia coli–H. pylori shuttle vector conferring kanamycin resistance. Transformation of 33 H. pylori strains was attempted with pHP1 purified from either E. coli or H. pylori, and was successfully introduced into only 11 strains. Digestion of H. pylori chromosomes with different restriction endonucleases (REs) showed that DNA methylation patterns vary substantially among strains. The strain most easily transformed, JP26, was found to have extremely low endogenous RE activity and to lack a restriction–modification (R–M) system, homologous to MboI, which is highly conserved among H. pylori strains. When we introduced this system to JP26, pHP1 from MboI.M+ JP26, but not from wild-type JP26, transformed MboI R−M+ JP26 and heterologous MboI R−M+ wild-type H. pylori strains. Parallel studies with pHP1 from dam+ and dam−E. coli strains confirmed these findings. These data indicate that the endogenous REs of H. pylori strains represent a critical barrier to interstrain plasmid transfer among H. pylori.
Introduction
Helicobacter pylori, a microaerophilic, Gram-negative bacterium that colonizes the human stomach, is a causative factor in peptic ulcer disease and gastric cancer (Blaser, 1999). H. pylori strains have a high degree of genetic diversity (Akopyanz et al., 1992; Fujimoto et al., 1994; Alm and Trust, 1999; Wang et al., 1999), including highly prevalent point mutations in conserved genes such as ureB (Kansau, 1996) and flaA (Suerbaum et al., 1998), mosaicism within genes such as vacA (Atherton et al., 1995) and the presence of non-conserved genes, such as the cag island (Tummuru et al., 1995; Censini et al., 1996; Akopyants et al., 1998a). H. pylori strains also vary in the presence of insertion sequences (Censini et al., 1996; Berg et al., 1997; Hook-Nikanne et al., 1998), plasmids (Kleanthous et al., 1991), homologues of restriction–modification (R–M) genes (Tomb et al., 1997; Akopyants et al., 1998b; Alm et al., 1999) and gene order (Jiang et al., 1996). Although endogenous mutation is an important source of polymorphism, horizontal gene transfer between H. pylori strains also contributes to the substantial diversity within H. pylori populations (Lawrence and Roth, 1996; Suerbaum et al., 1998; Atherton et al., 1999; Kersulyte et al., 1999; Wang et al., 1999).
Many H. pylori strains are known to be naturally competent for transformation in vitro by chromosomal DNA (Nedenskov-Sorensen et al., 1990; Segal and Tompkins, 1993; Tsuda et al., 1993; Wang et al., 1993; Kuipers et al., 1998). Plasmids that can replicate stably and autonomously can also be introduced into various H. pylori strains by natural transformation (Kleanthous et al., 1991; Lee et al., 1997; Heuermann and Haas, 1998). In Bacillus subtilis, another species naturally competent for transformation, competence requires the expression of dedicated genes subject to regulation by a network of signal transduction proteins (Dubnau, 1993; 1997). Several genes, including dprA (Ando et al., 1999; Smeets et al., 2000) and comB (Hofreuter et al., 1998; Smeets et al., 2000), play a major role in the transformation of H. pylori cells by either chromosomal or plasmid DNA.
Bacteria have evolved defences to prevent adulteration of their own DNA, including restriction endonucleases (REs) to recognize and cleave foreign DNA, which are paired with methylases used to differentiate self-DNA from foreign DNA (Meselson and Yuan, 1968; Arber, 1974). These R–M systems have been classified as types I, II or III, according to cofactor requirements, enzyme subunit composition, structure of the recognition sequences and position of DNA cleavage relative to the recognition sequences (Wilson, 1991). Type II R–M systems comprise an RE that cleaves DNA within a specific sequence 4–8 bp in length and a methyltransferase that specifically methylates the DNA at adenine (A) or cytosine (C) residues within the same sequence, thereby protecting these sequences from cleavage (Bennett and Halford, 1989; Bickle and Kruger, 1993; Pingoud and Jeltsch, 1997). Chromosomal modification in H. pylori and Escherichia coli (Phadnis et al., 1993) and restriction digestions (Q. Xu et al., unpublished results) indicate that H. pylori DNA is highly methylated at both A and C residues. Analysis of genomic sequences predicted 14 or 15 potential type II R–M systems for H. pylori strains 26695 and J99 respectively (Tomb et al., 1997; Alm et al., 1999). Variation in the complement of R–M systems accounts for many of the non-conserved genes among the H. pylori strains studied (Tomb et al., 1997; Akopyants et al., 1998b; Alm et al., 1999).
We have now sought to characterize the transformation of H. pylori by plasmids, and to determine whether there exist intraspecies barriers to horizontal gene transfer. Our experiments indicated that H. pylori strains have diverse methylation patterns and important intraspecies barriers to plasmid transformation. We provide evidence implicating the differing complements of R–M systems among strains in these barriers and suggest a biological role for this phenomenon.
Results
Frequency of transformation of H. pylori strains by chromosomal DNA
We first sought to determine whether the H. pylori strains selected for study were competent for transformation. Four H. pylori strains were used as recipients, with chromosomal DNA from streptomycin-resistant (StrR) mutants of each as a source of transforming DNA (Table 1). Each of the four strains could be transformed to StrR by each of the chromosomal DNA preparations, and the frequency of transformation (× 10−4) was a median of 5.9 transformants/µg DNA/cfu for HPK5, 3.3 for 84-183, 5.6 for 60190 and 2.6 for J188. Homologous and heterologous chromosomal DNA transformed recipient H. pylori cells with essentially identical frequencies. Thus, the recipient cells were fully competent for transformation, and no strain-specific barriers to transformation were observed under these conditions.
| H. pylori strain usedas DNA source | Transformation frequencyab for indicated recipient H. pylori strain (× 10−4) | |||
|---|---|---|---|---|
| HPK5 | 84-183 | 60190 | J188 | |
| HPK5 | 4.4 | 1.0 | 6.4 | 1.7 |
| 84-183 | 6.4 | 2.3 | 3.8 | 1.1 |
| 60190 | 5.6 | 5.1 | 7.8 | 3.4 |
| J188 | 6.1 | 4.2 | 3.7 | 4.6 |
- a . The transformation frequency was determined on the basis of streptomycin-resistant colonies/µg DNA/recipient cfu, with homologous transformations underlined.
- b . Data represent the arithmetic mean of two experiments. Negative controls with no donor DNA resulted in no Str R colonies.
Transformation of H. pylori strains with plasmid DNA
Plasmid pHP1 (KanR) from E. coli strain DH5α was introduced into H. pylori strain HPK5 by electroporation. pHP1 isolated from HPK5 (designated as pHP1HPK5) was then used to attempt the transformation of 32 other H. pylori strains, but only transformed three (84-183, J188 and JP26), and at a lower frequency than for the homologous strain (Table 2). Subsequently, pHP1 was isolated from each of these three strains and used to transform other strains. In total, 11 of the 33 H. pylori strains tested could be transformed naturally with a pHP1 preparation of H. pylori origin (Table 2). Each plasmid isolated from a transformed H. pylori strain subsequently transformed its parental strain substantially better than heterologous strains. Strain JP26 was the best recipient strain, being transformed by 10 of the 11 pHP1 preparations of H. pylori origin (Table 2) but, conversely, pHP1JP26 was able to transform only strain 88-29 in addition to the homologous strain (JP26). Of 33 strains tested, only three strains (J188, JP26 and A101) were naturally transformed by pHP1DH5α and at low frequency (Table 2).
| H. pylori donorof plasmidpHP1 | Number of transformants of recipient strainb | Totald | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DH5α | HPK5 | 84-183 | 60190 | J188 | 97-762 | JP6 | JP26 | B146 | B148 | A101 | 88-29 | 22 other strainsc | ||
| DH5α | 93333 | 0 | 0 | 0 | 8 | 0 | 0 | 9 | 0 | 0 | 7 | 0 | 0 | 3 |
| HPK5 (Japan) | 1 | 382 | 9 | 0 | 3 | 0 | 0 | 65 | 0 | 0 | 0 | 0 | 0 | 4 |
| 84-183 (US) | 0 | 0 | 4880 | 1796 | 59 | 0 | 0 | 30 | 1 | 0 | 6 | 0 | 0 | 6 |
| 60190 (UK) | 2 | 0 | 3900 | 4500 | 1 | 0 | 0 | 123 | 10 | 0 | 2 | 0 | 0 | 6 |
| J188 (USA) | 2 | 0 | 13 | 57 | 1480 | 3 | 0 | 3 | 28 | 2 | 2 | 0 | 0 | 8 |
| 97762 (India) | 1 | 0 | 0 | 0 | 0 | 370 | 278 | 6 | 5 | 0 | 2 | 0 | 0 | 5 |
| Jp6 (Japan) | 0 | 0 | 0 | 0 | 0 | 0 | 1082 | 1 | 43 | 0 | 0 | 1 | 0 | 4 |
| Jp26 (Japan) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 422 | 0 | 0 | 0 | 4 | 0 | 2 |
| B146 (USA) | 1 | 5 | 0 | 0 | 0 | 10 | 0 | 3 | 186 | 0 | 4 | 3 | 0 | 6 |
| B148 (USA) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 39 | 4 | 220 | 2 | 0 | 0 | 4 |
| A101 (USA) | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0 | 3 | 1 | 100 | 1 | 0 | 5 |
| 88-29 (Thailand) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | 140 | 0 | 2 |
| Totale | 7 | 2 | 4 | 3 | 5 | 3 | 3 | 11 | 8 | 3 | 8 | 5 | 0 | |
- a . 100 ng of pHP1 was introduced into each of the 11 donor H. pylori strains (but not successfully into 22 others) by selection of transformants for Kan R.
- b . Cells of each H. pylori recipient strain were incubated for 24 h with 100 ng of pHP1 purified from the specified donor strain. Homologous transformations are underlined. E. coli DH5α was transformed with 1 µg of each pHP1 preparation using the CaCl 2 method.
- c . Strains (location) are: J99 (USA), J166 (USA), 88-22 (USA), J178 (USA), B128 (USA) J195 (USA), J262 (USA), HPK1 (Japan), JP21 (Japan), JP25 (Japan), JP28 (Japan), 97-650 (India), 97-713 (India), 97-645 (India), CH4 (China), CH2 (China), 97-299 (Colombia), 97-146 (Colombia), 26695 (UK), 7767 (Zaire), 315 (Samoa), 549/91 (Finland).
- d . Total number of recipient H. pylori strains transformed by the specified pHP1 preparation.
- e . Total number of pHP1 preparations able to transform the specified recipient strain.
Presence of plasmids from wild-type H. pylori strains
The 33 strains were examined to determine whether they possessed native plasmids. Endogenous plasmids were found in four (HPK5, 97-762, B148 and 88-29) of the 11 strains that could be transformed by pHP1H. pylori and seven (88-22, CH4, 7767, 315, 97-713, 97-645 and B128) of the 22 strains that were not transformed by pHP1H. pylori. As the frequency of plasmids was the same in the transformable and non-transformable strains, and only one of the strains (B128) possessed the replicon present in pHP1 (Kleanthous et al., 1991), as determined by polymerase chain reaction (PCR) using specific primers (BA1087 and BA1088), we concluded that the presence of competing endogenous plasmids was not a substantial barrier to transformation by pHP1. (The sequences of all the primers in the text are available on request.)
Efficiency of H. pylori transformation by plasmid DNA using natural transformation or electroporation
Strains HPK5 and 60190 could be transformed by pHP1 that originated from the homologous strain, but not by the heterologous preparations (Tables 2 and 3). Next, we sought to introduce pHP1HPK5 and pHP160190 into strains HPK5 and 60190 using electroporation to attempt to overcome the barrier. Electroporation permitted the introduction of pHP1HPK5 into HPK5 4.7 times more frequently than by natural transformation and permitted the introduction of pHP160190 into 60190 11.1 times more frequently than by natural transformation. However, electroporation did not result in either of the heterologous transformations, indicating the substantial nature of the barrier to interstrain plasmid transfer in H. pylori.
| Recipient H.pylori strain | Source of pHP1 DNA | Transformation frequencya | |
|---|---|---|---|
| Natural transformation | Electroporation | ||
| HPK5 | Noneb | < 1.4 ± 0.4 × 10−8 | < 2.5 ± 0.4 × 10−8 |
| HPK5 | 7.8 ± 0.3 × 10−6 | 3.7 ± 0.4 × 10−5 | |
| 60190 | < 1.2 ± 0.6 × 10−8 | < 1.9 ± 0.7 × 10−8 | |
| 60190 | Noneb | < 1.3 ± 0.3 × 10−8 | < 1.6 ± 0.6 × 10−8 |
| HPK5 | < 1.6 ± 0.6 × 10−8 | < 1.7 ± 0.3 × 10−8 | |
| 60190 | 2.7 ± 0.4 × 10−6 | 3.0 ± 0.3 × 10−5 | |
- a . DNA used for transformation was from plasmid pHP1 isolated from the designated H. pylori strain. The transformation frequency was determined on the basis of Kan R colonies/µg DNA/recipient cfu. Data represent the arithmetic mean of two independent experiments ± the standard error of the mean.
- b . Recipient cells were transformed with buffer alone (without pHP1 DNA); no Kan R colonies were detected in any case.
Restriction digestion of chromosomal DNA and pHP1
We asked next whether the barriers to transformation might be related to the differential presence of REs. Digestion of chromosomal DNA from the 11 strains transformed by pHP1 showed differing patterns of susceptibility to 13 REs (Fig. 1 and Table 4). DNA from all strains tested was resistant to digestion by NlaIII, AvaI, BglI, PstI and SacI, indicating the presence of a cognate methylase, but all were susceptible to HindIII (Table 4). Identical restriction patterns were observed for strains 60190 and 84-183, consistent with their transformation frequency similarities (Table 4). Digestion of chromosomal DNA from seven strains (including genome-sequenced strains 26695 and J99) that could not be transformed by pHP1 showed a variety of patterns (Table 4). As expected, digestion of pHP1 isolated from the 11 transformed strains showed RE susceptibility patterns (Fig. 1) that paralleled those for the chromosomal DNA of the strain from which the plasmid was isolated. These results indicated that each H. pylori strain has its own varied complement of functioning R–M systems, and suggests that these differences among strains affect transformation by plasmids.
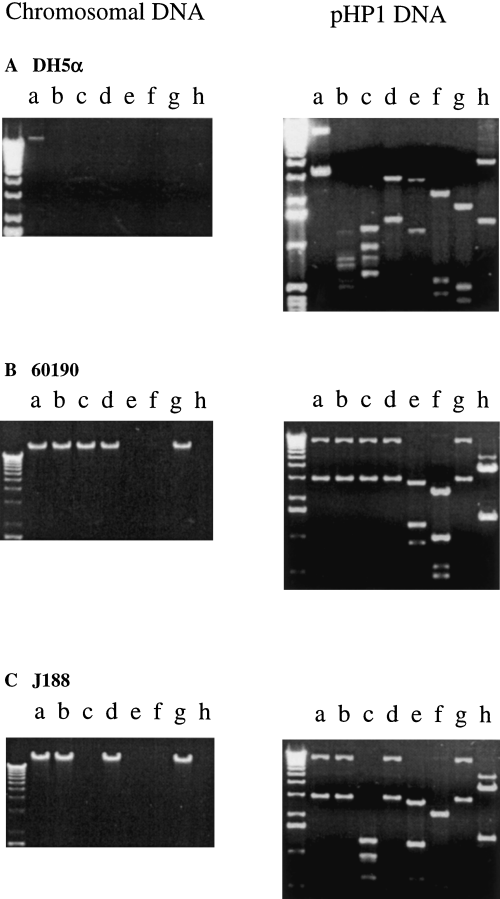
Restriction endonuclease digestions of chromosomal DNA (left) and pHP1 DNA (right) from E. coli DH5α (A) and H. pylori strains 60190 (B) and J188 (C). Lanes: a, uncut; b, NlaIII; c, MboII; d, HinfI; e, FokI; f, HaeIII; g, HpaII; and h, HindIII.
| Source ofchromosomal DNA | Restriction endonuclease used for digestion | Hpy188Id | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NlaIII | MboII | HinfI | FokI | HaeIII | HpaII | DdeI | HindIII | MboI | CviRIb | TaqIb | Hpy178IIIc | ||
| Strains transformable by pHP1 | |||||||||||||
| HPK5 | –e | – | + | – | + | – | – | + | – | + | + | – | + |
| 84-183 | – | – | – | + | + | – | + | + | – | + | + | – | + |
| 60190 | – | – | – | + | + | – | + | + | – | + | + | – | + |
| J188 | – | + | – | + | + | – | + | + | – | + | – | – | – |
| 97-762 | – | + | – | + | + | – | + | + | – | + | – | – | + |
| Jp6 | – | + | – | + | – | – | – | + | – | – | – | – | + |
| Jp26 | – | – | + | + | – | – | + | + | + | – | – | – | + |
| B146 | – | + | – | + | + | – | – | + | – | – | – | – | + |
| B148 | – | – | – | + | + | – | + | + | – | + | – | – | + |
| A101 | – | + | – | + | + | – | + | + | – | + | + | – | + |
| 8829 | – | + | + | – | + | – | – | + | + | – | – | – | + |
| Strains not transformable by pHP1 | |||||||||||||
| 26695 | – | – | – | + | + | + | + | + | – | + | + | + | + |
| J99 | – | + | – | + | + | – | + | + | – | + | – | – | + |
| 88–22 | – | – | + | + | + | – | – | + | – | – | – | – | + |
| J166 | – | + | + | + | + | – | + | + | – | – | – | + | – |
| J178 | – | – | – | – | – | – | + | + | – | + | – | – | + |
| JP21 | – | + | – | + | + | + | + | + | – | + | + | – | + |
| JP25 | – | + | – | + | – | – | – | + | – | + | + | – | – |
| JP28 | – | + | – | + | + | – | + | + | + | + | – | – | + |
- a . No strains were susceptible to NlaIII, AvaI, BglI, PstI or SacI.
- b . Isolated from JP26 (Q. Xu et al. unpublished results)
- c . Isolated from J178 (Q. Xu et al. unpublished results)
- d . Isolated from J188 (Q. Xu et al., submitted for publication)
- e .–, not susceptible to digestion; +, susceptible.
E. coli DH5α was transformed by six of the 11 pHP1 preparations from H. pylori strains (Table 2). pHP1 was isolated from each newly transformed DH5α isolate, and their restriction digestion patterns were compared. As expected, each of these plasmids was the same size and showed the same restriction digestion patterns as the original pHP1DH5α, indicating that deletion or rearrangement in H. pylori was not responsible for differences in interstrain transformation ability (data not shown).
MboI resistance of H. pylori
Re-examination of the 26695 genomic sequence showed that genes HP0091 and HP0092, previously annotated as hsdR and hsdM, in fact showed highest similarity to MboI.R (blast probability = 10−69) and to MboI.M (blast = 10−81) respectively. When we examined the susceptibility of H. pylori chromosomal DNA to MboI, we found that strain JP26, from which pHP1 preparations transformed the least other H. pylori strains (Table 2), was susceptible to digestion by MboI, whereas nearly all the other strains tested were resistant (Table 3). To determine the variation in susceptibility to MboI.R, we surveyed 100 H. pylori strains from different parts of the world [Japan (n = 40), India (n = 11), China (n = 4), Thailand (n = 7), USA (n = 13), Latin America (n = 13), Oceania (n = 6), Europe (n = 5) and Zaire (n = 1)]. Only three [JP26 (Japan), JP28 (Japan) and 88-29 (Thailand)] of the 100 H. pylori strains were susceptible to digestion by MboI. We then hypothesized that the lack of MboI methylation might account, at least in part, for the differences in transformation for JP26 compared with the other strains. For four strains with chromosomal DNA resistant to MboI digestion, evidence of the R–M system was demonstrated by PCR, whereas there was no evidence for the intact R–M genes in strains JP26 and JP28 with MboI-digestible DNA (Fig. 2A).
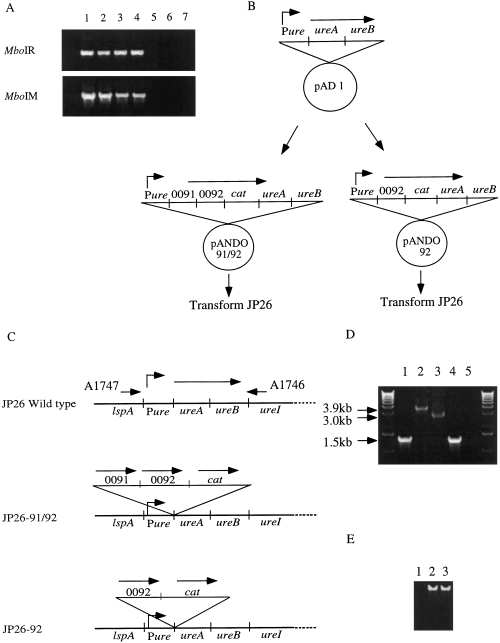
Assessment of the conservation of the MboI R–M system homologues (HP0091/HP0092) and creation of mutants of H. pylori strain JP26 into which the R–M homologues (HP0091/HP0092) or the methylase homologue (HP0092) alone were introduced in single copy on the bacterial chromosome.
A. PCR with HP0091 (MboI.R) locus primers C9065 and C9066 or HP0092 (MboI.M) locus primers BA3136 and BA3137. Lanes: 1, 26695; 2, J99; 3, B146; 4, JP25; 5, JP26; 6, JP28; 7, DNA (–).
B. Creation of pANDO91/92 and pANDO92. A fragment of the ureAB locus was introduced into pUC19, and then HP0091 and HP0092, or HP0091 alone, and cat were introduced sequentially to create pANDO91, 92 or pANDO92.
C. Schematic diagram of the construction of mutants of H. pylori strain JP26 in which HP0091and HP0092, or HP0092 alone, are introduced by allelic exchange by marker (cat) rescue. For each strain, the locus surrounding HP0091and HP0092, or HP0092, and ureAB is shown.
D. PCR with ureAB locus primers A1747 and A1746 to ascertain whether the mutants of JP26 were constructed correctly. Lane 1, JP26 wild type; lane 2, JP26-91/92; lane 3, JP26-92; lane 4, 26695 wild type; lane 5, DNA (–).
E. MboI digestion of H. pylori strain JP26 and its isogenic mutants. Lane 1, JP26 wild type; lane 2, JP26-91/92; lane 3, JP26-92. As expected, strains JP26-91/92 and JP26-92 were resistant to MboI digestion.
Impact of MboI R–M homologues on transformation efficiency
To determine whether the presence of the MboI R–M homologues affects the transformation frequency, we constructed mutants of JP26, in which we introduced both HP0091 and HP0092 to create JP26-91/92, or HP0092 (methylase homologue) alone to create JP26-92 (Fig. 2). We then transformed strains JP26-91/92 and JP26-92 with pHP1DH5α and isolated pHP1JP26-91/92 and pHP1JP26-92 respectively (Table 5). To study the function of HP0091, the predicted MboI.R homologue, we also constructed a mutant of H. pylori strain B146 in which cat was inserted into the HP0091 ORF (B146-91::cat). The colony formation and growth characteristics of the JP26-91/92, JP26-92 and B146-91::cat mutants were no different from wild-type strains JP26 and B146 (data not shown).
| Strain used as source of pHP1a | Number of transformants of recipient strainb | ||||
|---|---|---|---|---|---|
| JP26(MboI R−M−) | JP26-92 (MboI R−M+) | JP26-91/92 (MboI R+M+) | B146(MboI R+M+) | B146/91::cat(MboI R−M+) | |
| H. pylori JP26 (MboI R−M−) | 124 ± 14 | 104 ± 4 | 0 ± 0 | 0 ± 0 | 4 ± 1 |
| H. pylori JP26-92 (MboI R−M+) | 115 ± 11 | 201 ± 16 | 223 ± 9 | 4 ± 1 | 6 ± 1 |
| H. pylori JP26-91/92 (MboI R+M+) | 119 ± 10 | 203 ± 12 | 219 ± 18 | 6 ± 1 | 8 ± 1 |
| H. pylori B146 (MboI R+M+) | 5 ± 0.3 | 5 ± 1 | 7 ± 0 | 517 ± 23 | 514 ± 34 |
| E. coli DH5α (dam+) | 12 ± 1 | 8 ± 1 | 10 ± 1 | 9 ± 1 | 10 ± 1 |
| E. coli SCS110 (dam−) | 12 ± 3 | 11 ± 1 | 0 ± 0 | 0 ± 0 | 12 ± 1 |
- a . pHP1 from H. pylori strains (100 ng) or from E. coli strains (1 µg) was introduced into each of the recipient strains by selection of transformants for Kan R. In the absence of pHP1 (buffer alone), no KanR transformants were observed.
- b . Cells of each recipient strain were incubated for 24 h with pHP1 purified from the specified donor strain.
- Homologous transformations are underlined. The indicated value is the mean of three independent experiments ± the standard error of the mean.
We then asked whether disruption of HP0091 (MboI.R homologue) or the addition of HP0092 (MboI.M homologue) or both HP0091 and HP0092 affected the ability of H. pylori strains to be transformed by pHP1 preparations derived from these wild-type and mutant strains (Table 5). pHP1JP26 transformed cells of JP26 and JP26-92 (R−M+) but not JP26-91/92 (R+M+), indicating that the insertion of a functional MboI.R (HP0091) in those cells presented a new barrier to transformation (Table 5). As expected from previous results (Table 2), pHP1JP26 could not transform B146 cells; however, a few transformants were observed when HP0091 (MboI.R homologue) was interrupted in B146-91::cat (Table 5). The plasmids from the MboI.M+ JP26 strains, pHP1JP26-92 and pHPJP26-91/92, transformed all five H. pylori strains, with greater efficiency for the JP26 derivatives than for the B146 derivatives. Conversely, all five strains were also transformed by pHP1B146, although, as expected, the B146 derivatives were transformed more efficiently than the JP26 derivatives.
Transformation of H. pylori cells by pHP1 according to E. coli donor strain dam status
MboI.M and Dam both methylate adenines in the recognition sequence GATC. The use of E. coli strains that are either Dam+ (DH5α) or Dam− (SCS110) provided another means for testing the role of GATC-specific adenine methylation in H. pylori transformation barriers. pHP1DH5α transformed all five strains to an equal degree, consistent with Dam methylation of GATC (Table 5). In contrast, pHP1 from dam−E. coli strain SCS110 was unable to transform the HP0091+ (MboI.R+) strains JP26-91/92 and B146, whereas it transformed the HP0091− (MboI.R−) strains JP26, JP26-92 and B146-91::cat to the same degree as did pHP1DH5α. In total, these data confirm the importance of HP0091, the MboI.R homologue, as one barrier to transformation by pHP1 preparations from strains that lack MboI.M or Dam methylase activity.
Transformation of H. pylori cells by supercoiled, circular or linear plasmid DNA
We next asked whether the structure of donor pHP1 as supercoiled circular, religated (open) circular or linear DNA affected the ability of H. pylori cells to be transformed. For comparison, we also studied pHPT180, an E. coli vector unable to replicate in H. pylori. In pHPT180, aphA (KanR) inserted into ureB can be rescued by allelic exchange with the H. pylori chromosome. When H. pylori strain HPK5 was transformed by pHP1HPK5, there was little difference between the two circular forms [supercoiled 166 ± 25 (mean ± SE for four experiments); religated 193 ± 37 (mean ± SE for two experiments)], but substantially fewer transformants from the linearized plasmid (3 ± 0; mean ± SE for four experiments; P < 0.001 for both comparisons, Student's t-test, two-tailed). For suicide vector pHPT180, there was little difference in transforming ability between circular (325 ± 18; mean ± SE for three experiments) and linear (333 ± 26; mean ± SE for three experiments) DNA. Using streptomycin resistance as a marker, we found that as little as a 100 bp fragment encompassing the rpsl2 point mutation was sufficient to transform homologous or heterologous H. pylori cells.
Isolation and purification of restriction endonucleases
We next sought to characterize the REs of wild-type strain JP26. A crude extract from 10 g of cells was applied to heparin Hitrap, Q sepharose and HAP columns before two type II REs, Hpy26I and Hpy26II, were completely separated. Hpy26I was eluted from the Q sepharose column by NaCl at 0.25–0.27 M, whereas Hpy26II was eluted from the HAP column by potassium phosphate at about 0.1 M. The recognition site of Hpy26I was determined to be TGCA and, thus, is an isoschizomer of CviRI. HpyJP26II recognizes TCGA and is an isoschizomer of TaqI. Their specific activities were extremely low (< 20 units for HpyJP26I and ≈ 50–60 units for HpyJP26II), whereas other H. pylori strains generally exhibit total activities ≈ 100 times higher (Q. Xu et al., unpublished results). When chromosomal DNA from H. pylori strains was incubated with either of these purified REs, the DNA of several strains was protected, indicating the presence of cognate methylation (Table 4).
Discussion
In this study, we found strong barriers to transformation of H. pylori cells by plasmids (pHP1) from heterologous H. pylori strains. That chromosomal DNA transformed all strains well, and that each pHP1 derivative subsequently transformed its homologous wild-type strain better than heterologous strains indicates that the barriers were not the result of lack of competence of the host strains. That application of electroporation, although increasing the efficiency of homologous pHP1 transformation, had no detectable effect on heterologous transformations (Table 3) indicates that the strong barriers to transformation by heterologous plasmid DNA could not be easily overcome. In Neisseria gonorrhoeae, host-mediated restriction can prevent the acquisition of plasmid DNA by transformation, but not by conjugation (Stein et al., 1988). As H. pylori has a DNase-resistant conjugation-like mechanism (Kuipers et al., 1998), the role of host-mediated restriction for this mechanism of DNA exchange should be defined in future studies.
That chromosomal DNA from various H. pylori strains differs in susceptibility to restriction endonucleases indicates the differential functional presence of specific DNA methylases (Table 4). These data extend the observations on the two sequenced H. pylori strains (26695 and J99), which differ in the putative methylases present. As these methylases are often paired with cognate REs to form R–M systems, these data suggest that H. pylori strains differ in their complement of R–M systems, as proposed by the analysis of genomic data (Tomb et al., 1997; Akopyants et al., 1998b; Alm et al., 1999) and as indicated by recent biochemical studies (Q. Xu et al., unpublished). However, methylases may be present in H. pylori cells, whereas the cognate RE is absent or partially deleted (Tomb et al., 1997; Alm et al., 1999; Figueirdo et al., 2000).
MboI digested chromosomal DNA from H. pylori strains JP26, JP28 and 88-29, but not from 97 other H. pylori strains. Plasmids pHP1JP26 and pHP188-29 could only transform strains JP26 and 88-29 (Table 2), which were presumed to lack a functioning MboI.R homologue as they did not have MboI-specific methylation. If most H. pylori strains possess a homologue to MboI.R, as indicated by analysis of representative strains (Fig. 2A), the lack of MboI methylation in strain JP26 would be consistent with the failure of pHP1JP26 to transform all H. pylori strains tested (except JP26 and 88-29). Our hypothesis that an H. pylori MboI-like R–M system represents a barrier to transformation could be tested by our fortuitous discovery that HP0091/0092, noted in genome strain 26695, was highly homologous to the MboI R–M system. By mutation of wild-type strains by introducing HP0091 and/or HP0092, or by deleting HP0091 (Fig. 2 and Table 5), we showed that MboI.R+ strains were transformed by pHP1 from MboI.M+ strains, but not from MboI.M− strains, whereas MboI.R− strains were transformed by pHP1, regardless of MboI.M status.
Most E. coli strains contain three site-specific DNA methylases (Dam, Dcm and EcoKI). As Dam (encoded by dam) methylates the adenines in GATC (Marinus, 1973; Geier, 1979), plasmid DNA isolated from dam+E. coli should be completely resistant to GATC cleavage by MboI. That MboI.R+H. pylori strains were transformed by pHP1DH5α but not pHP1SCS110, whereas MboI.R− strains were transformed by pHP1 from either strain, confirms the important role of GATC methylation in H. pylori–E. coli interspecies restriction barriers. Together, these transformation data, based upon both H. pylori and E. coli strains, indicate the important role of the MboI R–M homologues as barriers to the transformation of H. pylori by plasmids from heterologous strains. This proof of concept suggests that other R–M systems in H. pylori represent parallel barriers.
The studies with circular and linear plasmid DNA provide one explanation for the transformation frequency differences when heterologous chromosomal (Table 1) or plasmid (Table 2) DNA is used, as pHP1 DNA transformed well when circular, whether supercoiled or not, whereas linearizing the DNA essentially ablated transformation. Because pHP1, a shuttle vector, must autonomously replicate within H. pylori cells, preservation of the circular form was apparently essential, indicating that religation frequency is low or non-existent. These experiments demonstrate that RE digestion can greatly curtail transformation by linearizing the DNA of obligately self-replicating plasmids. In contrast, that linearization of suicide vector pHPT180 had essentially no effect was expected, as replication in H. pylori cells is neither possible nor required for transformation to occur. Although linear DNA is also subject to restriction digestion, we have now shown that the presence of homologous DNA flanking the point mutation site in rps12 (for StrR) need not be extensive to permit allelic exchange.
The low (CviRI and TaqI) RE activity in JP26 is consistent with its relatively efficient transformation by (heterologous) pHP1 from strains with other R–M systems and provides further support for the hypothesis that R–M systems have an important role as strain-specific barriers to H. pylori transformation. Although strain JP26 has multiple methylase activities (Table 4), their presence does not necessarily imply active cognate REs, as demonstrated by the studies of genome strains 26695 and J99 (Tomb et al., 1997; Alm et al., 1999) and our biochemical analyses of JP26. In the companion paper, Donahue et al. (2000) offer other direct experimental evidence for the importance of intraspecies restriction barriers in limiting the transformation of H. pylori strains and provide methods for overcoming these barriers, which should facilitate investigation in this field.
H. pylori is unique in the number and variety of R–M systems that each strain possesses (Tomb et al., 1997; Alm et al., 1999; Q. Xu et al., unpublished results), and it appears that few strains possess the same complement of R–M systems. That the diversity represents a simple parasitism by ‘selfish’ R–M systems is unlikely, based on both the interstrain variation and the presence of inactive RE genes in many strains (Figueirdo et al., 2000). An alternative hypothesis is that H. pylori has evolved a biological niche that uses the diverse R–M systems for its benefit as well. As H. pylori are naturally competent, and co-colonization of hosts leads to horizontal gene transfer (Suerbaum et al., 1998; Kersulyte et al., 1999), one advantage of strains possessing different systems may be to provide limits to transformation. In that way, one strain cannot directly overwhelm the other via transformation, but two (or more) H. pylori populations can co-exist, with the potential for continuous intraspecies genetic exchange over the long course (decades) of colonization of a single host.
Experimental procedures
Bacterial strains
The strains and plasmids used in this study are listed in Table 6. E. coli was grown routinely at 37°C in Luria–Bertani (LB) broth or agar supplemented with ampicillin (100 µg ml−1) and/or kanamycin (25 µg ml−1), when appropriate. H. pylori strains were grown on trypticase soy agar (TSA) with 5% sheep blood (BBL) or Brucella serum (BS) (BBL) agar with 10% newborn calf serum (Intergen) at 37°C in a 5% CO2 atmosphere. We selected strains that were streptomycin resistant (Strr), as described previously (Ando et al., 1999); these strains had an A128G point mutation in codon 43 of rps12, resulting in a lysine to arginine change conferring streptomycin resistance (D. A. Israel et al., unpublished results). In subsequent experiments, antibiotic-resistant H. pylori transformants were selected with 25 µg ml−1 kanamycin or 20 µg ml−1 streptomycin, as appropriate.
| Designation | Relevant characteristics | Other genotypes | Country where isolated or reference |
|---|---|---|---|
| H. pylori | |||
| HPK5 | Wild type | cagA + iceA1 vacAs1am1 | Japan |
| HPK5 StrR KanR | StrR, KanR | HPK5 StrR/pHP1 | This study |
| 84-183 | Wild type | cagA + iceA2 vacAs1bm1 | USA |
| 84-183 KanR | KanR | 84-183/pHP1 | This study |
| HPK1 | Wild type | cagA + iceA1 vacAs1am1 | Japan |
| 26695 | Wild type | cagA + iceA1 vacAs1m1 | UK |
| 60190 | Wild type | cagA + iceA1 vacAs1am1 | UK |
| 60190 KanR | KanR | 60190/pHP1 | This study |
| J166 | Wild type | cagA + iceA1 vacAs1bm1 | USA |
| 88-22 | Wild type | cagA − iceA2 vacAs2m2 | USA |
| CH4 | Wild type | cagA + iceA1 vacAs1am2 | China |
| 97-650 | Wild type | cagA + iceA2 vacAs1am1 | India |
| 7767 | Wild type | cagA + iceA1 vacAs2m2 | Zaire |
| 315 | Wild type | cagA − iceA1 vacAs1am2 | Samoa |
| 549/91 | Wild type | cagA + iceA1 vacAs1am1 | Finland |
| 97-299 | Wild type | cagA + iceA2 vacAs1bm1 | Colombia |
| J178 | Wild type | cagA + iceA2 vacAs1am1 | USA |
| J188 | Wild type | cagA − iceA2 vacAs2m2 | USA |
| J188 KanR | KanR | 8/pHP1 | This study |
| J99 | Wild type | cagA + iceA2 vacAs1bm1 | USA |
| CH2 | Wild type | cagA + iceA1 vacAs1am1 | China |
| 97-146 | Wild type | cagA + iceA1 vacAs1am1 | Colombia |
| 97-762 | Wild type | cagA + iceA2 vacAs1am1 | India |
| 97-762 KanR | KanR | 97-762/pHP1 | This study |
| 97-713 | Wild type | cagA + iceA2 vacAs1am1 | India |
| 97-645 | Wild type | cagA + iceA1 vacAs1am1 | India |
| JP6 | Wild type | cagA + iceA1 vacAs1cm1 | Japan |
| JP6 KanR | KanR | JP6/pHP1 | This study |
| JP21 | Wild type | cagA + iceA1 vacAs1cm1 | Japan |
| JP25 | Wild type | cagA + iceA1 vacAs1cm1 | Japan |
| JP26 | Wild type | cagA + iceA1 vacAs1cm1 | Japan |
| JP26 KanR | KanR | JP26/pHP1 | This study |
| JP26-91/92 | CamR | JP26, Pure-HP0091, HP0092-catureAB | This study |
| JP26-92 | CamR | JP26, Pure-HP0092-cat′ureAB | This study |
| JP26-91/92 KanR | CamR, KanR | JP26-91/92/pHP1 | This study |
| JP26-92 KanR | CamR, KanR | JP26-92/pHP1 | This study |
| B128 | Wild type | cagA + iceA1 vacAs1am1 | USA |
| B146 | Wild type | cagA − iceA2 vacAs2m2 | USA |
| B146 KanR | KanR | B146/pHP1 | This study |
| B146-91::cat | CamR | B146/HP0091:: cat | This study |
| B148 | Wild type | cagA + iceA2 vacAs1am2 | USA |
| B148 KanR | KanR | B148/pHP1 | This study |
| A101 | Wild type | cagA + iceA1 vacAs1am2 | USA |
| A101 KanR | KanR | A101/pHP1 | This study |
| 88-29 | Wild type | cagA + iceA1 vacAs1cm1 | Thailand |
| 88-29 KanR | KanR | 88-29/pHP1 | This study |
| JP28 | Wild type | cagA + iceA1 vacAs1cm1 | Japan |
| J195 | Wild type | cagA − iceA2 vacAs2m2 | USA |
| J262 | Wild type | cagA − iceA2 vacAs2m2 | USA |
| E. coli | |||
| DH5α | endA1 hsdR17 (rK−mK+) supE44 thi-1 recA gyrA (Nalr) relA1 Δ(argF-lacZYA)U169 deoR [Ø80 dLacδ (lacZ) M15] | Hanahan (1983) | |
| SCS110 | rpsL (Strr) thr leu endA thi-1 lacY galK galT ara tonA tsx dam dcm supE44Δ(lac-proAB) [F′traD36 proAB lacIqZΔ M15] | Stratagene | |
| Plasmids | |||
| pHP1 | H. pylori–E. coli shuttle vector (AmpR, KanR) | Kleanthous et al. (1991) | |
| pHPT180 | aphA in ureB of pHPT177 (AmpR, KanR) | Takeuchi et al. (1998) and unpublished resultsa | |
| pBluescript II | 2.9 kb cloning vector, AmpR, SK(–) | Stratagene | |
| pUC19 | ColE1 (AmpR, KanR) | Phamacia | |
| pAD1 | pUC19, Pure′ureAB | Ando et al. (1999) | |
| pBSC103 | AmpR, CamR | Wang and Taylor (1990) | |
| pANDO91/92 | pUC19, Pure-HP0091-HP0092-cat′ureAB | This study | |
| pANDO92 | pUC19, Pure-HP0092-cat′ureAB | This study | |
| pANDO91 | pBluescript II SK–, HP0091::cat | This study | |
- a . pHPT177 includes a 12.8 kb fragment containing the entire urease gene operon (ureA to ureH) from strain HPK5. Tsuda et al. (1993) inserted a 1.3 kb aphA cassette into pHPT177 to create pHPT180.
DNA and protein techniques
Standard molecular techniques were used (Sambrook et al., 1989). H. pylori chromosomal DNA was prepared from cells of each strain after 48 h growth on two agar plates, as described previously (Wilson, 1995). Plasmid DNA was prepared from H. pylori after 48 h growth, or from E. coli after overnight cultures using a midi-prep protocol (Qiagen) according to the manufacturer's instructions. All columns used for protein purification were obtained from Pharmacia, if not specifically indicated. PCR reactions were performed by standard methods in a reaction volume of 50 µl containing 0.5 U of Taq (Qiagen), 1.5 mM MgCl2 and 200 ng of each primer.
Transformation of strains with chromosomal DNA
H. pylori strains HPK5, 84-183, 60190 and J188 were tested for their ability to be transformed by chromosomal DNA from spontaneous streptomycin-resistant derivatives (point mutation at codon 43 of rps12) of these same four H. pylori strains. Recipient H. pylori cells were harvested from 48 h growth on a single agar plate into 1.0 ml of phosphate-buffered saline (PBS, pH 7.4), then centrifuged at 5000 g for 5 min, and the pellet was resuspended in 300 µl of PBS. Each transformation mixture, consisting of 25 µl of recipient cells and 30 ng of donor DNA, was spotted onto a TSA plate, then incubated overnight at 37°C in 5% CO2. The transformation mixture was harvested into 1.0 ml of PBS, and 100 µl of appropriate 10-fold serial dilutions was plated on BS streptomycin (Str) and TSA plates. All plates were incubated for 4 days at 37°C in an atmosphere with 5% CO2. Transformation frequency was determined by counting the number of StrR transformants and total viable cells. In other experiments, we used PCR products of 100 (BA5474 and BA5475), 224 (AN307 and C7434) and 395 (AN307 and AN308) bp encompassing the rps12 codon 43 point mutation to attempt transformation.
Transformation of strains with plasmid DNA
H. pylori strain HPK5 was transformed to KanR by electroporation (Kuipers et al., 1998) with pHP1, a hybrid H. pylori–E. coli shuttle plasmid that carries the aphA cassette conferring KanR (Kleanthous et al., 1991). pHP1 was isolated from the transformed HPK5 cells by alkaline lysis (Qiagen) and designated pHP1HPK5. Transformation of 32 H. pylori strains (Table 6) with pHP1HPK5 was then attempted. The methods for transformation were identical to those for chromosomal DNA, except that each transformation mixture, consisting of 25 µl of recipient cells and 100 ng of plasmid DNA, was spotted onto a TSA plate, and transformants were selected on BS Kan plates. Transformants were expanded, and cells were harvested from 48 h growth on two plates into 1.0 ml of PBS and centrifuged at 5000 g for 5 min. Plasmid DNA was prepared using QIAprep spin miniprep kits (Qiagen). Plasmid pHP1 recovered from 10 other strains was subsequently used to attempt transformation of all 32 strains. In addition, transformation of strains HPK5 and 60190 with pHP1HPK5 or pHP160190 by electroporation was attempted, as described previously (Kuipers et al., 1998). E. coli DH5α or SCS110 was transformed by the calcium chloride method (Seidman et al., 1997), with pHP1 from transformed H. pylori strains as plasmid donors.
Restriction endonuclease digestion of H. pylori chromosomal DNA
Chromosomal DNA from 19 H. pylori strains was subjected to digestion with the REs NlaIII, MboII, HinfI, FokI, HaeIII, HpaII, DdeI, HindIII, MboI, CvirI, TaqI, Hpy178III and Hpy188I. All enzymes were from New England Biolabs or prepared as part of the investigation of H. pylori REs. For each reaction, 400 ng of DNA was incubated with the enzyme and appropriate buffer for 2 h. In addition, for a total of 100 clinical H. pylori isolates from different parts of the world, chromosomal DNA was prepared and subjected to digestion with MboI. After incubation, digestion patterns were compared by electrophoresis on a 1.0% agarose gel. pHP1 derived from E. coli DH5α or H. pylori strains that had been successfully transformed was subjected to digestion with the REs NlaIII, MboII, HinfI, FokI, HaeIII, HpaII, DdeI or HindIII and analysed as described above.
PCR for MboI-like R–M system
As the genome sequences of two H. pylori strains indicate a variable set of R–M systems in independent H. pylori strains, we determined whether the MboI-like RM system was present in H. pylori by PCR. Based on the 26695 sequence, for HP0091 (MboI.R homologue), we used primer pair C9065 and C9066, and for HP0092 (MboI.M homologue), we used primer pair BA3136 and BA3137.
Introduction of MboI R–M homologues HP0091/0092 or MboI.M homologue HP0092 to JP26
As JP26 DNA was found to be sensitive to MboI digestion, we introduced the MboI R–M homologues (HP0091/HP0092) or the MboI.M homologue (HP0092) alone to JP26. Portions of the H. pylori urease operon (ureAB) and its promoter region (Pure) were amplified from strain 26695 by PCR using primers described in Table 6, and these products were ligated into pUC19 to create pAD1 (Ando et al., 1999). HP0091 and HP0092 or HP0092 alone were amplified from chromosomal DNA from strain 26695, using primers BA3135 and BA3137, or BA3136 and BA3137, respectively, and ligated into pAD1 between Pure and ureAB. The chloramphenicol resistance cassette (cat) was isolated from SmaI- and EcoRV-digested pBSC103 (Wang and Taylor, 1990) and ligated between HP0091/HP0092 or HP0092 and ureAB to create pANDO91/92 or pANDO92 (Fig. 2B). JP26 was transformed with pANDO91/92 or pANDO92, resulting in JP26-91/92, or JP26-92 (Fig. 2C). The identities of JP26-91/92 and JP26-92 were confirmed by PCR using primers A1747 and A1746 (Ando et al., 1999) and MboI digestion of chromosomal DNA (Fig. 2D and E).
Disruption of MboI.R homologue in H. pylori strain B146
Because chromosomal DNA from H. pylori strain B146 was found to be resistant to MboI digestion (see below) by PCR using primer pairs C9065 and C9066, and BA3136 and BA3137, we determined whether genes HP0091 and HP0092 were present. We then disrupted the MboI.R homologue of strain B146. The HP0091 ORF of strain 26695 was amplified using primers BA3138 and BA3139, and the product was ligated into pBluescript and used to transform into E. coli DH5α. A unique EcoRI site was created by inverse PCR with primers BA3140 and BA3141. The CamR cassette (cat) was amplified from pBSC103 using primers C8935 and C8936, which also added EcoRI restriction sites. This cassette was ligated into the inverse PCR product to disrupt the HP0091 ORF, creating pANDO91. H. pylori strain B146 was transformed to chloramphenicol resistance with pANDO91 to create B146-91::cat. Chromosomal DNA was isolated from strain B146-91::cat, and the insertion of the cat cassette within HP0091 in the transformants was confirmed by PCR using primers BA3429 and BA3137.
Transformation using supercoiled, circular or linear plasmid DNA
To determine whether the structure of donor pHP1 affected the ability of H. pylori cells to be transformed, we linearized pHP1HPK5 by KpnI digestion. Linear pHP1HPK5 was also religated to produce open circular pHP1. For comparison, we used pHPT180 (kindly provided by T. Nakazawa) as a suicide vector control. pHPT180, derived from pHPT177 (Takeuchi et al., 1998), contains an aphA cassette inserted at the BamHI site within H. pylori ureB (T. Nakazawa, personal communication). Linearized pHPT180 was produced by NdeI digestion. For transformation, 100 ng of the pHP1HPK5 or pHPT180 preparations was incubated with HPK5 cells, and transformants were selected for KanR.
Purification of restriction endonucleases from JP26
Heparin Hitrap, Q sepharose and HAP columns were used to purify REs from H. pylori strain JP26. Strain JP26 was grown in Brucella broth for 48 h; 10 g of cells were harvested by centrifugation, and the cell pellet was stored at −70°C before use. To begin purification of endonucleases, the frozen cells were thawed on ice, resuspended in ice-cold buffer A [20 mM Tris-HCl, 0.5 mM EDTA, 1 mM dithiothreitol (DTT), pH 7.5], then sonicated until approximately 50 µg protein g−1 cells was released. The lysate was centrifuged, and the supernatant was applied to a 5 ml heparin Hitrap column. The column was washed with 50 ml of buffer A containing 0.05 M NaCl and eluted with a linear gradient of 0.05–0.8 M NaCl in buffer A in a total volume of 55 ml. Fractions were assayed for endonuclease activity by incubation at 37°C for 1 h in NEB buffer 4 (50 mM KOAc, 20 mM TrisOAc, 10 mM Mg(OAc)2, 1 mM DTT, pH 7.9), using 1.0 µg of phage λ or T7 DNA as substrate, and examined by electrophoresis on agarose gels. The fractions containing endonuclease activity were pooled, diluted to 50 mM NaCl with buffer A and applied to a 7 ml Q sepharose column, which was eluted with the same NaCl gradient as the first column in a total volume of 75 ml. Fractions were assayed for endonuclease activity, which revealed the activity of Hpy26I. The passthrough fraction was applied to a 7 ml HAP column, eluted with a linear 0.025–0.4 M gradient of potassium phosphate (pH 7.5) in the presence of 0.2 M NaCl in a total volume of 75 ml. Fractions were assayed for endonuclease activity, which revealed the activity of Hpy26II. After endonuclease assays, the purified REs were collected and stored at 4°C.
Determination of recognition sequences of purified REs
To map the recognition sequences of the purified REs, fractions containing endonuclease activity were used to cleave pUC19, pBR322 and φX174 DNA. Double digestions were also performed in the presence of a second RE that has a single known recognition site in the substrate DNA (EcoO109I, PstI and AlwNI for pUC19; ClaI, NruI, NdeI and PstI for pBR322; and PstI, SspI, NciI and StuI for φX174 (DNA). The recognition sequence of each RE was determined by mapping the locations of several RE cleavage sites in these DNAs based on the sizes of restriction fragments from single and double digestions and comparing the DNA sequences of these regions for homology, using the sites program (deHaseth et al., 1998) to generate potential recognition sequences. We then compared the predicted cleavage fragments of putative recognition sequences with the observed fragments produced by cleavage of various other substrate DNAs by the newly purified REs.
Acknowledgements
This work was supported in part by R01DK53707 from the National Institutes of Health and by the Medical Research Service of the Department of Veterans Affairs. We thank Drs Richard D. Morgan and Richard J. Roberts for their help and support, and T. Nakazawa and M. Tsuda for providing pHPT180.




