Entamoeba histolytica cysteine proteinases with interleukin-1 beta converting enzyme (ICE) activity cause intestinal inflammation and tissue damage in amoebiasis
Abstract
The protozoan parasite Entamoeba histolytica causes intestinal inflammation and ulceration. Amoebic trophozoites activate the transcription factor NF-κB in human intestinal epithelial cells, initiating an inflammatory response programme with resultant damage to the intestinal tissue. Amoebic cysteine proteinases have been proposed as important virulence factors for amoebiasis. To test the role of amoebic cysteine proteinases in the pathogenesis of amoebic colitis, human intestinal xenografts in SCID mice were infected with E. histolytica trophozoites expressing an antisense message to ehcp5. The cysteine proteinase-deficient amoeba failed to induce intestinal epithelial cell production of the inflammatory cytokines interleukin (IL)-1B and IL-8, and caused significantly less gut inflammation and damage to the intestinal permeability barrier. The critical role of amoebic cysteine proteinases in human gut inflammation and tissue damage may be explained by our discovery that amoebic cysteine proteinases possess IL-1B converting enzyme (ICE) activity. This ICE activity could contribute to intestinal inflammation by activating human pIL-1B released by damaged intestinal cells. These results demonstrate for the first time that amoebic cysteine proteinases are a key virulence factor in amoebic colitis, and provide a novel mechanism for their activity.
Introduction
The protozoan parasite Entamoeba histolytica causes amoebic dysentery and amoebic liver abscess, and is one of the leading causes of death from parasitic diseases worldwide (Walsh, 1986). Intestinal inflammation and ulceration are the hallmarks of amoebic dysentery (Kanani and Knight, 1969; Tucker et al., 1975; Stanley and Li, 1993). Studies in a human intestinal xenograft model of disease indicate that E. histolytica trophozoites can initiate an inflammatory response programme in human intestinal epithelial cells by activating the transcription factor NF-κB and stimulating the release of inflammatory mediators (Seydel et al., 1997; 1998). Intestinal epithelial cell production of interleukin (IL)-1B and IL-8 causes an influx of inflammatory cells into the intestinal mucosa with resultant tissue damage. Although the initiation of the host intestinal inflammatory response in vivo requires contact between intestinal cells and live amoebic trophozoites, human intestinal epithelial cells distal to the site of amoebic contact also produce cytokines, consistent with a role for soluble mediators, such as IL-1, in activating neighbouring epithelial cells (Seydel et al., 1997).
To identify amoebic molecules capable of inducing the human intestinal inflammatory response programme, we first looked at the E. histolytica neutral cysteine proteinases. These neutral cysteine proteinases are secreted in large quantities by the parasite and are established virulence factors in animal models of amoebic liver abscess (Li et al., 1995; Stanley et al., 1995; Bruchhaus et al., 1996; Ankri et al., 1999). A family of six genes encodes prepro-forms of E. histolytica cysteine proteinases (ehcp1–6), with approximately 90% of proteinase activity secondary to EhCP1, EhCP2 and EhCP5 (Bruchhaus et al., 1996). Here, we report that the expression of amoebic cysteine proteinases is necessary for E. histolytica to cause intestinal inflammation and tissue damage in amoebic colitis. We also find that amoebic cysteine proteinases have IL-1 converting enzyme (ICE) activity, which may play an important role in amoebic induction of intestinal inflammation.
Results
E. histolytica trophozoites deficient in cysteine proteinase production fail to induce gut inflammation in human intestine
To assess the role of E. histolytica cysteine proteinases in inducing intestinal inflammation and tissue damage, we transfected virulent E. histolytica HM1:IMSS trophozoites with plasmid pSA8, which permits episomal expression of an antisense message to ehcp 5. Under continuous G418 selection, this antisense construct reduces total amoebic cysteine proteinase expression to 10% of the levels seen in E. histolytica trophozoites transfected with the parent pEh-NeoAct plasmid (Ankri et al., 1998). Human intestinal xenografts in severe combined immunodeficient (SCID) mice were infected by intraluminal inoculation with pSA8-transfected amoebae, pEh-NeoAct-transfected amoebae or wild-type HM1:IMSS trophozoites. Four hours after their inoculation into human intestinal xenografts, both pSA8-transfected trophozoites and pEh-NeoAct-transfected trophozoites could be seen within the lumen of the gut adhering to the intestinal mucosa (Fig. 1A and B). This indicates that reduced expression of the E. histolytica cysteine proteinases did not obviously effect the ability of pSA8-transfected trophozoites to migrate through the mucin layer and reach the colonic mucosa. However, by 24 h after amoebic inoculation, wild-type E. histolytica trophozoites or pEh-NeoAct-transfected trophozoites had stimulated human intestinal xenograft production of human IL-1B and IL-8 (Fig. 2A and B). In contrast, pSA8-transfected amoebae failed to stimulate production of human IL-1B or IL-8 in infected human intestinal xenografts (Fig. 2A and B). Intestinal epithelial cell production of IL-1 and IL-8 is associated with an influx of neutrophils into the intestinal mucosa and lumen of the human intestinal xenograft (Seydel et al., 1997; 1998). The control pEh-Neo-Act-transfected amoebae provoked a significant inflammatory response, and amoebic trophozoites within mucosal and submucosal tissues were surrounded by neutrophils at 24 h (Fig. 1D). The inflammatory response to pSA8-transfected amoebae was much less prominent, even in areas with detectable mucosal damage (Fig. 1C). These findings were quantified by measuring intestinal tissue levels of the neutrophil/monocyte-specific enzyme myeloperoxidase (MPO). MPO levels were significantly lower in human intestinal xenografts infected with pSA8-transfected E. histolytica trophozoites compared with pEh-NeoAct-transfected amoebae or wild-type HM1:IMSS trophozoites (Fig. 2C), consistent with a reduction in inflammatory cell infiltration into intestinal xenografts infected with pSA8-transfected trophozoites.
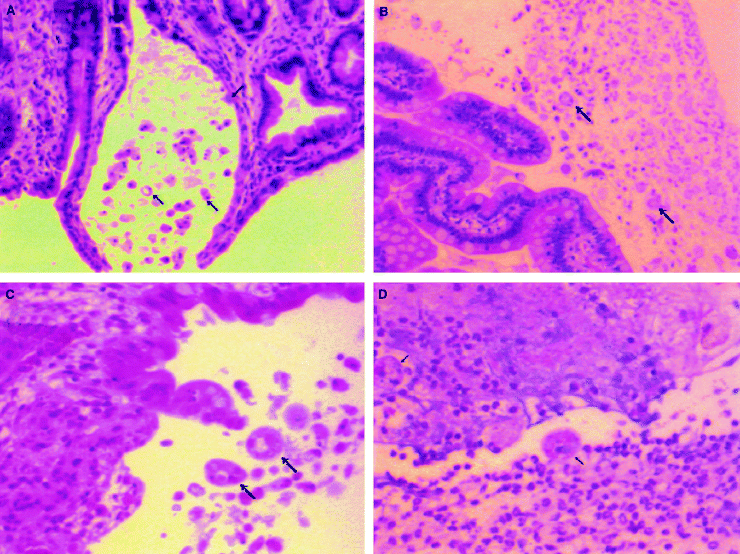
pSA8-transfected E. histolytica trophozoites survive within the intestinal lumen and bind to intestinal epithelial cells, but fail to induce intestinal inflammation. Histological sections of human intestine obtained 4 h after inoculation with pSA-8-transfected amoebae (A), 4 h after inoculation with pEh-NeoAct-transfected amoebae (B), 24 h after inoculation with pSA-8-transfected amoebae (C) or 24 h after inoculation with pEh-NeoAct-transfected amoebae (D). Selected E. histolytica trophozoites are indicated by the arrows. Haematoxylin and eosin stain, magnification 100× for (A) and (B); magnification 200× for (C) and (D).
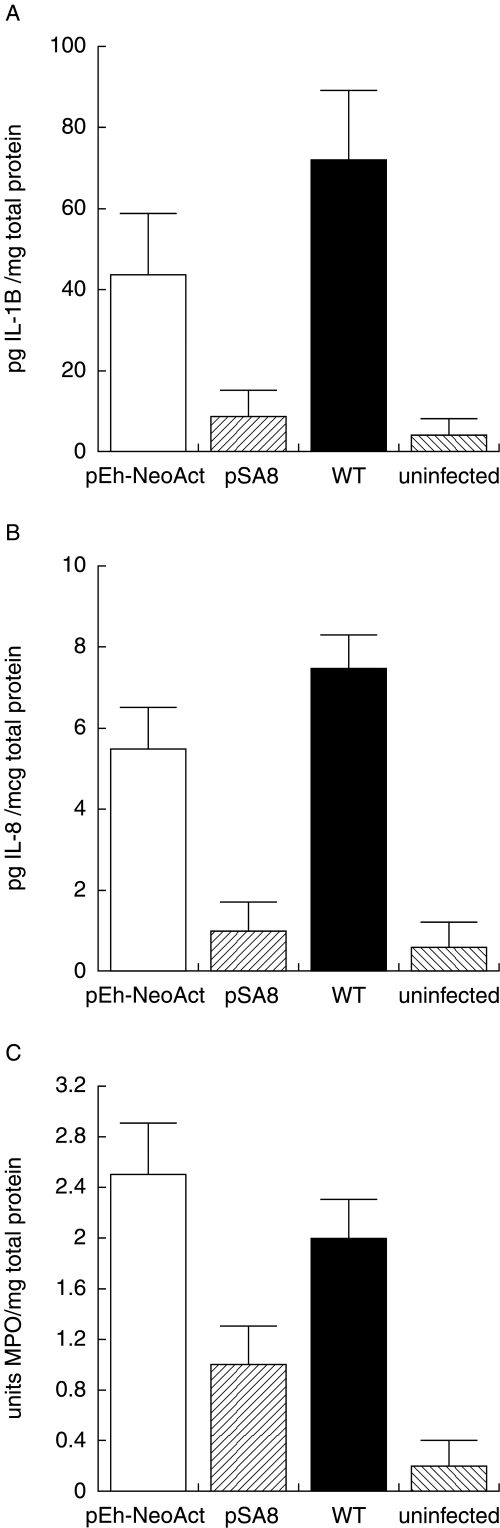
pSA8-transfected E. histolytica trophozoites fail to activate cytokine production or inflammation in infected human intestine.
A. Intestinal tissue levels of IL-1B, (B) IL-8 or (C) MPO, measured 24 h after infection with pSA8-transfected trophozoites, pEh-NeoAct-transfected trophozoites or wild-type HM1:IMSS trophozoites and levels in uninfected human intestinal xenografts. Values are the means ± SE of six to eight intestinal xenografts in each group. The P-values for the difference in the means between pSA8-transfected E. histolytica trophozoites and pEh-NeoAct-transfected trophozoites are P < 0.01 for IL-1B, P < 0.01 for IL-8 and P < 0.05 for MPO.
E. histolytica trophozoites deficient in amoebic cysteine proteinase production fail to damage human intestine
Human intestinal xenografts maintain the intestinal permeability barrier to the flow of higher molecular weight molecules, and fluoresceinated dextran (FITC-dextran) introduced into the lumen of the intestinal xenograft will not reach the systemic circulation of the SCID mouse (Seydel et al., 1998). However, when FITC-dextran is introduced into the lumen of a human intestinal xenograft that has been infected for 24 h with E. histolytica, there is a rapid (within 2 h) appearance of FITC-dextran in the circulation of the SCID mouse (Seydel et al., 1998). FITC-dextran introduced into the lumen of human intestinal xenografts infected for 24 h with pEh-NeoAct-transfected E. histolytica trophozoites appeared in the circulation of SCID mice within 2 h and was detectable at higher levels by 4 h (Fig. 2). This is the same pattern seen with infection with wild-type E. histolytica trophozoites (Seydel et al., 1998). In contrast, FITC-dextran introduced into the lumen of human intestinal xenografts infected for 24 h with pSA8-transfected E. histolytica trophozoites showed little or no flux into the systemic circulation of SCID mice at 2 or 4 h after inoculation (Fig. 3). The differences seen in intestinal barrier function could be correlated with histologic findings of much more severe mucosal and submucosal damage in human intestinal xenografts infected with the control pEh-Neo-Act-transfected trophozoites compared with human intestinal xenografts infected with pSA8-transfected amoebae (Fig. 1C and D). In contrast to the control pEh-Neo-Act-transfected amoebae, which invaded into submucosal tissues, pSA-8-transfected amoebae were rarely seen invading beyond the mucosal layer (Fig. 1D).

pSA8-transfected E. histolytica trophozoites do not damage the intestinal permeability barrier. The flux of FITC-dextran from the lumen of human intestinal xenografts into the circulation of SCID mice immediately before (0 h) FITC-dextran inoculation and 2 and 4 h later was measured in mice whose xenografts had been infected for 24 h with pSA-8-transfected E. histolytica trophozoites, pEh-NeoAct-transfected E. histolytica trophozoites, wild-type HM1:IMSS trophozoites or uninfected grafts. Values are the means ± SE of six to eight intestinal xenografts in each group.
E. histolytica cysteine proteinases have ICE activity
We next explored how E. histolytica cysteine proteinases cause intestinal inflammation and tissue damage. Lysates of amoebic trophozoites, which contain cysteine proteinase activity, do not cause intestinal inflammation or tissue damage when introduced into human intestinal xenografts (Seydel et al., 1997). In addition, pSA8-transfected E. histolytica trophozoites maintain the ability to lyse target cells, indicating that the failure of pSA8-transfected amoebic trophozoites to induce intestinal inflammation and damage was not secondary to an inability to kill host cells (Ankri et al., 1998). This suggested that cysteine proteinases might exert their effects after an initial phase of infection in which amoebic trophozoites damage or kill intestinal epithelial cells through contact-mediated cytotoxicity using the amoebapore molecule (Jarumilinta and Kradolfer, 1964; Ravdin et al., 1980; Bracha et al., 1999). Injured or necrotic epithelial cells release IL-1B in the form of the biologically inactive pIL-1B, which requires proteolytic cleavage by the IL-1 converting enzyme (ICE or caspase-1) to form active mIL-1B (Hogquist et al., 1991). We postulated that E. histolytica cysteine proteinases contribute to gut inflammation by proteolytically activating the pIL-1B released from damaged or necrotic intestinal epithelial cells (Kapur et al., 1993). The activated IL-1B produced by extracellular E. histolytica cysteine proteinases could then induce NF-kB activation and the resultant production of inflammatory mediators in nearby intestinal epithelial cells not in direct contact with amoebic trophozoites. We found that amoebic lysates could cleave 35S-labelled human pIL-1B into a species of approximately 18 kDa, the size of mIL-1B (Fig. 4A) (Thornberry et al., 1992). This cleavage was blocked by the specific cysteine proteinase inhibitor E-64 (trans-epoxysuccinyl-l-leucylamido-(4-guanidino)butane), but was not inhibited by the serine proteinase inhibitor phenylmethylsulphonyl fluoride (data not shown). Purified E. histolytica cysteine proteinase had identical activity, cleaving recombinant human pIL-1B into the 18 kDa molecule (Fig. 4B). Tryptic peptide analysis and amino acid sequencing of the separated peptides derived from the 18 kDa band indicated that cleavage of pIL-1B occurred between Ser-121 and Leu-122, creating a product that is five amino acids shorter than the product of ICE cleavage (Thornberry et al., 1992). This cleavage site is consistent with the requirement of the E. histolytica cysteine proteinase for a basic amino acid (Arg) in the P2 position (Scholze and Schulte, 1988). To determine whether the product of E. histolytica cysteine proteinase cleavage of pIL-1B was active, we used a biological assay with L929 cells, which will produce nitric oxide in response to stimulation with IL-1 and interferon-γ. Neither recombinant pIL-1B nor purified E. histolytica cysteine proteinase alone could stimulate nitrite production in L929 cells in the presence of interferon-γ (Fig. 4C). However, when recombinant pIL-1B was incubated with purified E. histolytica cysteine proteinases, the product stimulated a more than 10-fold increase in nitrite production from L929 cells. These data show that E. histolytica cysteine proteinases can convert human pIL-1B into a biologically active form of IL-1B.
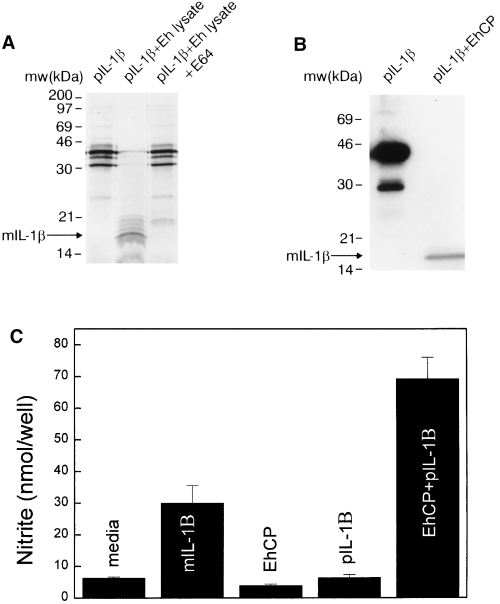
E. histolytica cysteine proteinases cleave pIL-1B to form the active cytokine.
A. E. histolytica lysates (Eh lysate) cleave 35S-labelled human pIL-1B into a species of approximately 18 kDa, the size of mature IL-1B. This cleavage is blocked by the specific cysteine proteinase inhibitor E-64.
B. Purified E. histolytica cysteine proteinase cleaves recombinant pIL-1B into the 18 kDa species.
C. Addition of pIL-1B incubated with purified E. histolytica cysteine proteinase (EhCP) to L929 cells induces nitrite production, whereas the addition of pIL-1B alone or EhCP alone fails to induce nitrite synthesis from L929 cells.
Discussion
Our results suggest a new model for amoebic pathogenesis, in which the interactions between E. histolytica virulence factors and specific components of the human intestinal inflammatory response contribute to disease. We have shown previously that E. histolytica activation of NF-κB in intestinal epithelial cells is required for inflammation and tissue damage in human intestinal disease, and that host neutrophils are the critical effector cells in this process (Seydel et al., 1997; 1998). In this study, we have identified the first parasite virulence factor for intestinal amoebiasis by showing that expression of E. histolytica cysteine proteinases is required for the induction of tissue damage and inflammation in a human intestinal xenograft model of amoebic dysentery. Cysteine proteinase-deficient amoebae maintained the ability to survive within the human intestinal xenograft and could bind to intestinal epithelial cells. However, quantitative studies of inflammation and intestinal permeability revealed that cysteine proteinase-deficient amoebae fail to stimulate production of inflammatory cytokines from intestinal epithelial cells, do not induce neutrophil influx into infected human intestine and do not damage the intestinal permeability barrier. These results were consistent with histological studies, which showed less inflammation and tissue damage in human intestinal xenografts infected with cysteine proteinase-deficient amoebae. The histological findings also suggested that cysteine proteinase-deficient amoebae may be defective in their ability to invade through mucosal tissue, as cysteine proteinase-deficient amoebae were rarely detected in the submucosal tissues.
There are multiple mechanisms by which E. histolytica cysteine proteinases may contribute to amoebic virulence in intestinal disease. As noted above, cysteine proteinases may be important in amoebic invasion through intestinal tissue, and their ability to cleave extracellular matrix proteins, such as laminin and fibronectin, may be key to the invasion process (Keene et al., 1986; Schulte and Scholze, 1989; Li et al., 1995). Cysteine proteinase-deficient amoebic trophozoites are defective in phagocytosis, and this may contribute to their reduced virulence in intestinal disease as well (Ankri et al., 1998).
Kapur et al. (1993) have shown previously that an extracellular cysteine proteinase from Streptococcus pyogenes could cleave pIL-1B to produce active IL-1B. We have now found that E. histolytica cysteine proteinases, which cleave at a different site from ICE, or the S. pyogenes proteinase can also function as an ICE-like protease and activate pIL-1B. This finding suggests an additional mechanism by which E. histolytica cysteine proteinases could contribute to intestinal inflammation. We postulate that amoebic trophozoites first bind to intestinal epithelial cells, than lyse those cells through the action of amoebapore (Leippe, 1997; Bracha et al., 1999). Lysed cells release pIL-1B, which is then cleaved by secreted amoebic cysteine proteinases to form the active mIL-1B. The mIL-1B then stimulates the production of inflammatory mediators in adjacent intestinal cells through a pathway involving activation of intestinal epithelial cell NF-κB (Seydel et al., 1997). Thus, amoebic cysteine proteinases would augment the intestinal inflammatory response and facilitate its spread beyond cells in direct contact with amoebic trophozoites.
Finally, the finding that E. histolytica cysteine proteinases can mimic the action of a member of the caspase family may have important implications for the pathogenesis of amoebic liver abscess as well. Studies using a murine model of amoebic liver abscess have shown that E. histolytica can induce apoptosis in hepatocytes by a Fas ligand-, tumour necrosis factor alpha-independent pathway (Seydel and Stanley, 1998). The ICE-like activity of amoebic cysteine proteinases suggests a mechanism whereby amoebic cysteine proteinases could activate the caspase cascade within hepatocytes, thus inducing death by apoptosis.
Experimental procedures
E. histolytica strains and transfection
E. histolytica trophozoites (strain HM1:IMSS), which have been passaged multiple times through murine and gerbil livers to maintain virulence, were transfected with the parent pEhNeo-Act plasmid or the pSA8 plasmid (containing an insert encoding 877 bp of the coding region of EhCP5 in antisense orientation) as described previously (Ankri et al., 1998). Cells were allowed to recover for 2–3 days, and G418 concentration gradually increased until it reached 60 µg ml−1. Trophozoites in the log phase of growth were used for all experiments.
Preparation of E. histolytica lysates
E. histolytica lysates were prepared from 1 × 106 trophozoites suspended in 2 ml of phosphate buffered saline (PBS) with 1% NP-40. Cysteine proteinase activity of lysates was measured using the chromophoric substrate benzyuloxycarbonyl-l-arginyl-l-arginine-p-nitroanilide (Z-Arg-Arg-pNA) as described previously (Ankri et al., 1998). One unit of proteinase activity is defined as the µmol of substrate digested min−1 mg−1 protein. Lysates obtained from our virulent wild-type E. histolytica HM1:IMSS lysates had mean cysteine proteinase activity of 62 U; lysates from pEh-NeoAct-transfected amoebae had mean cysteine proteinase activity of 55 U, whereas lysates from pSA8-transfected amoebae had mean cysteine proteinase activity of 6 U.
Generation of SCID-HU-INT mice and human xenograft infection
Human intestinal xenografts were engrafted onto the rear flanks and suprascapular regions of SCID mice (6–8 weeks old) as described previously (Seydel et al., 1997). Log-phase cultures of E. histolytica HM1:IMSS trophozoites transfected with pSA8 or pEh-NeoAct were chilled on ice for 10 min, pelleted by centrifugation at 500 g for 5 min and resuspended in B1-S-33 medium containing 60 µg ml−1 G418 at 106 trophozoites 100 µl−1. The amoebic suspension (100 µl) was injected directly into the lumen of the grafts via a 26-gauge needle.
Assay of human IL-1B and IL-8
Intestinal tissue samples for enzyme-linked immunosorbent assay (ELISA) of human IL-1B and human IL-8 were prepared by homogenizing tissue at 50 mg ml−1 in a solution of PBS containing 1 µg ml−1 each of aprotinin, leupeptin and pepstatin A (Sigma). After centrifugation at 12 000 g for 15 min, supernatants were processed for cytokine ELISAs according to the manufacturer's protocol (Endogen). The sensitivities were 1 pg ml−1 for IL-1B and 2 pg ml−1 for IL-8.
Assay of MPO activity and human intestinal xenograft permeability
MPO activity in lysates of human intestinal tissue was determined by measuring conversion of the substrate 1.28 mM 3,3′,5,5′-tetramethylbenzidine dihydrochloride (Sigma) exactly as described previously (Seydel et al., 1998). To measure intestinal permeability, 50 µl of FITC-dextran (Sigma) in endotoxin-free PBS at a concentration of 5 mg ml−1 solution was injected directly into the lumen of the human intestinal xenograft (Seydel et al., 1998). Two and 4 h after injection of the fluorophore, animals were bled, and 20 µl of blood was diluted into 400 µl of 150 mM NaCl−50 mM Tris, pH 10.3, and spun at 2000 g for 15 min. The supernatants were analysed on a Cytoflour 23000 fluorescent plate reader (Millipore).
Assay of pIL-1B proteolytic cleavage
The plasmid pSFFV-Neo containing the human pIL-1B sequence under a T7 promoter was provided by David Chaplin of Washington University. For in vitro translation, the plasmid was first linearized using the XbaI site, then the Promega TnT coupled rabbit reticulocyte lysate system (Promega) was used to generate 35S-labelled pIL-1B according to the manufacturer's instructions. A 1 µl aliquot of [35S]-methionine human pIL-1B was reacted with a 50 µl aliquot of E. histolytica HM1:IMSS lysate and incubated for 5 min at 30°C. Control samples were reacted with 50 µl of PBS rather than amoebic lysates. E-64 at a final concentration of 500 µM was added to some samples. A 10 µl aliquot of the mixture was resolved by SDS–PAGE and analysed by autoradiography. For prokaryotic expression of human pIL-1B, the pIL-1B coding sequence was cloned from pSFFV-Neo into the PET-30a(+) vector (Novagen) using the XbaI and HindIII sites. The human pIL-1B with six histidine residues at the N-terminus was purified on Ni-NTA agarose (Qiagen). Purity was assessed using SDS–PAGE and immunoblotting using rabbit anti-human IL-1B (Sigma). The purified recombinant human pIL-1B (100 ng) was mixed with purified E. histolytica cysteine proteinases (100 ng) in a total volume of 4.5 ml of PBS (pH 7.4) and incubated at 30°C for 30 min. A 25 µl aliquot of the mixture was resolved by SDS–PAGE, transferred to nitrocellulose, then reacted with a 20 µg ml−1 solution of rabbit anti-human IL-1B antibody (Sigma). The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit antibody (Sigma). E. histolytica cysteine proteinases were purified from lysates of E. histolytica HM1:IMSS on laminin–sepharose as described previously (Li et al., 1995). Purity was assessed using SDS–PAGE and immunoblotting with rabbit antiserum to recombinant EhCP1 (Li et al., 1995). The reaction products from the incubation of recombinant human pIL-1B and purified E. histolytica cysteine proteinase were separated by SDS–PAGE, and the 18 kDa band was excised and subjected to in-gel proteolytic digestion with trypsin. Peptides from the tryptic digestion were separated using microcapillary high-performance liquid chromatography (HPLC)/mass spectroscopy analysis and sequenced at the Harvard Microchemistry Facility (Dongre et al., 1997).
Assay of IL-B activity
Confluent stage L929 cells (grown in MEM with 10% heat-inactivated fetal calf serum) were plated on 96-well tissue culture plates at a density of 2 × 105 cells well−1 and incubated with recombinant interferon (IFN)-gamma (50 U well−1; R and D Systems) in the presence or absence of 100 ng well−1 recombinant human pIL-1B, 2 ng well−1 IL-1B (Sigma) and 100 ng well−1 purified E. histolytica cysteine proteinases for 24 h at 37°C under 5% CO2. To measure nitrite production, 100 µl of each sample was transferred to a new 96-well plate and reacted with 100 µl of developing reagent (equal volumes of 1.32% sulphanilamide in 60% acetic acid and 0.1% N-1-naphthyl-ethylenediamine-HCl). Sodium nitrite (1 mM) was diluted 1:10 with medium and serially diluted to generate the standard curve for nitrite concentration. The reaction was allowed to proceed for 10 min, and absorbance was read at 540 nM.
Acknowledgements
We thank Dr David Chaplin for providing the pSFFV-Neo plasmid, and the laboratory of Dr Robert Schreiber for assistance with the L929 cell assay. We also thank Dr Deborah Rubin from the Morphology Core of the Washington University Digestive Diseases Research Core Center for assistance with photomicroscopy. This study was supported by NIH grants AI30084 and AI01231 (S.L.S.), grant DK52574 from the Washington University Digestive Diseases Research Core Center, and the Center for the Study of Emerging Diseases, Jerusalem, Israel (D.M.). E.L. is a Burroughs Wellcome Scholar in toxicology. S.L.S. is a Burroughs Wellcome Scholar in molecular parasitology.




